ECG Signal Analysis for Detection and Diagnosis of Post-Traumatic Stress Disorder: Leveraging Deep Learning and Machine Learning Techniques
Abstract
1. Introduction
- This study introduces a novel, state-of-the-art approach for the classification of PTSD from ECG signals, which has not been addressed in previous research using either DL or ML methods.
- To improve feature representation, we optimized the performance of CNN architectures using scalogram-based time–frequency images. This method increased the ability to capture complex patterns associated with PTSD more effectively.
- 5-fold cross-validation (K-fold cross-validation) was used to address the difficulties arising from the datasets. This method increased the model’s generalization ability and provided a more balanced and robust performance.
- Compared with traditional ML methods, a clear superiority of DL models was observed.
- The method in this study achieved higher accuracy rates than other DL models and traditional ML methods. These results provide information that can contribute to the development of more effective diagnostic tools for detecting psychological disorders.
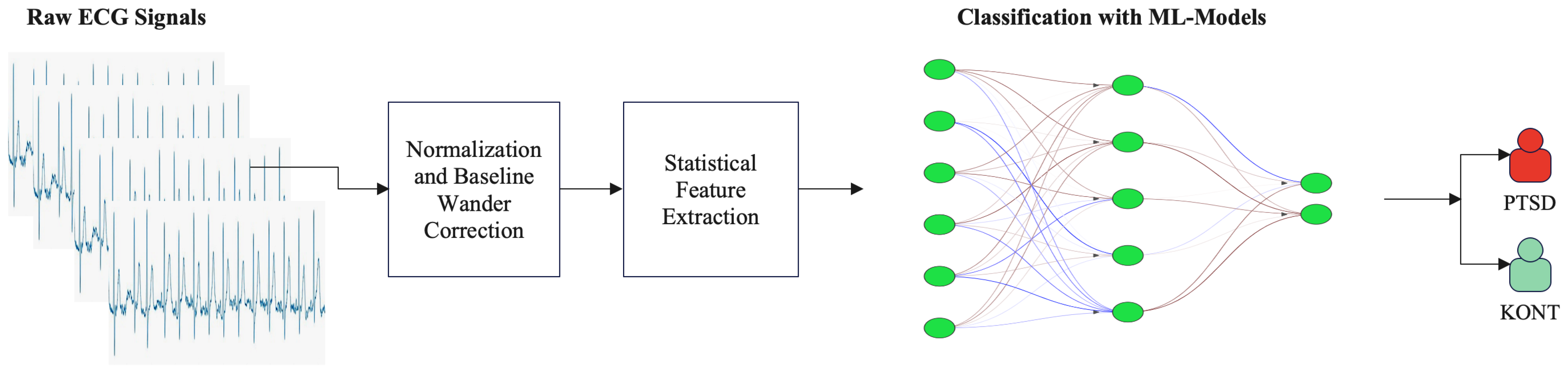
2. Materials and Methods
2.1. Data Acquisition
2.2. Signal Preprocessing
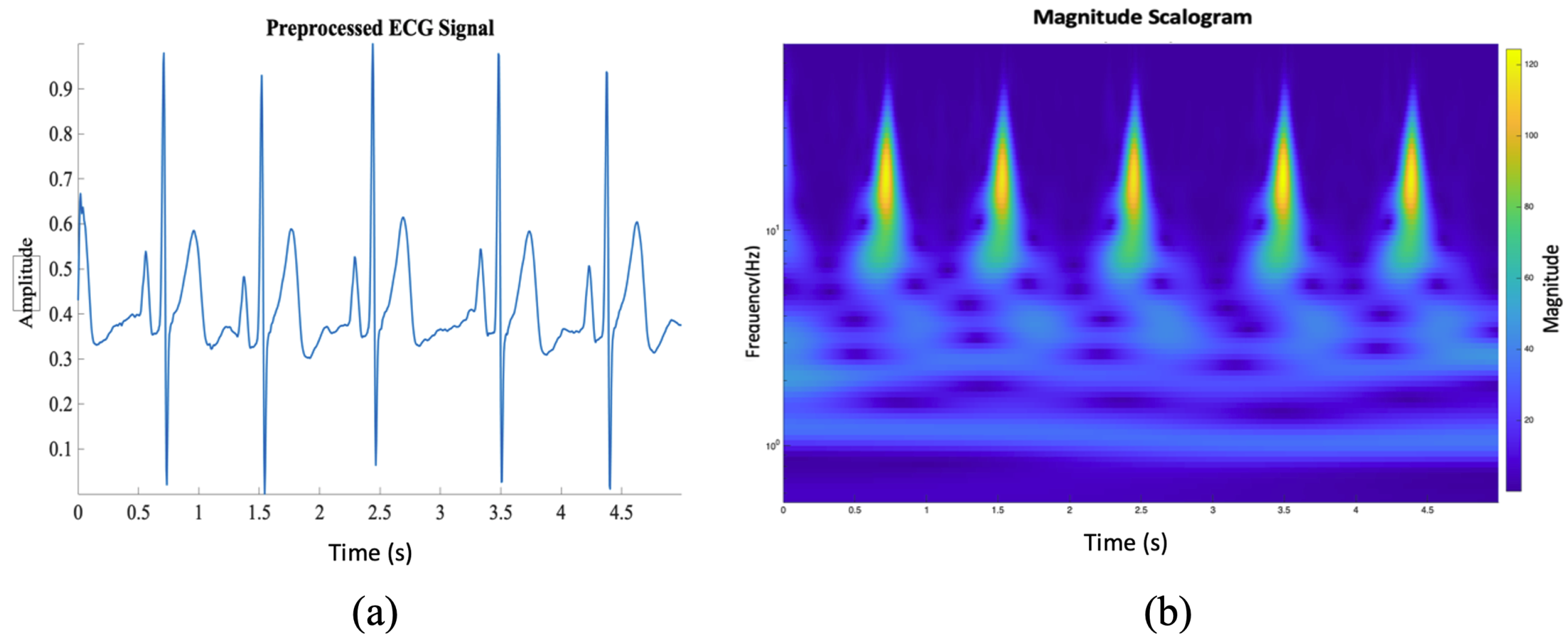
2.2.1. Normalization and Baseline Wander Correction
2.2.2. One-Dimensional ECG Signal into a Two-Dimensional Image
2.3. Performance Evaluation
3. Results
3.1. Machine Learning Classification
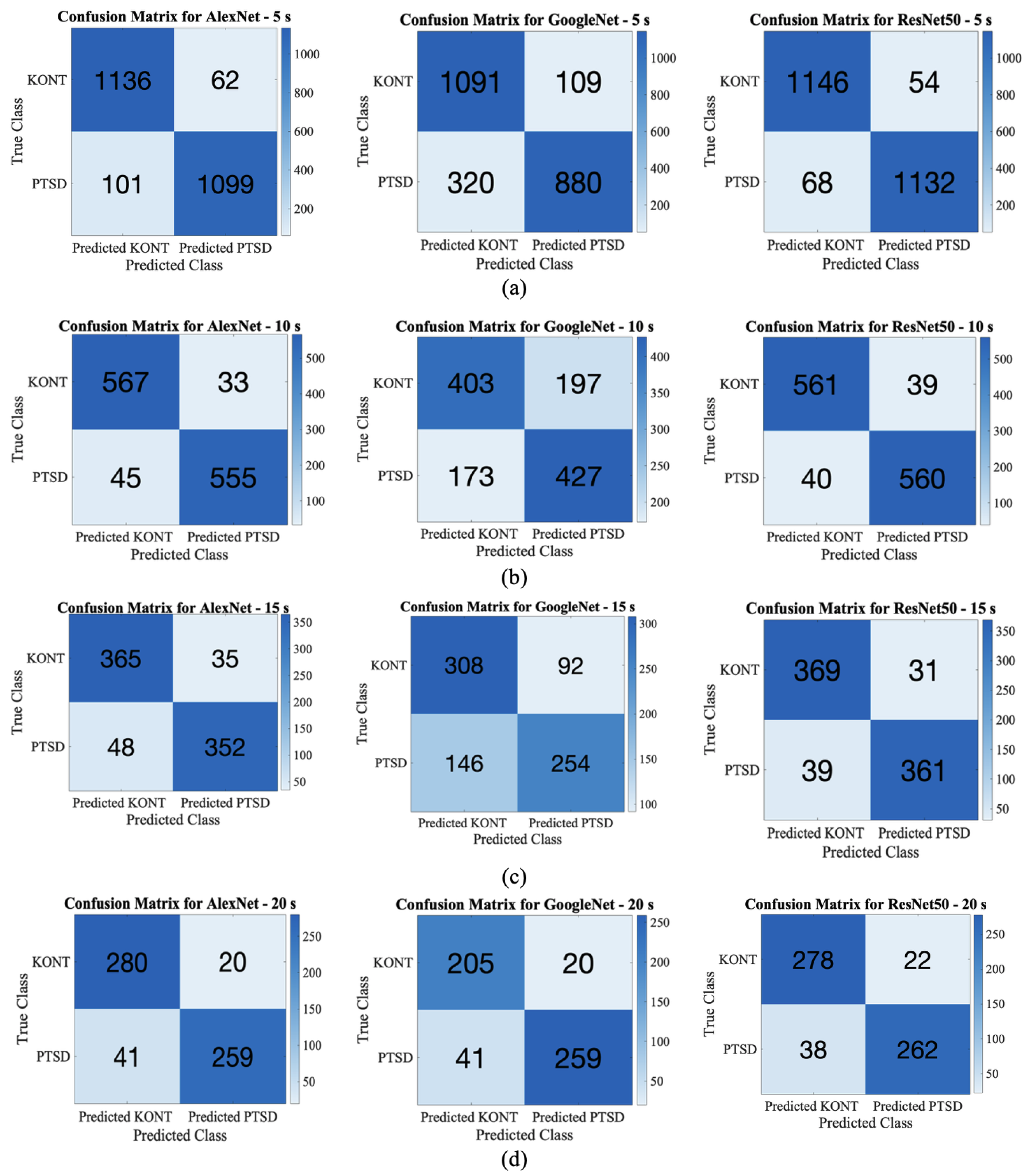
3.2. Deep Learning Classification
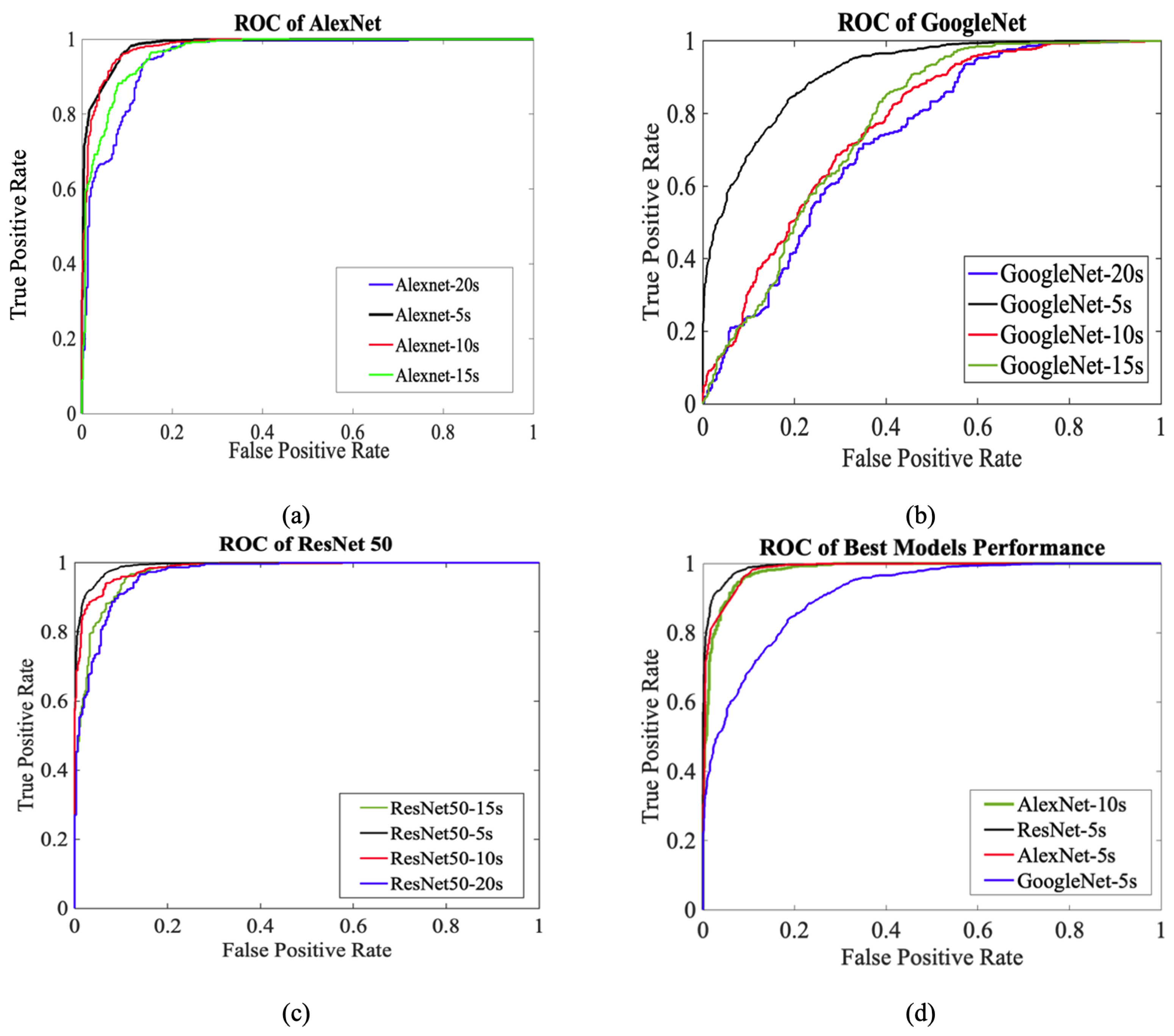
3.3. ROC Performance Evaluation
4. Discussion
5. Conclusions
6. Future Studies
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AUC | Area Under the Curve |
| BW | Baseline Wander |
| CHD | Coronary Heart Disease |
| CNN | Convolutional Neural Network |
| CVD | Cardiovascular Disease |
| CWT | Continuous Wavelet Transform |
| DL | Deep Learning |
| DNN | Deep Neural Network |
| ECG | Electrocardiogram |
| ERD | Emotion Regulation Difficulties |
| ERP | Event-Related Potential |
| HR | Heart Rate |
| ERP | Event-Related Potential |
| HR | Heart Rate |
| HRV | Heart Rate Variability |
| KNN | K-Nearest Neighbor |
| LDA | Linear Discriminant Analysis |
| MCC | Matthews Correlation Coefficient |
| ML | Machine Learning |
| PCL-C | PTSD Check List Civilian |
| PCI | Phase Locking Values |
| PLI | Power Line Interference |
| PTSD | Post-Traumatic Stress Disorder |
| ResNet | Residual Network |
| RMS | Root Mean Square |
| ROC | Receiver Operating Characteristic Curve |
| SGDM | Stochastic Gradient Descent with Momentum |
| SINAD | Signal-to-Noise and Distortion Ratio |
| SNR | Signal-to-Noise Ratio |
| STD | Standard Deviation |
| STFT | Short-Time Fourier Transform |
| SVM | Support Vector Machine |
| THD | Total Harmonic Distortion |
References
- Sherin, J.E.; Nemeroff, C.B. Post-traumatic stress disorder: The neurobiological impact of psychological trauma. Dialogues Clin. Neurosci. 2011, 13, 263–278. [Google Scholar] [CrossRef] [PubMed]
- Kessler, R.C.; Aguilar-Gaxiola, S.; Alonso, J.; Benjet, C.; Bromet, E.J.; Cardoso, G.; Degenhardt, L.; de Girolamo, G.; Dinolova, R.V.; Ferry, F. Trauma and PTSD in the WHO world mental health surveys. Eur. J. Psychotraumatol. 2017, 8, 1353383. [Google Scholar] [CrossRef]
- Gates, M.A.; Holowka, D.W.; Vasterling, J.J.; Keane, T.M.; Marx, B.P.; Rosen, R.C. Posttraumatic stress disorder in veterans and military personnel: Epidemiology, screening, and case recognition. Psychol. Serv. 2012, 9, 361. [Google Scholar] [CrossRef] [PubMed]
- GBD 2015 Mortality and Causes of Death Collaborators. Global, regional, and national life expectancy, all-cause mortality, and cause-specific mortality for 249 causes of death, 1980–2015: A systematic analysis for the Global Burden of Disease Study 2015. Lancet 2016, 388, 1459–1544. [Google Scholar] [CrossRef]
- Fisher, B.S. The effects of survey question wording on rape estimates: Evidence from a quasi-experimental design. Violence Against Women 2009, 15, 133–147. [Google Scholar] [CrossRef]
- Seligowski, A.V.; Webber, T.K.; Marvar, P.J.; Ressler, K.J.; Philip, N.S. Involvement of the brain–heart axis in the link between PTSD and cardiovascular disease. Depress. Anxiety 2022, 39, 663–674. [Google Scholar] [CrossRef] [PubMed]
- Tezuka, K.; Kubota, Y.; Ohira, T.; Nakano, H.; Maeda, M.; Yabe, H.; Yasumura, S.; Harigane, M.; Shimizu, Y.; Okada, T. Synergistic effect of history of cardiovascular disease and mental distress on post-traumatic stress disorder after the Great East Japan Earthquake: The Fukushima Health Management Survey. Int. J. Environ. Res. Public Health 2021, 18, 10283. [Google Scholar] [CrossRef]
- Roer, G.E.; Lien, L.; Bolstad, I.; Aaseth, J.O.; Abebe, D.S. The impact of PTSD on risk of cardiometabolic diseases: A national patient cohort study in Norway. BMC Psychiatry 2023, 23, 349. [Google Scholar] [CrossRef]
- Wesemann, U.; Sahebi, A.; Vogel, J.; Köhler, K.; Kupusovic, J.; Rassaf, T.; Siebermair, J. Post-traumatic stress disorder among COVID-19-affected high-risk cardiac patients. Int. Health 2024, 16, 120–122. [Google Scholar] [CrossRef]
- Crum-Cianflone, N.F.; Bagnell, M.E.; Schaller, E.; Boyko, E.J.; Smith, B.; Maynard, C.; Ulmer, C.S.; Vernalis, M.; Smith, T.C. Impact of combat deployment and posttraumatic stress disorder on newly reported coronary heart disease among US active duty and reserve forces. Circulation 2014, 129, 1813–1820. [Google Scholar] [CrossRef]
- Magsi, H.; Sodhro, A.H.; Al-Rakhami, M.S.; Zahid, N.; Pirbhulal, S.; Wang, L. A novel adaptive battery-aware algorithm for data transmission in IoT-based healthcare applications. Electronics 2021, 10, 367. [Google Scholar] [CrossRef]
- Bui, N.-T.; Byun, G.-S. The comparison features of ECG signal with different sampling frequencies and filter methods for real-time measurement. Symmetry 2021, 13, 1461. [Google Scholar] [CrossRef]
- Rahman, M.M.; Kakavand, B.; Van Wurm, W.; Holman, W.L.; Movahed, M.R.; Taebi, A. From video to vital signs: A new method for contactless multichannel seismocardiography. NPJ Cardiovasc. Health 2025, 2, 1. [Google Scholar] [CrossRef]
- Hao, T.; Zheng, X.; Wang, H.; Xu, K.; Chen, S. Linear and nonlinear analyses of heart rate variability signals under mental load. Biomed. Signal Process. Control 2022, 77, 103758. [Google Scholar] [CrossRef]
- Ding, J.; Tang, Y.; Chang, R.; Li, Y.; Zhang, L.; Yan, F. Reduction in the motion artifacts in noncontact ECG measurements using a novel designed electrode structure. Sensors 2023, 23, 956. [Google Scholar] [CrossRef]
- Mir, H.Y.; Singh, O. ECG denoising and feature extraction techniques—A review. J. Med. Eng. Technol. 2021, 45, 672–684. [Google Scholar] [CrossRef]
- Mourad, T. ECG denoising based on 1-D double-density complex DWT and SBWT. In The Stationary Bionic Wavelet Transform and its Applications for ECG and Speech Processing; Springer: Berlin/Heidelberg, Germany, 2022; pp. 31–50. [Google Scholar] [CrossRef]
- Acharya, U.R.; Fujita, H.; Oh, S.L.; Hagiwara, Y.; Tan, J.H.; Adam, M. Application of deep convolutional neural network for automated detection of myocardial infarction using ECG signals. Inf. Sci. 2017, 415, 190–198. [Google Scholar] [CrossRef]
- Kiranyaz, S.; Avci, O.; Abdeljaber, O.; Ince, T.; Gabbouj, M.; Inman, D.J. 1D convolutional neural networks and applications: A survey. Mech. Syst. Signal Process. 2021, 151, 107398. [Google Scholar] [CrossRef]
- Magrupov, T.M.; Nematov, S.K.; Talatov, Y.T. ECG processing and analysis technique based on neural network learning vector quantization. Chem. Technol. Control Manag. 2020, 2020, 15–22. [Google Scholar] [CrossRef]
- McDonald, A.D.; Sasangohar, F.; Jatav, A.; Rao, A.H. Continuous monitoring and detection of post-traumatic stress disorder (PTSD) triggers among veterans: A supervised machine learning approach. IISE Trans. Healthc. Syst. Eng. 2019, 9, 201–211. [Google Scholar] [CrossRef]
- Wu, Y.; Mao, K.; Dennett, L.; Zhang, Y.; Chen, J. Systematic review of machine learning in PTSD studies for automated diagnosis evaluation. NPJ Ment. Health Res. 2023, 2, 16. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Ouyang, H.; Jiao, R.; Cheng, S.; Zhang, H.; Shang, Z.; Jia, Y.; Yan, W.; Liu, W. The application of machine learning techniques in posttraumatic stress disorder: A systematic review and meta-analysis. NPJ Digit. Med. 2024, 7, 121. [Google Scholar] [CrossRef] [PubMed]
- Cakmak, A.S.; Alday, E.A.P.; Da Poian, G.; Rad, A.B.; Metzler, T.J.; Neylan, T.C.; House, S.L.; Beaudoin, F.L.; An, X.; Stevens, J.S. Classification and prediction of post-trauma outcomes related to PTSD using circadian rhythm changes measured via wrist-worn research watch in a large longitudinal cohort. IEEE J. Biomed. Health Inform. 2021, 25, 2866–2876. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Lei, D.; Qin, K.; Pinaya, W.H.; Suo, X.; Li, W.; Li, L.; Kemp, G.J.; Gong, Q. Using deep learning to classify pediatric posttraumatic stress disorder at the individual level. BMC Psychiatry 2021, 21, 535. [Google Scholar] [CrossRef]
- Shim, M.; Lee, S.-H.; Hwang, H.-J. Functional connectivity-based EEG features to assist the diagnosis of post-traumatic stress disorder patients. In Proceedings of the 9th International Winter Conference on Brain-Computer Interface (BCI), Gangwon, Repubilc of Korea, 22–24 February 2021; pp. 1–4. [Google Scholar] [CrossRef]
- Banerjee, D.; Islam, K.; Xue, K.; Mei, G.; Xiao, L.; Zhang, G.; Xu, R.; Lei, C.; Ji, S.; Li, J. A deep transfer learning approach for improved post-traumatic stress disorder diagnosis. Knowl. Inf. Syst. 2019, 60, 1693–1724. [Google Scholar] [CrossRef]
- Burrus, C.S.; Gopinath, R.A.; Guo, H. Wavelets and Wavelet Transforms; Prentice Hall Publications: Houston, TX, USA, 1998. [Google Scholar]
- Qiu, C.; Li, H.; Qi, C.; Li, B. Enhancing ECG classification with continuous wavelet transform and multi-branch transformer. Heliyon 2024, 10, e26147. [Google Scholar] [CrossRef]
- Aabdalla, I.D.; Vasumathi, D. Wavelet Scattering Transform for ECG Cardiovascular Disease Classification. Int. J. Artif. Intell. Appl. 2024, 15, 101–113. [Google Scholar] [CrossRef]
- Ganguly, B.; Das, A.; Ghosal, A.; Das, D.; Chatterjee, D.; Rakshit, D.; Das, E. A non-invasive approach for fetal arrhythmia detection and classification from ECG signals. In Proceedings of the 2020 IEEE VLSI Device Circuit and System (VLSI DCS), Kolkata, India, 18–19 July 2020. [Google Scholar] [CrossRef]
- Herff, C.; Krusienski, D.J. Extracting features from time series. In Fundamentals of Clinical Data Science; Springer: Berlin/Heidelberg, Germany, 2019; pp. 85–100. [Google Scholar] [CrossRef]
- Barandas, M.; Folgado, D.; Fernandes, L.; Santos, S.; Abreu, M.; Bota, P.; Liu, H.; Schultz, T.; Gamboa, H. TSFEL: Time series feature extraction library. SoftwareX 2020, 11, 100456. [Google Scholar] [CrossRef]
- Teja, K.; Tiwari, R.; Mohanty, S. Adaptive denoising of ECG using EMD, EEMD and CEEMDAN signal processing techniques. J. Phys. Conf. Ser. 2020, 1706, 012077. [Google Scholar] [CrossRef]
- Khan, M.U.; Aziz, S.; Naqvi, S.Z.H.; Rehman, A. Classification of coronary artery diseases using electrocardiogram signals. In Proceedings of the 2020 International Conference on Emerging Trends in Smart Technologies (ICETST), Karachi, Pakistan, 26–27 March 2020. [Google Scholar] [CrossRef]
- Kavitha, M.; Akhila, S.; Kannan, A. Design and Implementation of a Second Order Continuous-Time ∑Δ Modulator for ECG Signal Acquisition. Eng. Technol. Appl. Sci. Res. 2023, 13, 10128–10133. [Google Scholar] [CrossRef]
- Wang, X.; Yang, J. A Systematic Method Combining Rotated Convolution and State Space Augmented Transformer for Digitizing and Classifying Paper ECGs. Symmetry 2025, 17, 120. [Google Scholar] [CrossRef]
- Shmilovitz, D. On the definition of total harmonic distortion and its effect on measurement interpretation. IEEE Trans. Power Deliv. 2005, 20, 526–528. [Google Scholar] [CrossRef]
- Alotaiby, T.N.; Alrshoud, S.R.; Alshebeili, S.A.; Aljafar, L.M. ECG-Based Subject Identification Using Statistical Features and Random Forest. J. Sens. 2019, 2019, 6751932. [Google Scholar] [CrossRef]
- Singh, A.K.; Krishnan, S. ECG signal feature extraction trends in methods and applications. BioMed Eng. Online 2023, 22, 22. [Google Scholar] [CrossRef]
- Al-Saadany, D.; Attallah, O.; Elzaafarany, K.; Nasser, A. A machine learning framework for fetal arrhythmia detection via single ECG electrode. In Proceedings of the International Conference on Computational Science, London, UK, 21–23 June 2022. [Google Scholar] [CrossRef]
- Wahab, A.; Khan, U.S.; Nawaz, T.; Shah, S.T.H.; Izhar, U.; Zeb, A. An optimized Impulse Factor-based VMD-EMD approach to improve SSVEP accuracy for BCI Systems. Results Eng. 2025, 25, 104203. [Google Scholar] [CrossRef]
- Fjellström, C.; Nyström, K. Deep learning, stochastic gradient descent and diffusion maps. J. Comput. Math. Data Sci. 2022, 4, 100054. [Google Scholar] [CrossRef]
- Anbalagan, T.; Nath, M.K.; Vijayalakshmi, D.; Anbalagan, A. Analysis of various techniques for ECG signal in healthcare, past, present, and future. Biomed. Eng. Adv. 2023, 6, 100089. [Google Scholar] [CrossRef]
- Tharwat, A. Classification assessment methods. Appl. Comput. Inform. 2021, 17, 168–192. [Google Scholar] [CrossRef]
- Schultebraucks, K.; Yadav, V.; Shalev, A.Y.; Bonanno, G.A.; Galatzer-Levy, I.R. Deep learning-based classification of posttraumatic stress disorder and depression following trauma utilizing visual and auditory markers of arousal and mood. Psychol. Med. 2022, 52, 957–967. [Google Scholar] [CrossRef]
- Taha, B.; Kirk, M.; Ritvo, P.; Hatzinakos, D. Detection of post-traumatic stress disorder using learned time-frequency representations from pupillometry. In Proceedings of the ICASSP 2021–2021 IEEE International Conference on Acoustics, Speech and Signal Processing (ICASSP), Toronto, ON, Canada, 6–11 June 2021; pp. 3950–3954. [Google Scholar] [CrossRef]
- Beykmohammadi, A.; Ghanbari, Z.; Moradi, M.H. PTSD Diagnosis using Deep Transfer Learning: An EEG Study. In Proceedings of the 2022 29th National and 7th International Iranian Conference on Biomedical Engineering (ICBME), Tehran, Iran, 24–25 November 2022; pp. 9–13. [Google Scholar] [CrossRef]
- Bhattacharya, J.; Gupta, A.; Dretsch, M.; Denney, T.; Deshpande, G. A Reliable Clinical Decision Support System for Post Traumatic Stress Disorder using functional Magnetic Resonance Imaging data. IEEE Trans. Artif. Intell. 2024, 5, 5605–5615. [Google Scholar] [CrossRef]
- Vali, M.; Nezhad, H.M.; Kovacs, L.; Gandomi, A.H. Machine learning algorithms for predicting PTSD: A systematic review and meta-analysis. BMC Med. Inform. Decis. Mak. 2025, 25, 34. [Google Scholar] [CrossRef] [PubMed]
- Portugal, L.C.L.; Ramos, T.C.; Fernandes, O.; Bastos, A.F.; Campos, B.; Mendlowicz, M.V.; da Luz, M.; Portella, C.; Berger, W.; Volchan, E.; et al. Machine learning applied to fMRI patterns of brain activation in response to mutilation pictures predicts PTSD symptoms. BMC Psychiatry 2023, 23, 719. [Google Scholar] [CrossRef] [PubMed]
- Quatieri, T.F.; Wang, J.; Williamson, J.R.; DeLaura, R.; Talkar, T.; Solomon, N.P.; Lanius, R.A.; Ros, T. An emotion-driven vocal biomarker-based PTSD screening tool. IEEE Open J. Eng. Med. Biol. 2023, 5, 621–626. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.K.; Sinha, R. An investigation on the audio-video data based estimation of emotion regulation difficulties and their association with mental disorders. IEEE Access 2023, 11, 74324–74336. [Google Scholar] [CrossRef]
- Shahzad, M.N.; Ali, H.; Saba, T.; Rehman, A.; Kolivand, H.; Bahaj, S.A. Identifying patients with PTSD utilizing resting-state fMRI data and neural network approach. IEEE Access 2021, 9, 107941–107954. [Google Scholar] [CrossRef]
- Josephine Mary Juliana, M.; Sudha, G.F.; Nakkeeran, R. An atypical approach toward PTSD diagnosis through speech-based emotion modeling using CNN-LSTM. In Proceedings of Trends in Electronics and Health Informatics: TEHI 2021; Springer: Berlin/Heidelberg, Germany, 2022; pp. 291–309. [Google Scholar] [CrossRef]
- Terpou, B.A.; Shaw, S.B.; Théberge, J.; Férat, V.; Michel, C.M.; McKinnon, M.C.; Lanius, R.A.; Ros, T. Spectral decomposition of EEG microstates in post-traumatic stress disorder. Neuroimage Clin. 2022, 35, 103135. [Google Scholar] [CrossRef] [PubMed]
- Shim, M.; Lee, S.-H.; Hwang, H.-J. A novel neurophysiological feature based on quantifying EEG data for separating of patients in psychiatric disorders with comorbidities. In Proceedings of the 2022 10th International Winter Conference on Brain-Computer Interface (BCI), Gangwon, Repubilc of Korea, 21–23 February 2022. [Google Scholar] [CrossRef]
- Elouaham, S.; Dliou, A.; Laaboubi, M.; Latif, R.; Elkamoun, N.; Zougagh, H. Filtering and analyzing normal and abnormal electromyogram signals. Indones. J. Electr. Eng. Comput. Sci. 2020, 20, 176–184. [Google Scholar] [CrossRef]
- Anbalagan, T.; Nath, M.K. AF Identification From Time–Frequency Analysis of ECG Signal Using Deep Neural Networks. IEEE Sens. Lett. 2024, 8, 6010604. [Google Scholar] [CrossRef]


| Segment Length | Samples per Scalogram | Scalograms per Record | Total Records | Total Scalograms |
|---|---|---|---|---|
| 5 s | 1000 | 60 | 40 | 2400 |
| 10 s | 2000 | 30 | 40 | 1200 |
| 15 s | 3000 | 20 | 40 | 800 |
| 20 s | 4000 | 15 | 40 | 600 |
| Feature (No) | Description | Mathematical Formula |
|---|---|---|
| Clearance Factor [30] | Detects impulsive abnormalities in ECG morphology | |
| Skewness [31] | Identifies asymmetric waveform patterns | |
| Peak Value [32] | Captures extreme events like R-peaks | |
| RMS [33] | Represents overall signal energy | |
| Shape Factor [34] | Describes overall ECG waveform shape | |
| Crest Factor [35] | Indicates sharp transitions like QRS complexes | |
| SINAD [36] | Evaluates ECG signal quality, including noise artifacts | |
| SNR [37] | Assesses clarity of the ECG signal | |
| THD [38] | Identifies signal distortions or repetitive noise patterns | |
| Standard Deviation [39] | Detects variability in ECG waveforms | |
| Mean [40] | Baseline reference of ECG amplitude | |
| Kurtosis [41] | Detects sharp features like arrhythmic spikes | |
| Impulse Factor [42] | Sensitivity to sudden spikes such as premature beats |
| ML-Model | Accuracy (%) | Precision (%) | Recall (%) | Specificity (%) | F1-Score (%) | MCC | Prevalence (%) |
|---|---|---|---|---|---|---|---|
| Linear Discriminant | 72.50 | 73.68 | 70.00 | 75.00 | 71.79 | 0.4505 | 0.5 |
| Ensemble | 70.00 | 75.00 | 60.00 | 80.00 | 66.67 | 0.4082 | 0.5 |
| SVM (Linear) | 65.00 | 68.75 | 55.00 | 75.00 | 61.11 | 0.3061 | 0.5 |
| KNN (Cubic) | 65.00 | 66.67 | 60.00 | 70.00 | 63.16 | 0.3051 | 0.5 |
| Trilayered Neural Network | 62.50 | 60.87 | 70.00 | 0.55 | 65.12 | 0.2528 | 0.5 |
| Naïve Bayes | 57.50 | 67.00 | 70.00 | 75.00 | 0.7179 | 0.1549 | 0.5 |
| Parameter | Value/Setting | Description and Importance |
|---|---|---|
| Class Structure | Two Classes | The model is trained to classify data into two categories, enabling binary classification and distinguishing between two distinct groups. |
| Mini-Batch Size | 20 | The model processes 20 samples at a time for each weight update, balancing memory usage and training stability. Smaller batches introduce noise, improving generalization. |
| Max Epochs | 8 | The model iterates over the entire training dataset a maximum of 8 times, preventing overfitting by limiting dataset exposure. |
| Optimization Algorithm | SGDM | SGDM with Momentum accelerates learning and reduces oscillations during parameter optimization. |
| Learning Rate | 0.0001 | Controls the step size for weight updates during training. A small learning rate ensures stable and precise updates, avoiding overshooting the optimal solution. |
| Validation Frequency | 10 | The model is evaluated on the validation dataset every 10 training steps to monitor performance, detect overfitting, and ensure generalization. |
| Model | Learnable Parameters (M) | No. of Layers (MATLAB) | Fine-Tuning | Segment Length (s) | Training Time (per Fold, min) |
|---|---|---|---|---|---|
| AlexNet | 60.9 | 25 | Final 3 layers replaced | 5 | 29 ± 2 |
| 10 | 21 ± 2 | ||||
| 15 | 14 ± 2 | ||||
| 20 | 7 ± 2 | ||||
| GoogLeNet | 6.9 | 144 | Final 3 layers replaced | 5 | 55 ± 5 |
| 10 | 30 ± 5 | ||||
| 15 | 18 ± 5 | ||||
| 20 | 13 ± 5 | ||||
| ResNet50 | 25.5 | 177 | Final 3 layers replaced | 5 | 130 ± 5 |
| 10 | 60 ± 5 | ||||
| 15 | 41 ± 5 | ||||
| 20 | 32 ± 5 |
| DL-Model | Accuracy (%) | Precision (%) | Recall (%) | Specificity (%) | F1-Score (%) | MCC | Prevalence (%) |
|---|---|---|---|---|---|---|---|
| AlexNet_5s | 93.21 | 94.66 | 91.85 | 94.82 | 93.10 | 0.8645 | 50.00 |
| GoogleNet_5s | 82.1 | 88.98 | 73.33 | 90.92 | 80.40 | 0.6526 | 50.00 |
| ResNet50_5s | 94.92 | 95.45 | 94.33 | 95.50 | 94.89 | 0.8983 | 50.00 |
| AlexNet_10s | 93.50 | 94.39 | 92.50 | 94.50 | 93.43 | 0.8701 | 50.00 |
| GoogleNet_10s | 69.17 | 68.43 | 71.17 | 67.17 | 69.77 | 0.3836 | 50.00 |
| ResNet50_10s | 93.42 | 93.49 | 93.13 | 93.50 | 93.41 | 0.8683 | 50.00 |
| AlexNet_15s | 89.63 | 90.96 | 88.00 | 91.25 | 89.45 | 0.7921 | 50.00 |
| GoogleNet_15s | 70.25 | 73.41 | 63.50 | 77.00 | 68.10 | 0.4087 | 50.00 |
| ResNet50_15s | 91.25 | 92.09 | 90.25 | 92.25 | 91.16 | 0.8251 | 50.00 |
| AlexNet_20s | 89.83 | 92.83 | 86.33 | 93.33 | 89.46 | 0.7986 | 50.00 |
| GoogleNet_20s | 67.33 | 67.69 | 66.33 | 68.33 | 67.00 | 0.3467 | 50.00 |
| ResNet50_20s | 90.00 | 92.25 | 87.33 | 92.67 | 89.73 | 0.8011 | 50.00 |
| DL-Models | AUC |
|---|---|
| AlexNet-5s | 0.980 |
| AlexNet-10s | 0.981 |
| AlexNet-15s | 0.965 |
| AlexNet-20s | 0.952 |
| GoogleNet-5s | 0.911 |
| GoogleNet-10s | 0.764 |
| GoogleNet-15s | 0.765 |
| GoogleNet-20s | 0.730 |
| ResNet50-5s | 0.991 |
| ResNet50-10s | 0.984 |
| ResNet50-15s | 0.973 |
| ResNet50-20s | 0.966 |
| Author, Year | Dataset Type | Method | Classifier Used | Performance | Strengths | Limitations |
|---|---|---|---|---|---|---|
| Yang et al., 2021 [25] | fMRI | Graph theory + DL | SVM | Accuracy = 71.2% | Combines brain imaging with ML | Requires expensive equipment |
| Banerjee et al., 2019 [27] | Speech | Frequency feature extraction | DBN + Transfer Learning | Accuracy = 74.99% | Non-invasive and portable | Affected by environment |
| Schultebraucks et al., 2020 [46] | Video + Audio | Interviews | DNN | AUC = 0.90 | Rich multimodal input | Complex preprocessing |
| Taha et al., 2021 [47] | Pupillometry | STFT | CNN | Accuracy = 80.42% | Objective signal-based method | Specialized hardware needed |
| Shim et al., 2021 [26] | EEG | Functional connectivity: PLV + graph metrics | SVM | Accuracy = 70.35%, AUC = 0.85 | Widely used EEG markers | Sensitive to artifacts |
| Beykmohammadi et al., 2022 [48] | EEG | CWT | VGG16 | Accuracy = 78.93% | Deep features from time–frequency | Moderate accuracy |
| Bhattacharya et al., 2024 [49] | fMRI | Triplet-based feature learning | Not Stated | Certainty ≥95% for pure class | Novel framework | Unclear metrics |
| Vali et al., 2025 [50] | Mixed (military, trauma, disaster) | Systematic review/meta-analysis | Random Forest, XGBoost | AUC: 0.745–0.96 | Broad scope; high AUCs | - |
| Portugal et al., 2023 [51] | fMRI | Pattern recognition + regression | GPR | Predicted PTSD symptoms accurately | Biomarkers identified, contextual fMRI | Small sample, limited generalizability |
| Quatieri et al., 2023 [52] | Speech (PCL-C) | Emotion-based vocal biomarkers | Emotion-Filtered Acoustic Model | AUC = 0.80 | Emotion-driven boost in accuracy | Civilian scale, moderate accuracy |
| Gupta et al., 2023 [53] | Audio-video + questionnaires | ERD estimation | RF, SVM, Logistic Regression | Correlates well with PTSD severity | Explores gender bias, latent traits | Small sample, incomplete metrics |
| Shahzad et al., 2021 [54] | rs-fMRI | Resting-state fMRI | ANN | Accuracy = 94.5% | High accuracy; regional insights | Expensive, small sample |
| Josephine et al., 2022 [55] | Speech | Mel spectrogram, emotion recognition | CNN-LSTM | Accuracy = 98.68% | High accuracy, non-invasive | Indirect labels, clinical validation needed |
| Terpou et al., 2022 [56] | EEG | Spectral decomposition | SVM | Accuracy = 76% | Frequency-specific insight | Moderate accuracy, signal noise |
| Shim et al., 2022 [57] | EEG | ERP mean amplitude | SVM | Accuracy = 73.33% | Cognitive trait differentiation | One feature, small dataset |
| This study | ECG | CWT (scalogram); statistical features | CNN (AlexNet, GoogLeNet, ResNet); ML (SVM, KNN, Ensemble) | Accuracy = 94.92%, AUC = 0.99 | Accessible signal; first to apply ECG in PTSD DL/ML | Multiclass extension needed |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ebrahimpour Moghaddam Tasouj, P.; Soysal, G.; Eroğul, O.; Yetkin, S. ECG Signal Analysis for Detection and Diagnosis of Post-Traumatic Stress Disorder: Leveraging Deep Learning and Machine Learning Techniques. Diagnostics 2025, 15, 1414. https://doi.org/10.3390/diagnostics15111414
Ebrahimpour Moghaddam Tasouj P, Soysal G, Eroğul O, Yetkin S. ECG Signal Analysis for Detection and Diagnosis of Post-Traumatic Stress Disorder: Leveraging Deep Learning and Machine Learning Techniques. Diagnostics. 2025; 15(11):1414. https://doi.org/10.3390/diagnostics15111414
Chicago/Turabian StyleEbrahimpour Moghaddam Tasouj, Parisa, Gökhan Soysal, Osman Eroğul, and Sinan Yetkin. 2025. "ECG Signal Analysis for Detection and Diagnosis of Post-Traumatic Stress Disorder: Leveraging Deep Learning and Machine Learning Techniques" Diagnostics 15, no. 11: 1414. https://doi.org/10.3390/diagnostics15111414
APA StyleEbrahimpour Moghaddam Tasouj, P., Soysal, G., Eroğul, O., & Yetkin, S. (2025). ECG Signal Analysis for Detection and Diagnosis of Post-Traumatic Stress Disorder: Leveraging Deep Learning and Machine Learning Techniques. Diagnostics, 15(11), 1414. https://doi.org/10.3390/diagnostics15111414






