Triage-HF Validation in Heart Failure Clinical Practice: Importance of Episode Duration
Abstract
1. Introduction
2. Objectives
3. Materials and Methods
3.1. Study Population
3.2. TriageHF © Algorithm Description
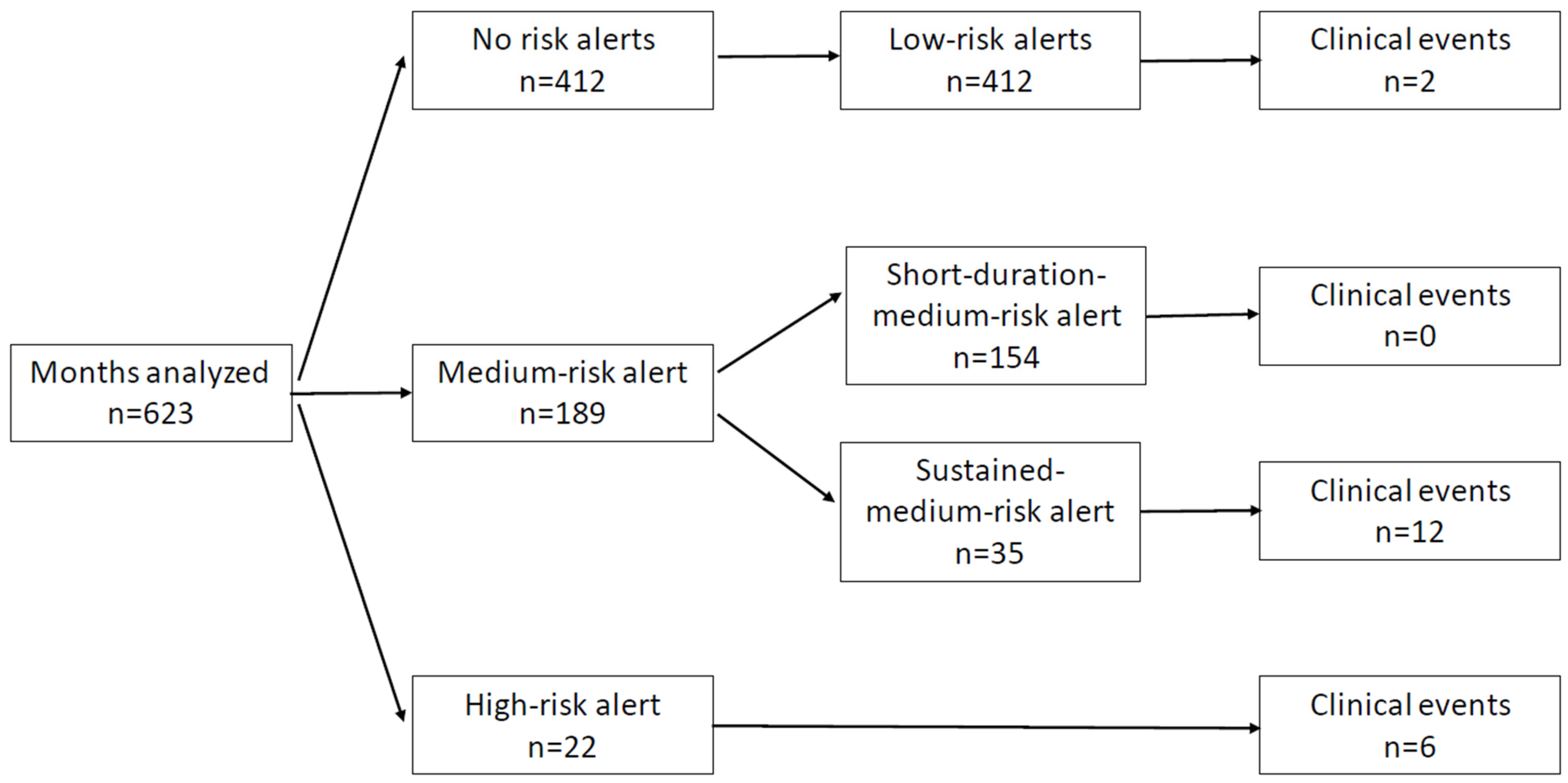
3.3. Care Link Data Analytics and Alert Categorization
3.4. Clinical Follow-Up
3.5. Statistical Analysis
3.6. Ethical Aspects
4. Results
4.1. Clinical Characteristics of the Study Population
4.2. Clinical Events in Follow-Up
4.3. Predictive Capacity of the TriageHF © Algorithm
5. Discussion
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Čelutkienė, J.; Chioncel, O.; et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur. Heart J. 2021, 36, 3599–3726. [Google Scholar] [CrossRef] [PubMed]
- Formiga, F.; Chivite, D.; Manito, N.; Casas, S.; Llopis, F.; Pujol, R. Hospitalization due to acute heart failure. Role of the precipitating factors. Int. J. Cardiol. 2007, 120, 237–241. [Google Scholar] [CrossRef]
- Zeppenfeld, K.; Tfelt-Hansen, J.; de Riva, M.; Winkel, B.G.; Behr, E.R.; Blom, N.A.; Charron, P.; Corrado, D.; Dagres, N.; de Chillou, C.; et al. 2022 ESC Guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death. Eur. Heart J. 2022, 43, 3997–4126. [Google Scholar] [CrossRef]
- Flórez, J.P.; García, D.; Valverde, I.; Rubín, J.; Pérez, D.; González-Vasserot, M.; Reguero, J.; de la Hera, J.M.; Avanzas, P.; Gómez, J.; et al. Role of syncope in predicting adverse outcomes in patients with suspected Brugada syndrome undergoing standardized flecainide testing. EP Eur. 2017, 20, f64–f71. [Google Scholar]
- García-Iglesias, D.; de Cos, F.J.; Romero, F.J.; Polana, S.; Rubín, J.M.; Pérez, D.; Reguero, J.; de la Hera, J.M.; Avanzas, P.; Gómez, J.; et al. Spectral analysis of the QT interval increases the prediction accuracy of clinical variables in Brugada syndrome. J. Clin. Med. 2019, 8, 1629. [Google Scholar] [CrossRef]
- Iglesias, D.G.; Gutiérrez, N.R.; De Cos, F.J.; Calvo, D. Analysis of the High-Frequency Content in Human QRS Complexes by the Continuous Wavelet Transform: An Automatized Analysis for the Prediction of Sudden Cardiac Death. Sensors 2018, 18, 560. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, J.C.; Kottkamp, H.; Zabel, M.; Aliot, E.; Kreutzer, U.; Bauer, A.; Schuchert, A.; Neuser, H.; Schumacher, B.; Hindricks, G. Automatic home monitoring of implantable cardioverter defibrillators. Europace 2008, 10, 729–735. [Google Scholar] [CrossRef]
- Nägele, H.; Lipoldová, J.; Oswald, H.; Klein, G.; Elvan, A.; Vester, E.; Bauer, W.; Bondke, H.; Reif, S.; Daub, C.; et al. Home monitoring of implantable cardioverter-defibrillators: Interpretation reliability of the second-generation “IEGM Online” system. Europace 2015, 17, 584–590. [Google Scholar] [CrossRef]
- Watanabe, E.; Yamazaki, F.; Goto, T.; Asai, T.; Yamamoto, T.; Hirooka, K.; Sato, T.; Kasai, A.; Ueda, M.; Yamakawa, T.; et al. Remote Management of Pacemaker Patients with Biennial In-Clinic Evaluation: Continuous Home Monitoring in the Japanese At-Home Study: A Randomized Clinical Trial. Circ. Arrhythm. Electrophysiol. 2020, 13, e007734. [Google Scholar] [CrossRef]
- Mairesse, G.H.; Braunschweig, F.; Klersy, K.; Cowie, M.R.; Leyva, F. Implementation and reimbursement of remote monitoring for cardiac implantable electronic devices in Europe: A survey from the health economics committee of the European Heart Rhythm Association. Europace 2015, 17, 814–818. [Google Scholar] [CrossRef]
- Parthiban, N.; Esterman, A.; Mahajan, R.; Twomey, D.J.; Pathak, R.K.; Lau, D.H.; Roberts-Thomson, K.C.; Young, G.D.; Sanders, P.; Ganesan, A.N. Remote monitoring of implantable cardioverter-defibrillators: A systematic review and meta-analysis of clinical outcomes. J. Am. Coll. Cardiol. 2015, 65, 2591–2600. [Google Scholar] [CrossRef] [PubMed]
- Klersy, C.; Boriani, G.; De Silvestri, A.; Mairesse, G.H.; Braunschweig, F.; Scotti, V.; Balduini, A.; Cowie, M.R.; Leyva, F. Effect of telemonitoring of cardiac implantable electronic devices on healthcare utilization: A meta-analysis of randomized controlled trials in patients with heart failure. Eur. J. Heart Fail. 2016, 18, 195–204. [Google Scholar] [CrossRef] [PubMed]
- Michowitz, Y.; Kronborg, M.B.; Glikson, M.; Nielsen, J.C. 2021 ESC Guidelines on cardiac pacing and cardiac resynchronization therapy. Eur. Heart J. 2021, 42, 3427–3520. [Google Scholar] [CrossRef]
- Boehmer, J.P.; Hariharan, R.; Devecchi, F.G.; Smith, A.L.; Molon, G.; Capucci, A.; An, Q.; Averina, V.; Stolen, C.M.; Thakur, P.H.; et al. A Multisensor Algorithm Predicts Heart Failure Events in Patients with Implanted Devices. JACC Heart Fail. 2017, 5, 216–225. [Google Scholar] [CrossRef]
- Gula, L.J.; Wells, G.A.; Yee, R.; Koehler, J.; Sarkar, S.; Sharma, V.; Skanes, A.C.; Sapp, J.L.; Redfearn, D.P.; Manlucu, J.; et al. A novel algorithm to assess risk of heart failure exacerbation using ICD diagnostics: Validation from RAFT. Hear. Rhythm. 2014, 11, 1626–1631. [Google Scholar] [CrossRef]
- Burri, H.; da Costa, A.; Quesada, A.; Ricci, R.P.; Favale, S.; Clementy, N.; Boscolo, G.; Villalobos, F.S.; Stefano, L.M.d.S.; Sharma, V.; et al. Risk stratification of cardiovascular and heart failure hospitalizations using integrated device diagnostics in patients with a cardiac resynchronization therapy defibrillator. EP Eur. 2018, 20, e69–e77. [Google Scholar] [CrossRef]
- Feijen, M.; Egorova, A.D.; Beeres, S.L.M.A.; Treskes, R.W. Early Detection of Fluid Retention in Patients with Advanced Heart Failure: A Review of a Novel Multisensory Algorithm, HeartLogic. Sensors 2021, 21, 1361. [Google Scholar] [CrossRef]
- Morgan, J.M.; Kitt, S.; Gill, J.; McComb, J.M.; Ng, G.A.; Raftery, J.; Roderick, P.; Seed, A.; Williams, S.G.; Witte, K.K.; et al. Remote management of heart failure using implantable electronic devices. Eur. Heart J. 2017, 38, 2352–2360. [Google Scholar] [CrossRef]
- Whellan, D.J.; Ousdigian, K.T.; Al-Khatib, S.M.; Pu, W.; Sarkar, S.; Porter, C.B.; Pavri, B.B.; O’Connor, C.M. Combined heart failure device diagnostics identify patients at higher risk of subsequent heart failure hospitalizations: Results from PARTNERS HF study. J. Am. Coll. Cardiol. 2010, 55, 1803–1810. [Google Scholar] [CrossRef]
- Perego, G.B.; Landolina, M.; Vergara, G.; Lunati, M.; Zanotto, G.; Pappone, A.; Lonardi, G.; Speca, G.; Iacopino, S.; Varbaro, A.; et al. Implantable CRT device diagnostics identify patients with increased risk for heart failure hospitalization. J. Interv. Card. Electrophysiol. 2008, 23, 235–242. [Google Scholar] [CrossRef]
- Capucci, A.; Santini, L.; Favale, S.; Pecora, D.; Petracci, B.; Calò, L.; Molon, G.; Cipolletta, L.; Bianchi, V.; Schirripa, V.; et al. Preliminary experience with the multisensor HeartLogic algorithm for heart failure monitoring: A retrospective case series report. ESC Heart Fail. 2019, 6, 308–318. [Google Scholar] [CrossRef] [PubMed]
- Santini, L.; D’Onofrio, A.; dello Russo, A.; Calò, L.; Pecora, D.; Favale, S.; Petracci, B.; Molon, G.; Bianchi, V.; De Ruvo, E.; et al. Prospective evaluation of the multisensor HeartLogic algorithm for heart failure monitoring. Clin. Cardiol. 2020, 43, 691–697. [Google Scholar] [CrossRef]
- López-Azor, J.C.; de la Torre, N.; Carmena, M.D.G.-C.; Pérez, P.C.; Munera, C.; Clement, I.M.; León, R.C.; Álvarez-García, J.; Pachón, M.; Ynsaurriaga, F.A.; et al. Clinical Utility of HeartLogic, a Multiparametric Telemonitoring System, in Heart Failure. Card. Fail. Rev. 2022, 8, e13. [Google Scholar] [CrossRef]
- Virani, S.A.; Sharma, V.; McCann, M.; Koehler, J.; Tsang, B.; Zieroth, S. Prospective evaluation of integrated device diagnostics for heart failure management: Results of the TRIAGE-HF study: Integrated device diagnostics for heart failure management. ESC Heart Fail 2018, 5, 809–817. [Google Scholar] [CrossRef]
- Ahmed, F.Z.; Taylor, J.K.; Green, C.; Moore, L.; Goode, A.; Black, P.; Howard, L.; Fullwood, C.; Zaidi, A.; Seed, A.; et al. Triage-HF Plus: A novel device-based remote monitoring pathway to identify worsening heart failure. ESC Heart Fail. 2019, 7, 107–116. [Google Scholar] [CrossRef]
- Cowie, M.R.; Sarkar, S.; Koehler, J.; Whellan, D.J.; Crossley, G.H.; Tang, W.H.W.; Abraham, W.T.; Sharma, V.; Santini, M. Development and validation of an integrated diagnostic algorithm derived from parameters monitored in implantable devices for identifying patients at risk for heart failure hospitalization in an ambulatory setting. Eur. Heart J. 2013, 34, 2472–2480. [Google Scholar] [CrossRef]
- Okumura, K.; Sasaki, S.; Kusano, K.; Mine, T.; Fujii, K.; Iwasa, A.; Sunagawa, O.; Yamabe, H.; Takahashi, N.; Ishii, S.; et al. Evaluation of an Integrated Device Diagnostics Algorithm to Risk Stratify Heart Failure Patients—Results from the SCAN-HF Study. Circ. J. 2020, 84, 1118–1123. [Google Scholar] [CrossRef]
- Lee, D.S.; Straus, S.E.; Farkouh, M.E.; Austin, P.C.; Taljaard, M.; Chong, A.; Fahim, C.; Poon, S.; Cram, P.; Smith, S.; et al. Trial of an Intervention to Improve Acute Heart Failure Outcomes. N. Engl. J. Med. 2023, 388, 22–32. [Google Scholar] [CrossRef]
- Wilkoff, B.L.; Filippatos, G.; Leclercq, C.; Gold, M.R.; Hersi, A.S.; Kusano, K.; Mullens, W.; Felker, G.M.; Kantipudi, C.; El-Chami, M.F.; et al. Adaptive versus conventional cardiac resynchronisation therapy in patients with heart failure (AdaptResponse): A global, prospective, randomised controlled trial. Lancet 2023, 402, 1147–1157. [Google Scholar] [CrossRef]
- Jabbour, S.; Fouhey, D.; Shepard, S.; Valley, T.S.; Kazerooni, E.A.; Banovic, N.; Wiens, J.; Sjoding, M.W. Measuring the Impact of AI in the Diagnosis of Hospitalized Patients: A Randomized Clinical Vignette Survey Study. JAMA 2023, 330, 2275–2284. [Google Scholar] [CrossRef]
- Infeld, M.; Wahlberg, K.; Cicero, J.; Plante, T.B.; Meagher, S.; Novelli, A.; Habel, N.; Krishnan, A.M.; Silverman, D.N.; LeWinter, M.M.; et al. Effect of Personalized Accelerated Pacing on Quality of Life, Physical Activity, and Atrial Fibrillation in Patients with Preclinical and Overt Heart Failure with Preserved Ejection Fraction: The myPACE Randomized Clinical Trial. JAMA Cardiol. 2023, 8, 213–221. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, F.Z.; Sammut-Powell, C.; Kwok, C.S.; Tay, T.; Motwani, M.; Martin, G.P.; Taylor, J.K. Remote CIED monitoring Remote monitoring data from cardiac implantable electronic devices predicts all-cause mortality. Europace 2022, 24, 245–255. [Google Scholar] [CrossRef] [PubMed]
- Tedeschi, A.; Palazzini, M.; Trimarchi, G.; Conti, N.; Di Spigno, F.; Gentile, P.; D’Angelo, L.; Garascia, A.; Ammirati, E.; Morici, N.; et al. Failure Management Through Telehealth: Expanding Care and Connecting Hearts. J. Clin. Med. 2024, 13, 2592. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2023; Available online: https://www.R-project.org/ (accessed on 1 October 2020).

| Variable | Total Sample (N = 39) | Clinical Events (N = 14) | No Clinical Events (N = 25) | p |
|---|---|---|---|---|
| Clinical Features | ||||
| Sex | 30 (76.92%) | 11 (78.57%) | 19 (76%) | 1 |
| Age (Years) | 67.47 ± 9.91 | 71.01 | 65.495 | 0.1 |
| Hypertension | 22 (56.41%) | 10 (71.43%) | 12 (48%) | 0.281 |
| Diabetes mellitus | 14 (35.9%) | 5 (35.71%) | 9 (36%) | 1 |
| Dyslipidemia | 25 (64.1%) | 8 (57.24%) | 17 (68%) | 0.741 |
| Smoking | 27 (56.41%) | 8 (57.24%) | 19 (76%) | 0.389 |
| Chronic pulmonary dis. | 7 (17.95%) | 3 (21.43%) | 4 (16%) | 1 |
| Renal insufficiency | 11 (28.21%) | 5 (35.71%) | 6 (24%) | 0.683 |
| Hepatopathy | 2 (5.13%) | 0 (0%) | 2 (8%) | 0.742 |
| Sleep apnea | 5 (12.82%) | 3 (21.43%) | 2 (8%) | 0.481 |
| Peripheral vasculopathies | 8 (20.51%) | 1 (7.14%) | 7 (28%) | 0.257 |
| Ischemic DCM | 21 (53.85%) | 7 (50%) | 14 (56%) | 0.979 |
| Primary prevention | 33 (84.62%) | 12 (85.71%) | 21 (84%) | 1 |
| Atrial fibrillation | 18 (46.15%) * | 9 (64.29%) | 9 (36%) | 0.011 |
| NYHA class II | 22 (64.1%) | 5 (35.71%) | 17 (68%) | 0.063 |
| NYHA class III | 14 (28.2%) | 7 (50%) | 7 (28%) | 0.305 |
| NYHA class IV | 3 (7.7%) | 2 (14.29%) | 1 (4%) | 0.596 |
| Follow-up months | 17.93 ± 9.81 | 21.56 | 15.89 | 0.116 |
| Drug treatment | ||||
| Diuretics | 31 (79.49%) * | 14 (100%) | 17 (68%) | 0.05 |
| ECAIs/ARBs | 26 (66.67%) | 11 (78.57%) | 15 (60%) | 0.409 |
| Sacubutril | 9 (23.08%) * | 0 (0%) | 9 (36%) | 0.03 |
| Beta-blockers | 36 (92.31%) | 12 (85.71%) | 24 (96%) | 0.596 |
| AMRs | 11 (28.21%) | 2 (14.29%) | 9 (36%) | 0.283 |
| Clinical events | ||||
| Death | 2 (5.13%) * | 4 (28.57%) | 0 (0%) | 0.023 |
| Hospital admission | 7 (17.95%) * | 7 (50%) | 0 (0%) | 0.001 |
| Emergency consultation | 4 (10.26%) * | 4 (28.57%) | 0 (0%) | 0.023 |
| Outpatient consultation | 7 (17.95%) * | 4 (28.57%) | 0 (0%) | 0.023 |
| HF Clinical Event | No HF Clinical Event | HR | p | |
|---|---|---|---|---|
| Low-risk | 2 (0.48%) | 419 (99.52%) | ||
| Medium-risk short-duration | 0 (0%) | 35 (100%) | 18.45 (17.71–19.2) | |
| Sustained medium-risk | 12 (7.79%) | 142 (92.21%) | 24.23 (23.49–24.98) | <0.001 |
| High-risk | 6 (27.27%) | 16 (72.73%) | 72.84 (72.02–73.66) | <0.001 |
| Sustained Medium-Risk | High-Risk | |
|---|---|---|
| Sensitivity (%) | 90 | 30 |
| Specificity (%) | 75.85 | 97.35 |
| PPV (%) | 11.25 | 27.27 |
| NPV (%) | 99.55 | 97.67 |
| TriageHF Alerts | No TriageHF Alerts | p | |||
|---|---|---|---|---|---|
| N = 31 | N = 8 | ||||
| Mean/Num. | Std. Dev./Perc. | Mean/Num. | Std. Dev./Perc. | ||
| Clinical Features | |||||
| Sex (male) | 25 | 80.65 | 5 | 62.5 | 0.538 |
| Age (years) | 66.65 | 10.116 | 70.65 | 8.93 | 0.29 |
| High blood pressure | 18 | 58.06 | 4 | 50 | 0.992 |
| Diabetes mellitus | 13 | 41.94 | 1 | 12.5 | 0.257 |
| Dyslipidemia | 19 | 61.29 | 6 | 75 | 0.759 |
| Smoking | 21 | 67.74 | 6 | 75 | 1 |
| Chronic pulmonary dis. | 4 | 12.9 | 3 | 37.5 | 0.272 |
| Renal insufficiency | 9 | 29.03 | 2 | 25 | 1 |
| Hepatopathy | 2 | 6.45 | 0 | 0 | 1 |
| Sleep apnea | 3 | 9.68 | 2 | 25 | 0.574 |
| Peripheral vasculopathies | 7 | 22.58 | 1 | 12.5 | 0.89 |
| Ischemic DCM | 18 | 58.06 | 3 | 37.5 | 0.521 |
| Primary prevention | 27 | 87.1 | 6 | 75 | 0.767 |
| Atrial fibrillation | 15 | 48.39 | 3 | 37.5 | 0.001 |
| NYHA class III | 9 | 29.03 | 5 | 62.5 | 0.178 |
| NYHA class IV | 2 | 6.45 | 1 | 12.5 | 1 |
| Follow-up (months) | 19.18 | 9.07 | 13.05 | 11.67 | 0.199 |
| Drug Treatment | |||||
| Diuretics | 23 | 74.19 | 8 | 100 | 0.262 |
| ECAIs/ARBs | 22 | 70.97 | 4 | 50 | 0.483 |
| Sacubitril | 7 | 22.58 | 2 | 25 | 1 |
| Beta-blockers | 29 | 93.55 | 7 | 87.5 | 1 |
| AMRs | 9 | 29.03 | 2 | 25 | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
García Iglesias, D.; Oloriz, D.L.; Pérez Diez, D.; Calvo Cuervo, D.; Álvarez Velasco, R.; Junco-Vicente, A.; Rubín López, J.M. Triage-HF Validation in Heart Failure Clinical Practice: Importance of Episode Duration. Diagnostics 2025, 15, 1476. https://doi.org/10.3390/diagnostics15121476
García Iglesias D, Oloriz DL, Pérez Diez D, Calvo Cuervo D, Álvarez Velasco R, Junco-Vicente A, Rubín López JM. Triage-HF Validation in Heart Failure Clinical Practice: Importance of Episode Duration. Diagnostics. 2025; 15(12):1476. https://doi.org/10.3390/diagnostics15121476
Chicago/Turabian StyleGarcía Iglesias, Daniel, David Ledesma Oloriz, Diego Pérez Diez, David Calvo Cuervo, Rut Álvarez Velasco, Alejandro Junco-Vicente, and José Manuel Rubín López. 2025. "Triage-HF Validation in Heart Failure Clinical Practice: Importance of Episode Duration" Diagnostics 15, no. 12: 1476. https://doi.org/10.3390/diagnostics15121476
APA StyleGarcía Iglesias, D., Oloriz, D. L., Pérez Diez, D., Calvo Cuervo, D., Álvarez Velasco, R., Junco-Vicente, A., & Rubín López, J. M. (2025). Triage-HF Validation in Heart Failure Clinical Practice: Importance of Episode Duration. Diagnostics, 15(12), 1476. https://doi.org/10.3390/diagnostics15121476






