Is UA/HDL-C a Reliable Surrogate Marker for Fatty Liver? A Comparative Evaluation with Metabolic Scores in a Mexican Population: The Genetics of Atherosclerotic Disease Study
Abstract
1. Introduction
2. Materials and Methods
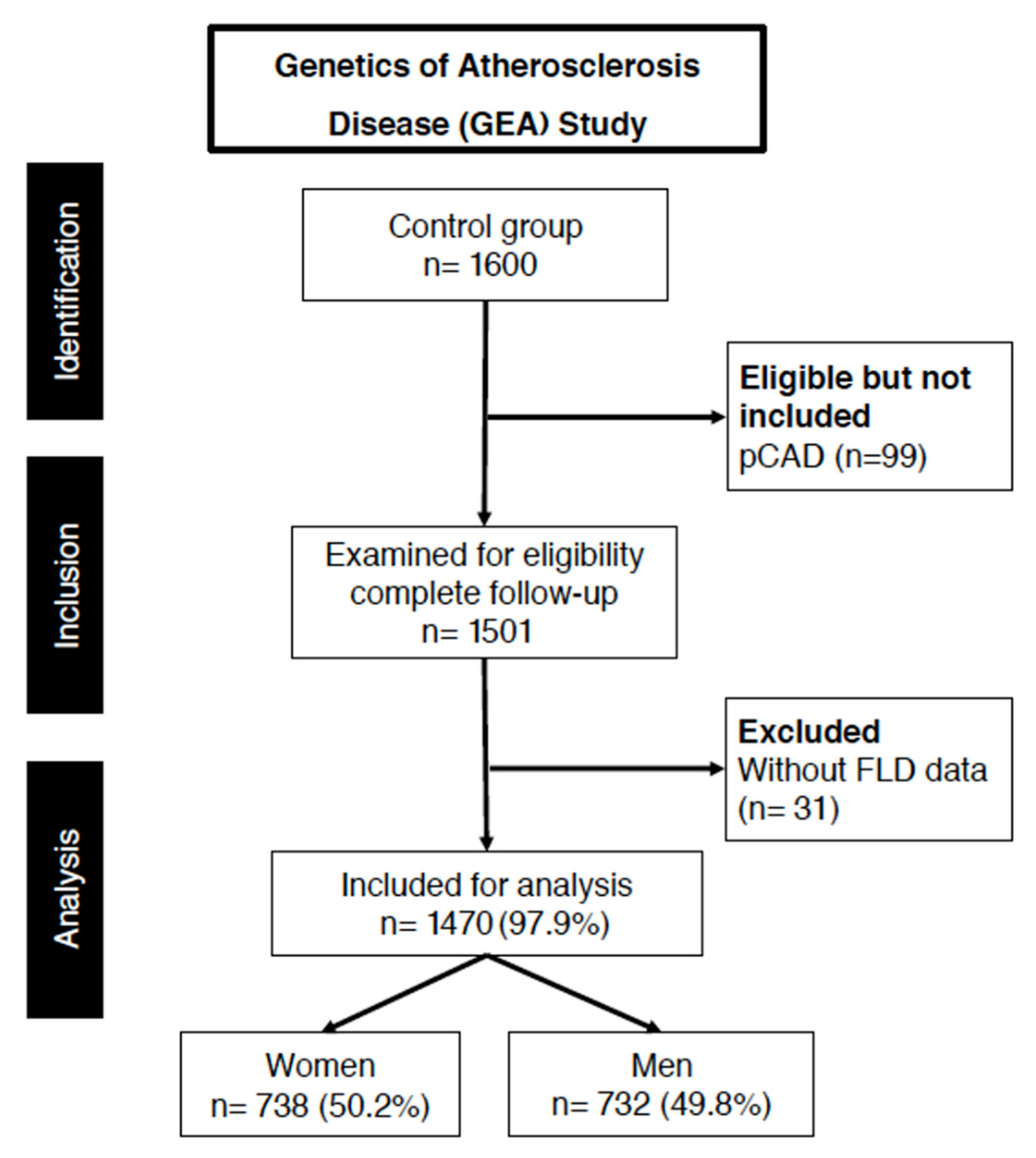
2.1. Study Population
2.2. Computed Tomography Assessment
2.3. Liver Fat Diagnosis
2.4. Cardiometabolic Risk Factors
2.5. Laboratory Analyses
2.6. Comparison of Indices for Predicting FLD
- 1.
- Fatty liver index (FLI):
- 2.
- NAFLD score (FLS):
- 3.
- Lipid Accumulation Product (LAP):
- 4.
- Hepatic Steatosis Index (HSI):
2.7. Statistical Analysis
2.8. Protein–Protein Interaction Network Analysis
2.9. Ethical Statement
3. Results
3.1. Population Characteristics
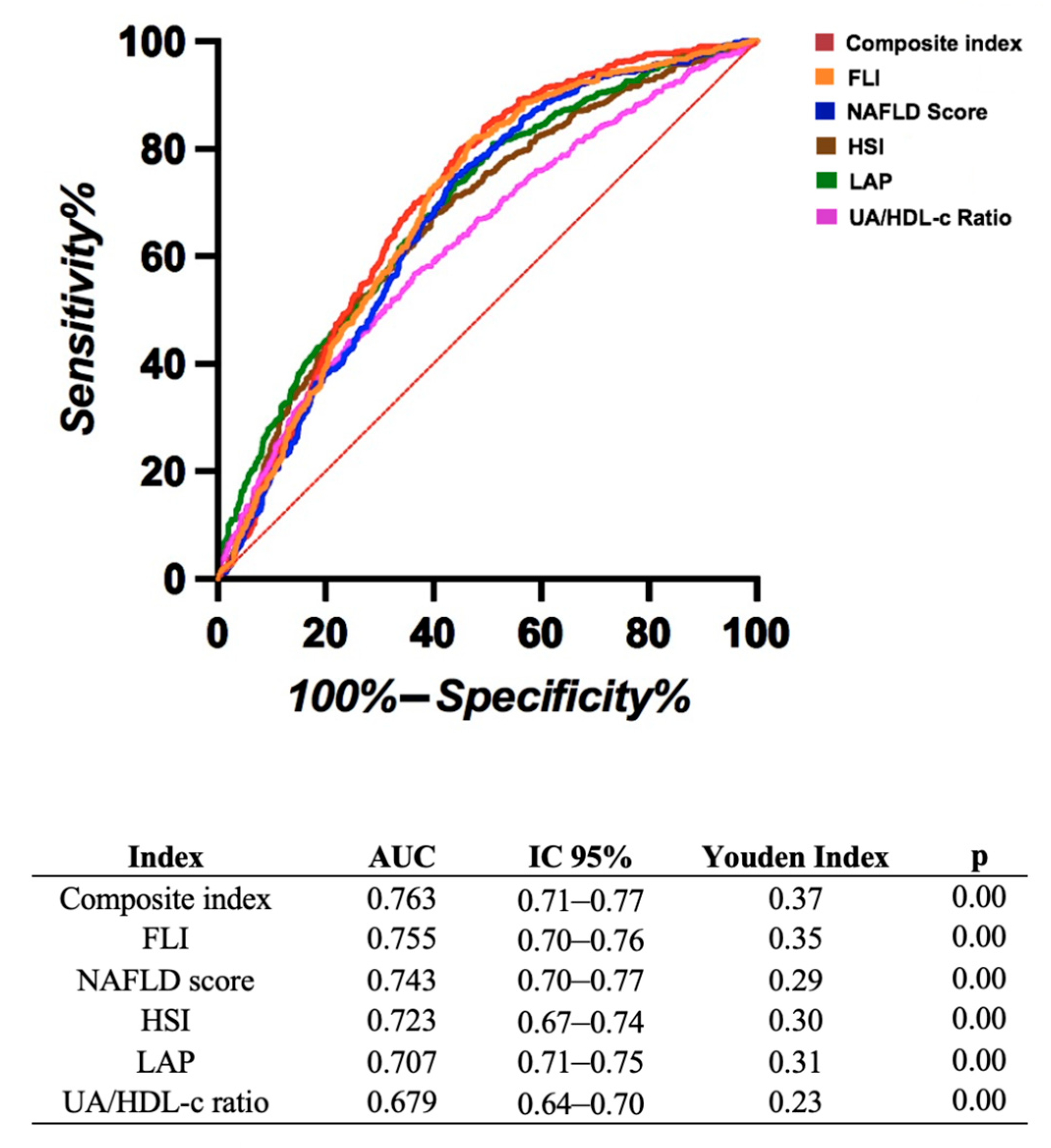
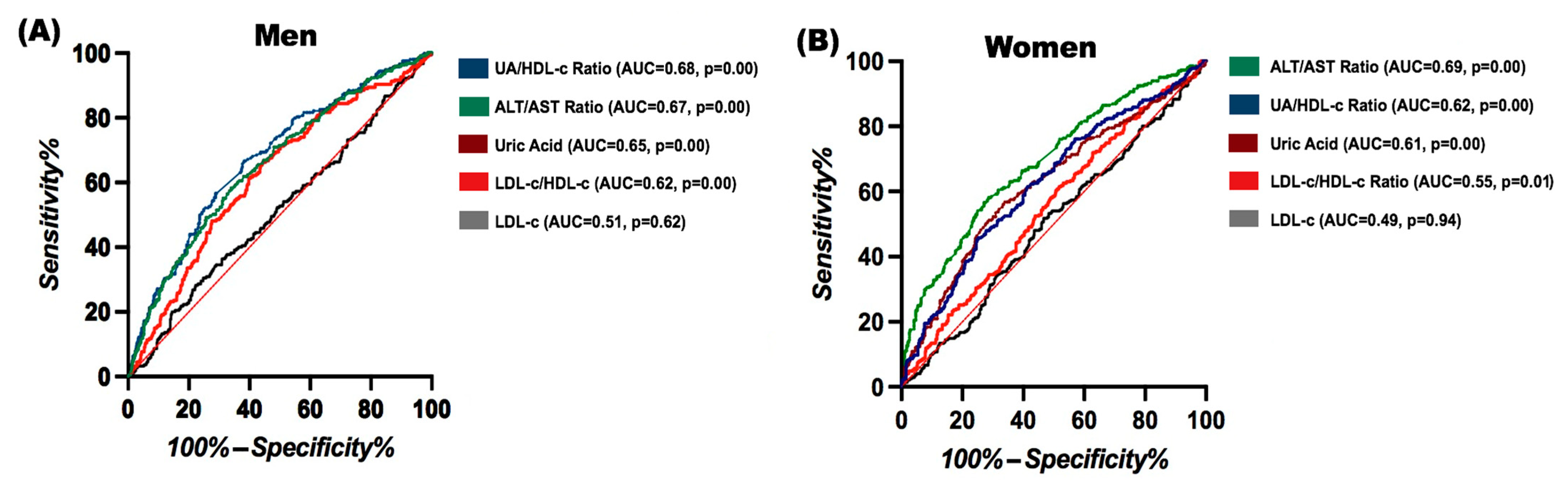
3.2. Comparison of Ratios and Indices for Predicting FLD
4. Discussion
5. Limitations
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Younossi, Z.; Anstee, Q.M.; Marietti, M.; Hardy, T.; Henry, L.; Eslam, M.; George, J.; Bugianesi, E. Global burden of NAFLD and NASH: Trends, predictions, risk factors and prevention. Nat. Rev. Gastroenterol. Hepatol. 2018, 15, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Sepulveda-Villegas, M.; Roman, S.; Rivera-Iñiguez, I.; Ojeda-Granados, C.; Gonzalez-Aldaco, K.; Torres-Reyes, L.A.; Jose-Abrego, A.; Panduro, A. High prevalence of nonalcoholic steatohepatitis and abnormal liver stiffness in a young and obese Mexican population. PLoS ONE 2019, 14, e0208926. [Google Scholar] [CrossRef] [PubMed]
- Huh, Y.; Cho, Y.J.; Nam, G.E. Recent Epidemiology and Risk Factors of Nonalcoholic Fatty Liver Disease. J. Obes. Metab. Syndr. 2022, 31, 17. [Google Scholar] [CrossRef]
- Sayiner, M.; Koenig, A.; Henry, L.; Younossi, Z.M. Epidemiology of Nonalcoholic Fatty Liver Disease and Nonalcoholic Steatohepatitis in the United States and the Rest of the World. Clin. Liver Dis. 2016, 20, 205–214. [Google Scholar] [CrossRef]
- Polyzos, S.A.; Kountouras, J.; Mantzoros, C.S. Obesity and nonalcoholic fatty liver disease: From pathophysiology to therapeutics. Metabolism 2019, 92, 82–97. [Google Scholar] [CrossRef] [PubMed]
- Portillo-Sanchez, P.; Bril, F.; Maximos, M.; Lomonaco, R.; Biernacki, D.; Orsak, B.; Subbarayan, S.; Webb, A.; Hecht, J.; Cusi, K. High prevalence of nonalcoholic fatty liver disease in patients with type 2 diabetes mellitus and normal plasma aminotransferase levels. J. Clin. Endocrinol. Metab. 2015, 100, 2231–2238. [Google Scholar] [CrossRef]
- Rojano-Ramón, R.; Calderón-Garcidueñas, A.L.; Ruiz-Ramos, R.; López-Amador, N. Frequency of non-alcoholic fatty liver disease in a series of forensic cases in Mexico. Horiz. Sanit. 2021, 20, 169–177. [Google Scholar] [CrossRef]
- Barton Duell, P.; Welty, F.K.; Miller, M.; Chait, A.; Hammond, G.; Ahmad, Z.; Cohen, D.E.; Horton, J.D.; Pressman, G.S.; Toth, P.P.; et al. Nonalcoholic Fatty Liver Disease and Cardiovascular Risk: A Scientific Statement from the American Heart Association. Arterioscler. Thromb. Vasc. Biol. 2022, 42, E168–E185. [Google Scholar] [CrossRef]
- Marušić, M.; Paić, M.; Knobloch, M.; Liberati Pršo, A.M. NAFLD, Insulin Resistance, and Diabetes Mellitus Type 2. Can. J. Gastroenterol. Hepatol. 2021, 2021, 6613827. [Google Scholar] [CrossRef]
- Tomah, S.; Alkhouri, N.; Hamdy, O. Nonalcoholic fatty liver disease and type 2 diabetes: Where do Diabetologists stand? Clin. Diabetes Endocrinol. 2020, 6, 9. [Google Scholar] [CrossRef]
- Jäger, S.; Jacobs, S.; Kröger, J.; Stefan, N.; Fritsche, A.; Weikert, C.; Boeing, H.; Schulze, M.B. Association between the fatty liver index and risk of type 2 diabetes in the EPIC-Potsdam study. PLoS ONE 2015, 10, e0124749. [Google Scholar] [CrossRef] [PubMed]
- Dai, W.; Ye, L.; Liu, A.; Wen, S.W.; Deng, J.; Wu, X.; Lai, Z. Prevalence of nonalcoholic fatty liver disease in patients with type 2 diabetes mellitus: A meta-analysis. Medicine 2017, 96, e8179. [Google Scholar] [CrossRef] [PubMed]
- Yoo, J.J.; Cho, E.J.; Chung, G.E.; Chang, Y.; Cho, Y.; Park, S.H.; Jeong, S.-M.; Kim, B.-Y.; Shin, D.W.; Kim, Y.J.; et al. Nonalcoholic Fatty Liver Disease Is a Precursor of New-Onset Metabolic Syndrome in Metabolically Healthy Young Adults. J. Clin. Med. 2022, 11, 935. [Google Scholar] [CrossRef]
- Motta, B.M.; Masarone, M.; Torre, P.; Persico, M. From Non-Alcoholic Steatohepatitis (NASH) to Hepatocellular Carcinoma (HCC): Epidemiology, Incidence, Predictions, Risk Factors, and Prevention. Cancers 2023, 15, 5458. [Google Scholar] [CrossRef] [PubMed]
- Méndez-Sánchez, N.; Villa, A.R.; Chávez-Tapia, N.C.; Ponciano-Rodriguez, G.; Almeda-Valdés, P.; González, D.; Uribe, M. Trends in liver disease prevalence in Mexico from 2005 to 2050 through mortality data. Ann. Hepatol. 2005, 4, 52–55. [Google Scholar] [CrossRef]
- Bernal-Reyes, R.; Castro-Narro, G.; Malé-Velázquez, R.; Carmona-Sánchez, R.; González-Huezo, M.S.; García-Juárez, I.; Chávez-Tapia, N.; Aguilar-Salinas, C.; Aiza-Haddad, I.; Ballesteros-Amozurrutia, M.A.; et al. The Mexican consensus on nonalcoholic fatty liver disease. Rev. Gastroenterol. México 2019, 84, 69–99. [Google Scholar] [CrossRef]
- Chinchilla-López, P.; Ramírez-Pérez, O.; Cruz-Ramón, V.; Canizales-Quinteros, S.; Domínguez-López, A.; Ponciano-Rodríguez, G.; Sánchez-Muñoz, F.; Méndez-Sánchez, N. More Evidence for the Genetic Susceptibility of Mexican Population to Nonalcoholic Fatty Liver Disease through PNPLA3. Ann. Hepatol. 2018, 17, 250–255. [Google Scholar] [CrossRef]
- Yuan, L.; Terrrault, N.A. PNPLA3 and nonalcoholic fatty liver disease: Towards personalized medicine for fatty liver. Hepatobiliary Surg. Nutr. 2020, 9, 353. [Google Scholar] [CrossRef]
- Alalwani, J.; Eljazzar, S.; Basil, M.; Tayyem, R. The impact of health status, diet and lifestyle on non-alcoholic fatty liver disease: Narrative review. Clin. Obes. 2022, 12, e12525. [Google Scholar] [CrossRef]
- Vidal-González, D.; Uribe, M.; Montalvo-Javé, E.E.; Nuño-Lámbarri, N. Assessment of non-alcoholic fatty liver disease by non-invasive methods: Present and future perspectives. Rev. Médica Hosp. General. México 2020, 83, 135–143. [Google Scholar] [CrossRef]
- Parente, D.B. Imaging methods in the assessment of nonalcoholic fatty liver disease. Radiol. Bras. 2020, 53, IX–X. [Google Scholar] [CrossRef] [PubMed]
- Khang, A.R.; Lee, H.W.; Yi, D.; Kang, Y.H.; Son, S.M. The fatty liver index, a simple and useful predictor of metabolic syndrome: Analysis of the Korea National Health and Nutrition Examination Survey 2010–2011. Diabetes Metab. Syndr. Obes. 2019, 12, 181. [Google Scholar] [CrossRef] [PubMed]
- Fennoun, H.; Mansouri, S.E.; Tahiri, M.; Haraj, N.E.; Aziz, S.E.; Hadad, F.; Hliwa, W.; Badr, W.; Chadli, A. Interest of hepatic steatosis index (HSI) in screening for metabolic steatopathy in patients with type 2 diabetes. Pan. Afr. Med. J. 2020, 37, 270. [Google Scholar] [CrossRef] [PubMed]
- Ebrahimi, M.; Seyedi, S.A.; Nabipoorashrafi, S.A.; Rabizadeh, S.; Sarzaeim, M.; Yadegar, A.; Mohammadi, F.; Bahri, R.A.; Pakravan, P.; Shafiekhani, P.; et al. Lipid accumulation product (LAP) index for the diagnosis of nonalcoholic fatty liver disease (NAFLD): A systematic review and meta-analysis. Lipids Health Dis. 2023, 22, 41. [Google Scholar] [CrossRef]
- Jeong, S.; Kim, K.; Chang, J.; Choi, S.; Kim, S.M.; Son, J.S.; Lee, G.; Kim, W.; Park, S.M. Development of a simple nonalcoholic fatty liver disease scoring system indicative of metabolic risks and insulin resistance. Ann. Transl. Med. 2020, 8, 1414. [Google Scholar] [CrossRef]
- Fan, J.; Wang, D. Serum uric acid and nonalcoholic fatty liver disease. Front. Endocrinol. 2024, 15, 1455132. [Google Scholar] [CrossRef]
- Zhou, Y.; Wei, F.; Fan, Y. High serum uric acid and risk of nonalcoholic fatty liver disease: A systematic review and meta-analysis. Clin. Biochem. 2016, 49, 636–642. [Google Scholar] [CrossRef]
- Zhang, Y.N.; Wang, Q.Q.; Chen, Y.S.; Shen, C.; Xu, C.F. Association between Serum Uric Acid to HDL-Cholesterol Ratio and Nonalcoholic Fatty Liver Disease in Lean Chinese Adults. Int. J. Endocrinol. 2020, 2020, 5953461. [Google Scholar] [CrossRef]
- Yazdi, F.; Baghaei, M.H.; Baniasad, A.; Naghibzadeh-Tahami, A.; Najafipour, H.; Gozashti, M.H. Investigating the relationship between serum uric acid to high-density lipoprotein ratio and metabolic syndrome. Endocrinol. Diabetes Metab. 2022, 5, e00311. [Google Scholar] [CrossRef]
- Zhu, W.; Liang, A.; Shi, P.; Yuan, S.; Zhu, Y.; Fu, J.; Zheng, T.; Wen, Z.; Wu, X. Higher serum uric acid to HDL-cholesterol ratio is associated with onset of non-alcoholic fatty liver disease in a non-obese Chinese population with normal blood lipid levels. BMC Gastroenterol. 2022, 22, 196. [Google Scholar] [CrossRef]
- Yin, J.; Zheng, C.; Li, Z.; Chang, Y.; Cao, L.; Qu, Y. Gender differences in the association between the uric acid to high-density lipoprotein cholesterol ratio and diabetes risk: A mediation analysis of c-reactive protein, triglycerides, and insulin resistance. Lipids Health Dis. 2024, 23, 409. [Google Scholar] [CrossRef] [PubMed]
- Longo, R.; Ricci, C.; Masutti, F.; Vidimari, R.; Crocé, L.S.; Bercich, L.; Tiribelli, C.; Dalla Palma, L. Fatty infiltration of the liver. Quantification by 1H localized magnetic resonance spectroscopy and comparison with computed tomography. Investig. Radiol. 1993, 28, 297–302. [Google Scholar] [CrossRef]
- Villarreal-Molina, T.; Posadas-Romero, C.; Romero-Hidalgo, S.; Antúnez-Argüelles, E.; Bautista-Grande, A.; Vargas-Alarcón, G.; Kimura-Hayama, E.; Canizales-Quinteros, S.; Juárez-Rojas, J.G.; Posadas-Sánchez, R.; et al. The ABCA1 Gene R230C Variant Is Associated with Decreased Risk of Premature Coronary Artery Disease: The Genetics of Atherosclerotic Disease (GEA) Study. PLoS ONE 2012, 7, e49285. [Google Scholar] [CrossRef] [PubMed]
- Cortés-Rubio, J.A.; Martín-Acicoya, D.; Candela-Fernández, M.; Alcanda-Renquel, E.; Vañó-Garrido-Arroquia, A.; Cortés-Costa, M. Validación del test FLI (Fatty Liver Index) para el diagnóstico de esteatosis hepática en pacientes con obesidad y/o diabetes mellitus para su utilización en Atención Primaria. Estudio FLIAP. Rev. Clínica Med. Familia 2023, 16, 325–329. [Google Scholar] [CrossRef]
- Koneru, K.; Bhatt, V.; Kakrani, A.; Edara, M.; Reddy, V.T.; Jawade, P.G. A study of non-alcoholic fatty liver disease-liver fat score in overweight and obese individuals. J. Fam. Med. Prim. Care 2022, 11, 4368. [Google Scholar] [CrossRef]
- Li, H.; Zhang, Y.; Luo, H.; Lin, R. The lipid accumulation product is a powerful tool to diagnose metabolic dysfunction-associated fatty liver disease in the United States adults. Front. Endocrinol. 2022, 13, 977625. [Google Scholar] [CrossRef]
- Priego-Parra, B.A.; Triana-Romero, A.; Martínez-Pérez, G.P.; Reyes-Diaz, S.A.; Ordaz-Alvarez, H.R.; Bernal-Reyes, R.; Icaza-Chávez, M.E.; Martínez-Vázquez, S.E.; Cano-Contreras, A.D.; Vivanco-Cid, H.; et al. Hepatic Steatosis Index (HSI): A Valuable Biomarker in Subjects with Metabolic Dysfunction-associated Fatty Liver Disease (MAFLD). Ann. Hepatol. 2024, 29, 101391. [Google Scholar] [CrossRef]
- Ge, S.X.; Jung, D.; Yao, R. ShinyGO: A graphical gene-set enrichment tool for animals and plants. Bioinformatics 2020, 36, 2628–3629. [Google Scholar] [CrossRef]
- Kanehisa, M.; Furumichi, M.; Sato, Y.; Matsuura, Y.; Ishiguro-Watanabe, M. KEGG: Biological systems database as a model of the real world. Nucleic Acids Res. 2025, 53, D672–D677. [Google Scholar] [CrossRef]
- Yang, X.; Lin, Y.; Huang, J.; Chi, Y.; Wu, Y.; Lin, S. Serum uric acid levels and prognosis of patients with non-alcoholic fatty liver disease. Sci. Rep. 2024, 14, 5923. [Google Scholar] [CrossRef]
- Bao, T.; Ying, Z.; Gong, L.; Du, J.; Ji, G.; Li, Z.; Gao, W.; Jiang, X.; Yang, H.; Huang, Y.; et al. Association between serum uric acid and nonalcoholic fatty liver disease in nonobese postmenopausal women: A cross-sectional study. Sci. Rep. 2020, 10, 10072. [Google Scholar] [CrossRef] [PubMed]
- Hoekstra, M.; Van Eck, M. High-density lipoproteins and non-alcoholic fatty liver disease. Atheroscler. Plus 2023, 53, 33. [Google Scholar] [CrossRef] [PubMed]
- Xuan, Y.; Wu, D.; Zhang, Q.; Yu, Z.; Yu, J.; Zhou, D. Elevated ALT/AST ratio as a marker for NAFLD risk and severity: Insights from a cross-sectional analysis in the United States. Front. Endocrinol. 2024, 15, 1457598. [Google Scholar] [CrossRef]
- Thakur, S.; Kumar, V.; Das, R.; Sharma, V.; Mehta, D.K. Biomarkers of Hepatic Toxicity: An Overview. Curr. Ther. Res. 2024, 100, 100737. [Google Scholar] [CrossRef] [PubMed]
- Bazmandegan, G.; Dehghani Mhasan Karimifard, M.; Kahnooji, M.; Balaee, P.; Zakeri, M.A.; Kamiab, Z. Uric acid to HDL ratio: A marker for predicting incidence of metabolic syndrome in patients with type II diabetes. Nutr. Metab. Cardiovasc. Dis. 2024, 34, 1014–1020. [Google Scholar] [CrossRef]
- Yu, X.; Sun, F.; Ming, J.; Liang, S.; Zhang, W.; Wang, L.; Li, Q.; Xu, Q.; Shi, L.; Gao, B.; et al. Serum uric acid to high-density lipoprotein cholesterol ratio is a promising marker for identifying metabolic syndrome in nondiabetic Chinese men. Postgrad. Med. 2023, 135, 741–749. [Google Scholar] [CrossRef]
- Kocak, M.Z.; Aktas, G.; Erkus, E.; Sincer, I.; Atak, B.; Duman, T. Serum uric acid to HDL-cholesterol ratio is a strong predictor of metabolic syndrome in type 2 diabetes mellitus. Rev. Assoc. Med. Bras. 2019, 65, 9–15. [Google Scholar] [CrossRef]
- Barquera, S.; Rivera, J.A. Obesity in Mexico: Rapid epidemiological transition and food industry interference in health policies. Lancet Diabetes Endocrinol. 2020, 8, 746. [Google Scholar] [CrossRef]
- Barquera, S.; Hernández-Barrera, L.; Trejo-Valdivia, B.; Shamah, T.; Campos-Nonato, I.; Rivera-Dommarco, J. Obesidad en México, prevalencia y tendencias en adultos. Ensanut 2018–19. Salud Pública México 2020, 62, 682–692. [Google Scholar] [CrossRef]
- Thirumalai, A.; Rubinow, K.B.; Page, S.T. An update on testosterone, HDL and cardiovascular risk in men. Clin. Lipidol. 2015, 10, 251. [Google Scholar] [CrossRef]
- Beazer, J.D.; Freeman, D.J. Estradiol and HDL Function in Women—A Partnership for Life. J. Clin. Endocrinol. Metab. 2021, 107, e2192. [Google Scholar] [CrossRef] [PubMed]
- Eun, Y.; Kim, I.Y.; Han, K.; Lee, K.N.; Lee, D.Y.; Shin, D.W.; Kang, S.; Lee, S.; Cha, H.-S.; Koh, E.-M.; et al. Association between female reproductive factors and gout: A nationwide population-based cohort study of 1 million postmenopausal women. Arthritis Res. Ther. 2021, 23, 304. [Google Scholar] [CrossRef] [PubMed]
- Guerra, S.; Mocciaro, G.; Gastaldelli, A. Adipose tissue insulin resistance and lipidome alterations as the characterizing factors of non-alcoholic steatohepatitis. Eur. J. Clin. Investig. 2022, 52, e13695. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Zhang, Q.; Zhou, M.; Li, B.; Zhao, J.; Bai, R.; Song, X.; Qin, W.; Zhang, Y. Correlation between serum uric acid to high-density lipoprotein cholesterol ratio and atrial fibrillation in patients with NAFLD. PLoS ONE 2024, 19, e0305952. [Google Scholar] [CrossRef]
- Nogueira, J.P.; Cusi, K. Role of Insulin Resistance in the Development of Nonalcoholic Fatty Liver Disease in People with Type 2 Diabetes: From Bench to Patient Care. Diabetes Spectr. 2024, 37, 20–28. [Google Scholar] [CrossRef]
- Bessone, F.; Razori, M.V.; Roma, M.G. Molecular pathways of nonalcoholic fatty liver disease development and progression. Cell Mol. Life Sci. 2018, 76, 99. [Google Scholar] [CrossRef]
- Chandrasekaran, P.; Weiskirchen, R. The Role of SCAP/SREBP as Central Regulators of Lipid Metabolism in Hepatic Steatosis. Int. J. Mol. Sci. 2024, 25, 1109. [Google Scholar] [CrossRef]
- Crudele, L.; De Matteis, C.; Piccinin, E.; Gadaleta, R.M.; Cariello, M.; Di Buduo, E.; Piazzolla, G.; Suppressa, P.; Berardi, E.; Sabbà, C.; et al. Low HDL-cholesterol levels predict hepatocellular carcinoma development in individuals with liver fibrosis. JHEP Rep. 2023, 5, 100627. [Google Scholar] [CrossRef]
- Rangel-Baltazar, E.; Cuevas-Nasu, L.; Shamah-Levy, T.; Rodríguez-Ramírez, S.; Méndez-Gómez-Humarn, I.; Rivera, J.A. Association between High Waist-to-Height Ratio and Cardiovascular Risk among Adults Sampled by the 2016 Half-Way National Health and Nutrition Survey in Mexico (ENSANUT MC 2016). Nutrients 2019, 11, 1402. [Google Scholar] [CrossRef]
| Variable | Female NO FLD n = 518 | Male NO FLD n = 464 | Female FLD n = 220 | Male FLD n = 268 | p1 | p2 |
|---|---|---|---|---|---|---|
| Age (years) | 54.00 (29.00–77.00) | 54.00 (26.00–81.00) | 53.00 (34.00–74.00) | 52.50 (29.00–75.00) | 0.54 | 0.33 |
| BMI (kg/m2) | 26.99 (17.26–39.84) | 27.07 (18.93–39.90) | 29.71 (20.33–39.86) | 29.59 (19.58–39.84) | <0.001 | <0.001 |
| WC (cm) | 88.20 (63.50–117.00) | 95.20 (73.20–132.30) | 95.65 (73.00–123.40) | 101.50 (76.40–130.80) | <0.001 | <0.001 |
| DBP (mmHg) | 71.00 (44.00–160.00) | 78.00 (55.00–174.00) | 73.50 (53.00–138.00) | 77.00 (55.00–158.00) | <0.001 | <0.001 |
| SBP (mmHg) | 108.00 (15.00–199.00) | 116.00 (87.00–199.00) | 111.50 (89.00–188.00) | 119.00 (95.00–181.00) | <0.001 | <0.001 |
| HDL-c (mg/dL) | 51.30 (22.00–97.60) | 41.00 (18.60–76.40) | 45.58 (22.36–100.80) | 38.61 (16.88–97.50) | <0.001 | <0.001 |
| LDL-c (mg/dL) | 116.06 (39.54–264.56) | 117.99 (18.52–280.07) | 117.93 (37.30–273.74) | 120.96 (45.21–233.45) | 0.55 | 0.95 |
| Tg (mg/dL) | 130.30 (40.00–540.10) | 151.25 (41.00–581.00) | 163.30 (50.00–735.00) | 177.65 (54.90–682.50) | <0.001 | <0.001 |
| Glucose (mg/dL) | 87.00 (58.00–391.00) | 90.00 (72.00–324.00) | 95.00 (62.00–271.00) | 95.00 (71.00–295.00) | <0.001 | <0.001 |
| HOMA-IR | 3.33 (0.22–27.08) | 3.30 (0.44–26.44) | 5.49 (1.07–22.91) | 5.34 (1.46–34.11) | <0.001 | <0.001 |
| C-RP (mg/L) | 1.50 (0.12–52.00) | 1.11 (0.15–124.00) | 3.06 (0.15–16.90) | 1.67 (0.15–14.50) | <0.001 | <0.001 |
| VAF (cm2) | 131 (23–446) | 168 (25–504) | 166 (53–389) | 208 (48–459) | <0.001 | <0.001 |
| Uric Acid (mg/dL) | 4.62 (2.07–9.20) | 6.12 (2.70–11.72) | 5.23 (2.36–8.68) | 6.76 (2.82–11.03) | <0.001 | <0.001 |
| Physical activity index | 7.85 (4.5–11.2) | 7.81 (4.5–12.2) | 7.82 (4.2–11) | 8.1 (5.5–10.5) | - | - |
| AST (U/L) | 22.00 (10.00–148.00) | 25.00 (11.00–78.00) | 27.00 (13.00–92.00) | 29.00 (12.00–114.00) | <0.001 | <0.001 |
| ALT (U/L) | 19.00 (4.00–220.00) | 23.00 (5.00–125.00) | 28.00 (8.00–151.00) | 35.00 (6.00–145.00) | <0.001 | <0.001 |
| GGT (U/L) | 20.00 (4.00–315.00) | 29.00 (7.00–363.00) | 27.00 (9.00–607.00) | 40.00 (6.90–286.00) | <0.001 | <0.001 |
| Ratios and Fatty liver Index | ||||||
| UA/HDL-C Ratio | 0.09 (0.03–0.36) | 0.15 (0.05–0.47) | 0.12 (0.05–0.28) | 0.17 (0.06–0.52) | <0.001 | <0.001 |
| ALT/AST Ratio | 0.89 (0.22–2.31) | 1.00 (0.23–3.33) | 1.07 (0.38–2.75) | 1.24 (0.26–3.63) | <0.001 | <0.001 |
| LDL/HDL Ratio | 2.23 (0.01–9.80) | 2.83 (0.66–7.04) | 2.72 (0.47–6.75) | 3.05 (0.92–6.87) | <0.001 | <0.001 |
| NAFLD Score | 10.57 (6.47–53.53) | 11.60 (8.22–45.20) | 11.79 (6.80–37.75) | 12.73 (8.73–41.26) | <0.001 | <0.001 |
| FLI | 4.72 (0.43–100.00) | 9.12 (0.50–100.00) | 12.71 (0.59–100.00) | 23.40 (0.45–100.00) | <0.001 | <0.001 |
| LAP | 34.26 (0.00–240.77) | 64.93 (12.86–307.49) | 55.27 (7.88–215.76) | 89.19 (15.54–464.66) | <0.001 | <0.001 |
| HSI | 36.89 (22.72–58.65) | 49.49 (34.71–69.88) | 43.89 (26.40–59.28) | 55.22 (39.99–69.80) | <0.001 | <0.001 |
| Comorbidities | ||||||
| MS % (n) | 11.5 (60) | 57 (269) | 29 (64) | 79 (214) | - | - |
| Smoking % (n) | 18.7 (97) | 25.4 (118) | 20.4 (45) | 23.1 (62) | - | - |
| Alcohol consuption % (n) | 0.4 (2) | 2.8 (13) | 0.5 (1) | 4.9 (13) | - | - |
| HTA % (n) | 7.1 (37) | 13.3 (62) | 8.1 (18) | 13.4 (36) | - | - |
| T2DM % (n) | 9.2 (48) | 11.4 (53) | 18.1 (40) | 19 (51) | - | - |
| Unadjusted Logistic Regression | ||||
|---|---|---|---|---|
| Variable | Coefficient | Standard Error | 95% CI | p |
| UA/HDL-c Ratio | 2.50 | 0.66 | 3.31–44.98 | <0.001 |
| FLI | 0.44 | 0.27 | 0.90–2.70 | <0.10 |
| NAFLD Score | 2.00 | 0.57 | 2.42–22.81 | <0.001 |
| LAP | 0.13 | 0.80 | 0.23–5.54 | 0.86 |
| HSI | 1.16 | 0.46 | 1.29–7.88 | 0.01 |
| ALT/AST Ratio | 3.85 | 1.17 | 4.71–470.41 | <0.001 |
| Adjusted Logistic Regression (Sex) | ||||
| Variable | Coefficient | Standard Error | 95% CI | p |
| UA/HDL-C ratio | 3.47 | 0.70 | 8.18–128.18 | <0.001 |
| FLI | 0.29 | 0.28 | 0.77–2.33 | 0.29 |
| NAFLD Score | 1.942 | 0.58 | 2.23–21.75 | <0.001 |
| LAP | −0.08 | 0.81 | 0.18–4.57 | 0.92 |
| HSI | 3.20 | 0.57 | 7.96–75.86 | <0.001 |
| ALT/AST Ratio | 3.28 | 1.14 | 2.79–253.23 | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Posadas-Sánchez, R.; Fuentevilla-Álvarez, G.; Vargas-Alarcón, G.; Cardoso-Saldaña, G.C. Is UA/HDL-C a Reliable Surrogate Marker for Fatty Liver? A Comparative Evaluation with Metabolic Scores in a Mexican Population: The Genetics of Atherosclerotic Disease Study. Diagnostics 2025, 15, 1419. https://doi.org/10.3390/diagnostics15111419
Posadas-Sánchez R, Fuentevilla-Álvarez G, Vargas-Alarcón G, Cardoso-Saldaña GC. Is UA/HDL-C a Reliable Surrogate Marker for Fatty Liver? A Comparative Evaluation with Metabolic Scores in a Mexican Population: The Genetics of Atherosclerotic Disease Study. Diagnostics. 2025; 15(11):1419. https://doi.org/10.3390/diagnostics15111419
Chicago/Turabian StylePosadas-Sánchez, Rosalinda, Giovanny Fuentevilla-Álvarez, Gilberto Vargas-Alarcón, and Guillermo C. Cardoso-Saldaña. 2025. "Is UA/HDL-C a Reliable Surrogate Marker for Fatty Liver? A Comparative Evaluation with Metabolic Scores in a Mexican Population: The Genetics of Atherosclerotic Disease Study" Diagnostics 15, no. 11: 1419. https://doi.org/10.3390/diagnostics15111419
APA StylePosadas-Sánchez, R., Fuentevilla-Álvarez, G., Vargas-Alarcón, G., & Cardoso-Saldaña, G. C. (2025). Is UA/HDL-C a Reliable Surrogate Marker for Fatty Liver? A Comparative Evaluation with Metabolic Scores in a Mexican Population: The Genetics of Atherosclerotic Disease Study. Diagnostics, 15(11), 1419. https://doi.org/10.3390/diagnostics15111419






