Neurological Outcomes in Late Preterm Infants: An Updated Review of Recent Research and Clinical Insights
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Birthweight and Growth Restriction
3.2. Nutrition
3.3. Cerebral Oxygenation
3.4. Hyperbilirubinemia
3.5. Neurological Assessment
3.6. Outcomes
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Correction Statement
Abbreviations
| AGA | Appropriate for gestational age |
| AIMS | Alberta Infant Motor Scale |
| ASQ-2 | Ages and Stages Questionnaires second Edition |
| BIND | Bilirubin-induced neurologic dysfunction |
| BPD | Bronchopulmonary dysplasia |
| BPF | Brain parenchymal fraction |
| BSID II/III | Bayley Scales of Infant and Toddler Development Second/Third Edition |
| CBFi | Index of microvascular cerebral blood flow |
| CC | Corpus callosum |
| cFTOE | Cerebral fractional tissue oxygen extraction |
| CMRO2i | Index of cerebral oxygen metabolism |
| CP | Cerebral palsy |
| crSO2 | Cerebral regional oxygen saturation |
| CS | Cramped–synchronized |
| DCS | Diffuse correlation spectroscopy |
| DD/MR | Developmental delay/mental retardation |
| EUGR | Extrauterine growth restriction |
| FA | Fractional anisotropy |
| FDNIRS-DCS | Functional diffuse near-infrared spectroscopy and diffuse correlation spectroscopy |
| FGR | Fetal growth restriction |
| FiO2 | Fraction of inspired oxygen |
| FSIQ | Full-Scale Intelligence Quotient |
| GA | Gestational age |
| GCC | Genu of the corpus callosum |
| GM | General movement |
| GMA | General Movements Assessment |
| HINE | Hammersmith Infant Neurological Examination |
| HR | Heart rate |
| IVH | Intraventricular hemorrhage |
| LISA/MIST | Less/minimally invasive surfactant administration/therapy |
| MABC-2 | Movement Assessment Battery for Children-2 |
| MDI | Mental Developmental Index |
| MK | Mean kurtosis |
| MOS-R | Motor optimality score—revised |
| MRI | Magnetic resonance imaging |
| NBNA | Neonatal Behavioral Neurological Assessment |
| NDI | Neurodevelopmental impairment |
| NICU | Neonatal intensive care unit |
| OEF | Cerebral oxygen extraction fraction |
| PCP | Primary care pediatrician |
| PDI | Psychomotor Developmental Index |
| PR | Poor repertoire |
| PPROM | Pre-labor preterm rupture of membranes |
| PVL | Periventricular leukomalacia |
| RBC | Red blood cell |
| rhEPO | Recombinant human erythropoietin |
| RK | Radial kurtosis |
| SpO2 | Peripheral arterial oxygen saturation |
| TEA | Term-equivalent age |
| TH | Thalamus |
| TIMP | Test of Infant Motor Performance |
| WBCs | White blood cells |
| WM | White matter |
| WMI | White matter injury |
| WPPSI-V | Wechsler Intelligence Scale for Children-V |
References
- Adams-Chapman, I. Neurodevelopmental outcome of the late preterm infant. Clin. Perinatol. 2006, 33, 947–964. [Google Scholar] [CrossRef] [PubMed]
- Bogdan, R.D.; Bohiltea, R.E.; Toma, A.I. Respiratory follow up of the premature neonates—Rationale and practical issues. J. Clin. Med. 2022, 11, 1746. [Google Scholar] [CrossRef]
- Woythaler, M. Neurodevelopmental outcomes of the late preterm infant. Semin. Fetal Neonatal Med. 2019, 24, 54–59. [Google Scholar] [CrossRef] [PubMed]
- Petrini, J.R.; Dias, T.; McCormick, M.C.; Massolo, M.L.; Green, N.S.; Escobar, G.J. Increased risk of adverse neurological development for late preterm infants. J. Pediatr. 2009, 154, 169–176. [Google Scholar] [CrossRef]
- Soloveichick, M.; Marschik, P.B.; Gover, A.; Molad, M.; Kessel, I.; Einspieler, C. Movement Imitation Therapy for Preterm Babies (MIT-PB): A Novel Approach to Improve the Neurodevelopmental Outcome of Infants at High-Risk for Cerebral Palsy. J. Dev. Phys. Disabil. 2020, 32, 587–598. [Google Scholar] [CrossRef]
- Triggs, T.; Crawford, K.; Hong, J.; Clifton, V.; Kumar, S. The influence of birthweight on mortality and severe neonatal morbidity in late preterm and term infants: An Australian cohort study. Lancet Reg. Health–West. Pac. 2024, 45, 101054. [Google Scholar] [CrossRef]
- Paulsen, H.; Ljungblad, U.W.; Riiser, K.; Evensen, K.A.I. Early neurological and motor function in infants born moderate to late preterm or small for gestational age at term: A prospective cohort study. BMC Pediatr. 2023, 23, 390. [Google Scholar] [CrossRef] [PubMed]
- Toma, A.I.; Dima, V.; Alexe, A.; Bojan, C.; Nemeș, A.F.; Gonț, B.F.; Arghirescu, A.; Necula, A.I.; Fieraru, A.; Stoiciu, R.; et al. Early Intervention Guided by the General Movements Examination at Term Corrected Age—Short Term Outcomes. Life 2024, 14, 480. [Google Scholar] [CrossRef]
- Mitha, A.; Chen, R.; Razaz, N.; Johansson, S.; Stephansson, O.; Altman, M.; Bolk, J. Neurological development in children born moderately or late preterm: National cohort study. BMJ 2024, 384, e075630. [Google Scholar] [CrossRef]
- Ericson, J.; Ahlsson, F.; Wackernagel, D.; Wilson, E. Equally Good Neurological, Growth, and Health Outcomes up to 6 Years of Age in Moderately Preterm Infants Who Received Exclusive vs. Fortified Breast Milk—A Longitudinal Cohort Study. Nutrients 2023, 15, 2318. [Google Scholar] [CrossRef]
- Assar, E.H.; Mohamed, N.N. Outcome of Neonatal Hyperbilirubinemia and Its Effect on Neurological System In Full term and Preterm Baby. Benha Med. J. 2024, 41, 8–18. [Google Scholar] [CrossRef]
- Toma, A.I.; Dima, V.; Alexe, A.; Rusu, L.; Nemeș, A.F.; Gonț, B.F.; Arghirescu, A.; Necula, A.; Fieraru, A.; Stoiciu, R. Correlations between Head Ultrasounds Performed at Term-Equivalent Age in Premature Neonates and General Movements Neurologic Examination Patterns. Life 2024, 14, 46. [Google Scholar] [CrossRef] [PubMed]
- Merino-Andrés, J.; Pérez-Nombela, S.; Álvarez-Bueno, C.; Hidalgo-Robles, Á.; Ruiz-Becerro, I.; Fernández-Rego, F.J. Neonatal hyperbilirubinemia and repercussions on neurodevelopment: A systematic review. Child Care Health Dev. 2023, 50, e13183. [Google Scholar] [CrossRef] [PubMed]
- Toma, A.I.; Dima, V.; Rusu, L.; Nemeș, A.F.; Gonț, B.F.; Arghirescu, A.; Necula, A.; Fieraru, A.; Stoiciu, R.; Andrășoaie, L.; et al. Cerebral Ultrasound at Term-Equivalent Age: Correlations with Neuro-Motor Outcomes at 12–24 Months Corrected Age. Children 2024, 12, 30. [Google Scholar] [CrossRef]
- Côté-Corriveau, G.; Simard, M.N.; Beaulieu, O.; Chowdhury, R.A.; Gagnon, M.M.; Gagnon, M.; Ledjiar, O.; Bernard, C.; Nuyt, A.M.; Dehaes, M.; et al. Associations between neurological examination at term-equivalent age and cerebral hemodynamics and oxygen metabo-lism in infants born preterm. Front. Neurosci. 2023, 17, 1105638. [Google Scholar] [CrossRef]
- Inder, T.E.; Volpe, J.J.; Anderson, P.J.; Ingelfinger, J.R. Defining the neurologic consequences of preterm birth. N. Engl. J. Med. 2023, 389, 441–453. [Google Scholar] [CrossRef]
- Zhang, C.; Zhu, Z.; Wang, K.; Moon, B.F.; Zhang, B.; Shen, Y.; Wang, Z.; Zhao, X.; Zhang, X. Assessment of brain structure and volume reveals neurodevelopmental abnormalities in preterm infants with low-grade intraventricular hemorrhage. Sci. Rep. 2024, 14, 5709. [Google Scholar] [CrossRef]
- Lubián-Gutiérrez, M.; Benavente-Fernández, I.; Marín-Almagro, Y.; Jiménez-Luque, N.; Zuazo-Ojeda, A.; Sánchez-Sandoval, Y.; Lubián-López, S.P. Corpus callosum long-term biometry in very preterm children related to cognitive and motor outcomes. Pediatr. Res. 2024, 96, 409–417. [Google Scholar] [CrossRef]
- Gonzalez-Moreira, E.; Harmony, T.; Hinojosa-Rodríguez, M.; Carrillo-Prado, C.; Juárez-Colín, M.E.; Gutiérrez-Hernández, C.C.; Carlier, M.E.M.; Cubero-Rego, L.; Castro-Chavira, S.A.; Fernández, T. Prevention of Neurological Sequelae in Preterm Infants. Brain Sci. 2023, 13, 753. [Google Scholar] [CrossRef]
- Bobba, P.S.; Weber, C.F.; Malhotra, A.; Bahtiyar, M.O.; Copel, J.; Taylor, S.N.; Ment, L.R.; Payabvash, S. Early brain microstructural development among preterm infants requiring caesarean section versus those delivered vaginally. Sci. Rep. 2023, 13, 21514. [Google Scholar] [CrossRef]
- Silveira, R.C.; Corso, A.L.; Procianoy, R.S. The influence of early nutrition on neurodevelopmental outcomes in preterm infants. Nutrients 2023, 15, 4644. [Google Scholar] [CrossRef] [PubMed]
- Song, I.G. Neurodevelopmental outcomes of preterm infants. Clin. Exp. Pediatr. 2022, 66, 281–287. [Google Scholar] [CrossRef]
- Wolfsberger, C.H.; Pichler-Stachl, E.; Höller, N.; Mileder, L.P.; Schwaberger, B.; Avian, A.; Urlesberger, B.; Pichler, G. Cerebral oxygenation immediately after birth and long-term out-come in preterm neonates—A retrospective analysis. BMC Pediatr. 2023, 23, 145. [Google Scholar] [CrossRef]
- Molloy, E.J.; El-Dib, M.; Soul, J.; Juul, S.; Gunn, A.J.; Bender, M.; Gonzalez, F.; Bearer, C.; Wu, Y.; Robertson, N.J.; et al. Neuroprotective therapies in the NICU in preterm infants: Present and future (Neonatal Neurocritical Care Series). Pediatr. Res. 2024, 95, 1224–1236. [Google Scholar] [CrossRef] [PubMed]
- Toma, A.I. Paediatric neurology: Standardization of neonatal assessment in Romania. Enfance 2023, 4, 333–338. [Google Scholar] [CrossRef]
- Zhou, M.; Wang, S.; Zhang, T.; Duan, S.; Wang, H. Neurodevelopmental outcomes in preterm or low birth weight infants with germinal matrix-intraventricular hemorrhage: A meta-analysis. Pediatr. Res. 2024, 95, 625–633. [Google Scholar] [CrossRef]
- Snir, A.; Zamstein, O.; Wainstock, T.; Sheiner, E. Long-term neurological outcomes of offspring misdiagnosed with fetal growth restriction. Arch. Gynecol. Obstet. 2024, 311, 245–250. [Google Scholar] [CrossRef]
- González-López, C.; Solís-Sánchez, G.; Lareu-Vidal, S.; Mantecón-Fernández, L.; Ibáñez-Fernández, A.; Rubio-Granda, A.; Suárez-Rodríguez, M. Variability in Definitions and Criteria of Extrauterine Growth Restriction and Its Association with Neurodevelopmental Outcomes in Preterm Infants: A Narrative Review. Nutrients 2024, 16, 968. [Google Scholar] [CrossRef]
- Johnson, S.; Evans, T.A.; Draper, E.S.; Field, D.J.; Manktelow, B.N.; Marlow, N.; Matthews, R.; Petrou, S.; E Seaton, S.; Smith, L.K.; et al. Neurodevelopmental outcomes following late and moderate prematurity: A population-based cohort study. Arch. Dis. Child. Fetal Neonatal Ed. 2015, 100, F301–F308. [Google Scholar] [CrossRef]
- Diggikar, S.; Trif, P.; Mudura, D.; Prasath, A.; Mazela, J.; Ognean, M.L.; Kramer, B.W.; Galis, R. Neonatal Hypoglycemia and Neurodevelopmental Outcomes—An Updated Systematic Review and Meta-Analysis. Life 2024, 14, 1618. [Google Scholar] [CrossRef]
- Diggikar, S.; Galis, R.; Nagesh, K.; Pandita, A.; Ognean, M.L.; Rüdiger, M.; Mazela, J.; Kramer, B.W. Surfactant therapy—The conundrum of which infant should be given, when, which drug in what dose via which route of administration? Semin. Fetal Neonatal Med. 2024, 29, 101568. [Google Scholar] [CrossRef] [PubMed]
- McCarthy, E.K.; Dempsey, E.M.; Kiely, M.E. Iron supplementation in preterm and low-birth-weight infants: A systematic review of intervention studies. Nutr. Rev. 2019, 77, 865–877. [Google Scholar] [CrossRef] [PubMed]
- Solis-Garcia, G.; Raghuram, K.; Augustine, S.; Ricci, M.F.; St-Hilaire, M.; Louis, D.; Makary, H.; Yang, J.; Shah, P.S. Hyperbiliru-binemia Among Infants Born Preterm: Peak Levels and Association with Neurodevelopmental Outcomes. J. Pediatr. 2023, 259, 113458. [Google Scholar] [CrossRef] [PubMed]
- Banihani, R.; Seesahai, J.; Asztalos, E.; Church, P.T. Neuroimaging at term equivalent age: Is there value for the preterm infant? A narrative summary. Children 2021, 8, 227. [Google Scholar] [CrossRef]
- Harmony, T. Outcome of infants at risk of brain damage after Katona neurohabilitation therapy. Int. J. Neurorehabilit. 2017, 4, 277. [Google Scholar] [CrossRef]
- Robertson, N.J.; Lingam, I.; Benner, E.J.; Thornton, C. Melatonin as a Therapy for Preterm Brain Injury: What Is the Evidence? Int. J. Mol. Sci. 2023, 24, 11806. [Google Scholar]
- Mead, E.C.; Wang, C.A.; Phung, J.; Fu, J.Y.; Williams, S.M.; Merialdi, M.; Jacobsson, B.; Lye, S.; Menon, R.; Pennell, C.E. The Role of Genetics in Preterm Birth. Reprod. Sci. 2023, 30, 3410–3427. [Google Scholar] [CrossRef]
- Chau, V.; Synnes, A.; Grunau, R.E.; Poskitt, K.J.; Brant, R.; Miller, S.P. Abnormal brain maturation in preterm neonates associated with adverse developmental outcomes. Neurology 2013, 81, 2082–2089. [Google Scholar] [CrossRef]
- Bohilțea, R.E.; Cioca, A.M.; Dima, V.; Ducu, I.; Grigoriu, C.; Varlas, V.; Furtunescu, F. Expectant management of PPROM improves neonatal outcome—A retrospective study of 562 patients. J. Clin. Med. 2021, 11, 214. [Google Scholar] [CrossRef]
- Hall, M.; Valencia, C.M.; Soma-Pillay, P.; Luyt, K.; Jacobsson, B.; Shennan, A. Effective and simple interventions to improve outcomes for preterm infants worldwide: The FIGO PremPrep-5 initiative. Int. J. Gynaecol. Obstet. 2024, 165, 929–935. [Google Scholar] [CrossRef]
- Jafarabady, K.; Shafiee, A.; Eshraghi, N.; Salehi, S.A.; Mohammadi, I.; Rajai, S.; Zareian, Z.; Movahed, F.; Bakhtiyari, M. Magnesium sulfate for fetal neuroprotection in preterm pregnancy: A me-ta-analysis of randomized controlled trials. BMC Pregnancy Childbirth 2024, 24, 519. [Google Scholar] [CrossRef] [PubMed]
- Dusing, S.C.; Tripathi, T. Long-term neurodevelopmental outcomes of infants born late preterm: A systematic review. Res. Rep. Neonatol. 2015, 5, 91–111. [Google Scholar] [CrossRef]
- Davis, M.B.E.; Leppert, M.M.O.; German, M.K.; Lehmann, M.C.U.; Adams-Chapman, M.I.; Disabilities, C.O.C.W.; APP Council on Children with Disabilities, Committee on Fetus and New-born. Primary Care Framework to Monitor Preterm Infants for Neurodevelopmental Outcomes in Early Childhood. Pediatrics 2023, 152, e2023062511. [Google Scholar] [CrossRef] [PubMed]
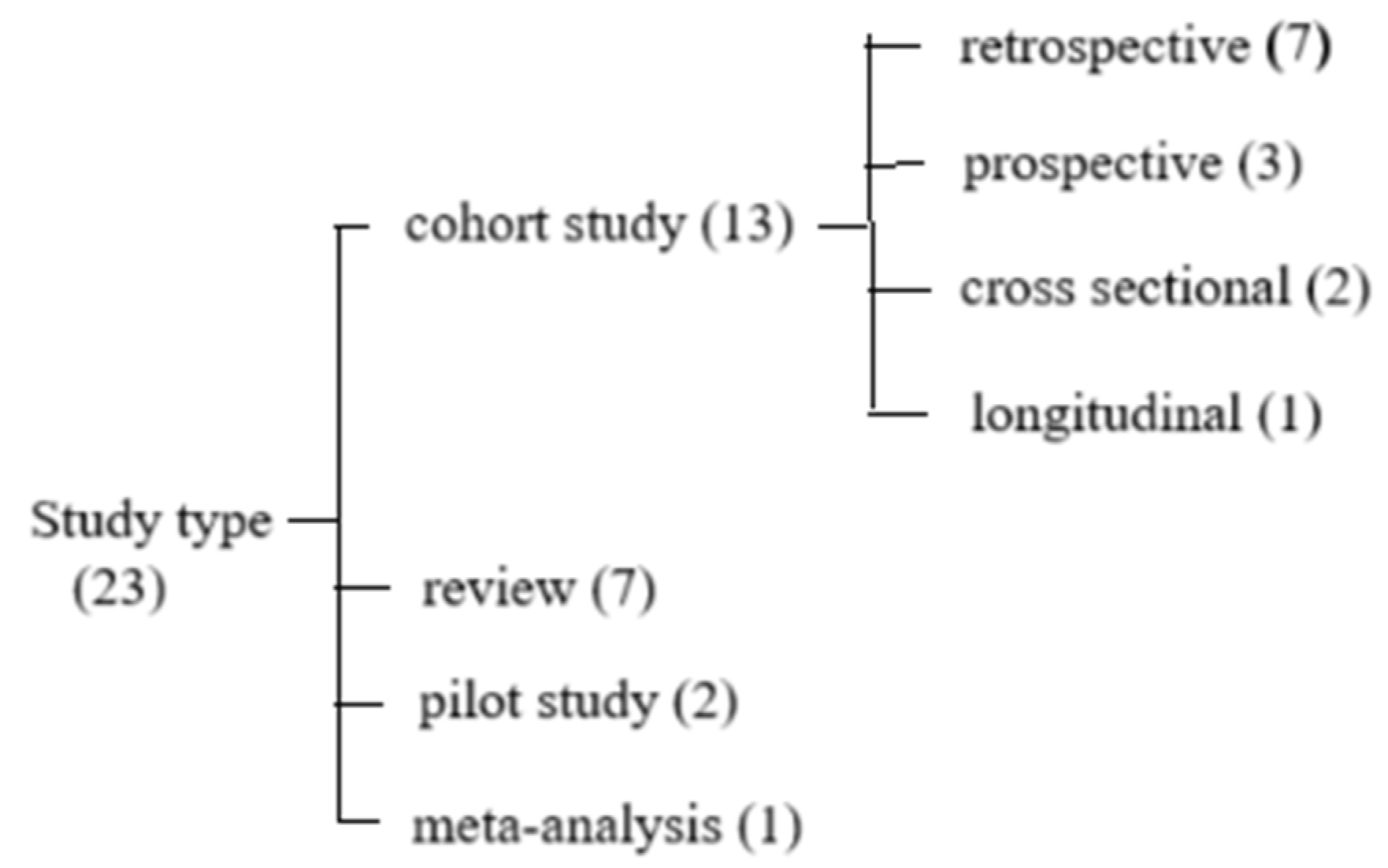
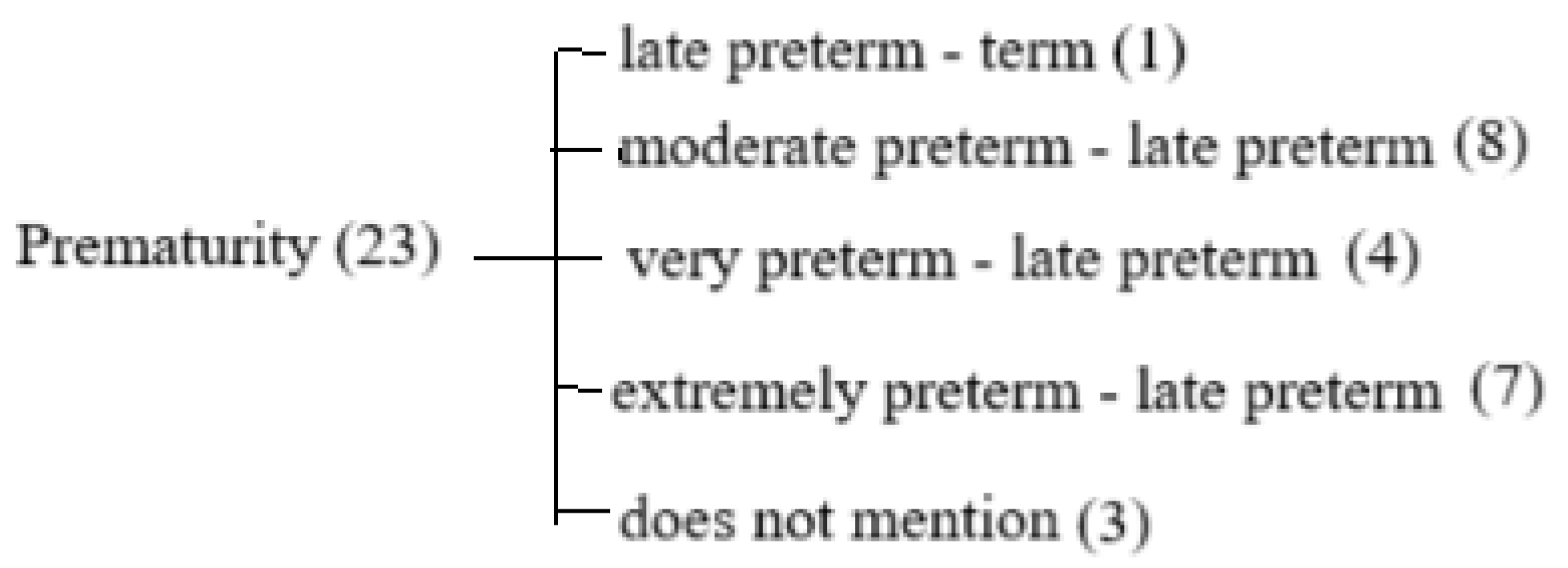
| Authors | Title | Year | Study Type | Country | Measurements | Findings |
|---|---|---|---|---|---|---|
| Wolfsberger et al. [23] | Cerebral oxygenation immediately after birth and long-term outcome in preterm neonates—a retrospective analysis. | 2023 | Retrospective cohort study | Austria | Population, crSO2, cFTOE, SpO2, HR | Preterm neonates with adverse outcomes had beside lower GA and also a lower crSO2, SpO2, and HR |
| Gonzalez-Moreira et al. [19] | Prevention of neurological sequelae in preterm infants | 2023 | Retrospective cohort study | Mexico | Population, MRI evaluation, follow-up at 3 years, MDI, PDI, Katona’s neurohabilitation therapy | Significantly better outcomes at 3 years old compared with no treatment; the presence of sepsis and the volumes of the corpus callosum and lateral ventricles at 3–4 months were significant predictors of developmental outcomes at 3 years |
| Bobba et al. [20] | Early brain microstructural development among preterm infants requiring caesarean section versus those delivered vaginally | 2023 | Retrospective cohort study | USA | Population, delivery type, GA at birth/scan, MRI evaluation, WM maturation, 5 min APGAR, birth weight Z-score, maternal pre-eclampsia, chorioamnionitis | Preterm infants delivered by C-section show delayed white matter maturation, especially in the corpus callosum and internal capsule, independent of other factors |
| Paulsen et al. [7] | Early neurological and motor function in infants born moderate to late preterm or small for gestational age at term: a prospective cohort study | 2023 | Prospective cohort study | Norway | Population, neurological function, motor performance (HINE, GMA, TIMP, AIMS) | Preterm infants scored lower in neurological and motor assessments (HINE, GMA, TIMP, AIMS) compared with term AGA infants, indicating higher risks of developmental delays |
| Côté-Corriveau et al. [15] | Associations between neurological examination at term-equivalent age and cerebral hemodynamics and oxygen metabolism in infants born preterm | 2023 | Prospective cohort study | Canada | Population, FDNIRS, DCS, CBFi, SpO2, OEF, CMRO2i | FDNIRS-DCS parameters were not linked to neurological exams at TEA, greater CBFi and CMRO2i increases from birth to TEA, correlated with higher GA |
| Ericson et al. [10] | Equally good neurological, growth, and health outcomes up to 6 years of age in moderately preterm infants who received exclusive vs. fortified breast milk—a longitudinal cohort study | 2023 | Longitudinal cohort study | Sweden | Neurological development, growth parameters, health outcomes | No significant differences in neurological, growth, or health outcomes were found between preterm infants fed exclusive vs. fortified breast milk up to six years of age |
| Toma et al. [12] | Correlations between head ultrasounds performed at term-equivalent age in premature neonates and general movements neurologic examination patterns | 2024 | Pilot study | Romania | Population, TEA evaluation, GM, head ultrasound measurements | The CS movement pattern was associated with larger ventricular index, increased lateral ventricle measurements, and reduced basal ganglia width; the PR pattern showed an increased sinocortical width and reduced pons diameter |
| Triggs et al. [6] | The influence of birthweight on mortality and severe neonatal morbidity in late preterm and term infants: an Australian cohort study. | 2024 | Retrospective cohort study | Australia | Population, birthweight centile, outcomes (stillbirth, neonatal mortality, severe neurological morbidity, no adverse outcome) | Low birthweight increases stillbirth risk and neonatal mortality, while high birthweight increases severe neurological or other severe morbidity |
| Snir et al. [27] | Long-term neurological outcomes of offspring misdiagnosed with fetal growth restriction | 2024 | Retrospective cohort study | Israel | Population, misdiagnosis rate | Falsely diagnosed infants had higher risks of movement disorders, cerebral palsy, and developmental disorders |
| Mitha et al. [9] | Neurological development in children born moderately or late preterm: national cohort study | 2024 | Retrospective cohort study | Sweden | Population, follow-up from birth until the date of first diagnosis of the neurodevelopmental outcome, death, emigration, 16th birthday/31 December 2019 | Late preterm have higher risks of adverse neurodevelopmental outcomes (any impairment; motor, cognitive, epileptic, visual, and hearing impairments, and severe or major neurodevelopmental impairment) |
| Zhang et al. [17] | Assessment of brain structure and volume reveals neurodevelopmental abnormalities in preterm infants with low-grade intraventricular hemorrhage | 2024 | Retrospective cohort study | China | Population, TEA evaluation, NBNA, MRI measurements | Preterm infants with low-grade IVH showed lower FA and MK values in the internal capsule and corpus callosum, changes linked to poorer neurodevelopmental outcomes at 40 weeks of corrected age |
| Toma et al. [14] | Cerebral ultrasound at term-equivalent age: Correlations with neuro-motor outcomes at 12–24 months corrected age | 2024 | Retrospective cohort study | Romania | Population, head ultrasound measurements, Amiel Tison neurologic examination | At 12 months, larger ventricular midbody and smaller basal ganglia were linked to abnormal motor outcomes; at 24 months, worse outcomes correlated with ventricular midbody >10.33 mm, smaller basal ganglia, reduced cortical depth, and immature gyral maturation |
| Lubián-Gutiérrez et al. [18] | Corpus callosum long-term biometry in very preterm children related to cognitive and motor outcomes | 2024 | Prospective cohort study | Spain | Population, MRI evaluation, CC, WPPSI-V, MABC-2 | Children with smaller CC sizes had lower cognitive scores (FSIQ < 85) and motor function deficits (MABC-2 < 15th percentile) |
| Assar and Nouran Nasef [11] | Outcome of neonatal hyperbilirubinemia and its effect on neurological system in full term and preterm baby | 2024 | Cross sectional cohort study | Egypt | Population, clinical parameters, BIND score, follow-up at 3 months | Higher adverse outcomes (62.2%), mortality (32.4%), and neurological issues (29.7%); BIND score >5 predicted risk with 90.5% sensitivity |
| Toma et al. [8] | Early intervention guided by the general movements examination at term corrected age—short term outcomes | 2024 | Pilot study | Romania | Population, GMA, MOS-R | Early intervention improved outcomes in premature infants with cramped–synchronized GM patterns |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Necula, A.-I.; Stoiciu, R.; Radulescu Botica, R.; Durdu, C.-E.; Bohiltea, R. Neurological Outcomes in Late Preterm Infants: An Updated Review of Recent Research and Clinical Insights. Diagnostics 2025, 15, 1514. https://doi.org/10.3390/diagnostics15121514
Necula A-I, Stoiciu R, Radulescu Botica R, Durdu C-E, Bohiltea R. Neurological Outcomes in Late Preterm Infants: An Updated Review of Recent Research and Clinical Insights. Diagnostics. 2025; 15(12):1514. https://doi.org/10.3390/diagnostics15121514
Chicago/Turabian StyleNecula, Andreea-Ioana, Roxana Stoiciu, Razvan Radulescu Botica, Cristiana-Elena Durdu, and Roxana Bohiltea. 2025. "Neurological Outcomes in Late Preterm Infants: An Updated Review of Recent Research and Clinical Insights" Diagnostics 15, no. 12: 1514. https://doi.org/10.3390/diagnostics15121514
APA StyleNecula, A.-I., Stoiciu, R., Radulescu Botica, R., Durdu, C.-E., & Bohiltea, R. (2025). Neurological Outcomes in Late Preterm Infants: An Updated Review of Recent Research and Clinical Insights. Diagnostics, 15(12), 1514. https://doi.org/10.3390/diagnostics15121514







