Real-Time MR-Guided Lumbosacral Periradicular Injection Therapy Using a 0.55 T MRI System: A Phantom Study
Abstract
1. Introduction
2. Methods
2.1. Study Design
2.2. Phantom of Lumbosacral Spine: Lumbar Training Phantom, CIRS Model 034 by Sun Nuclear
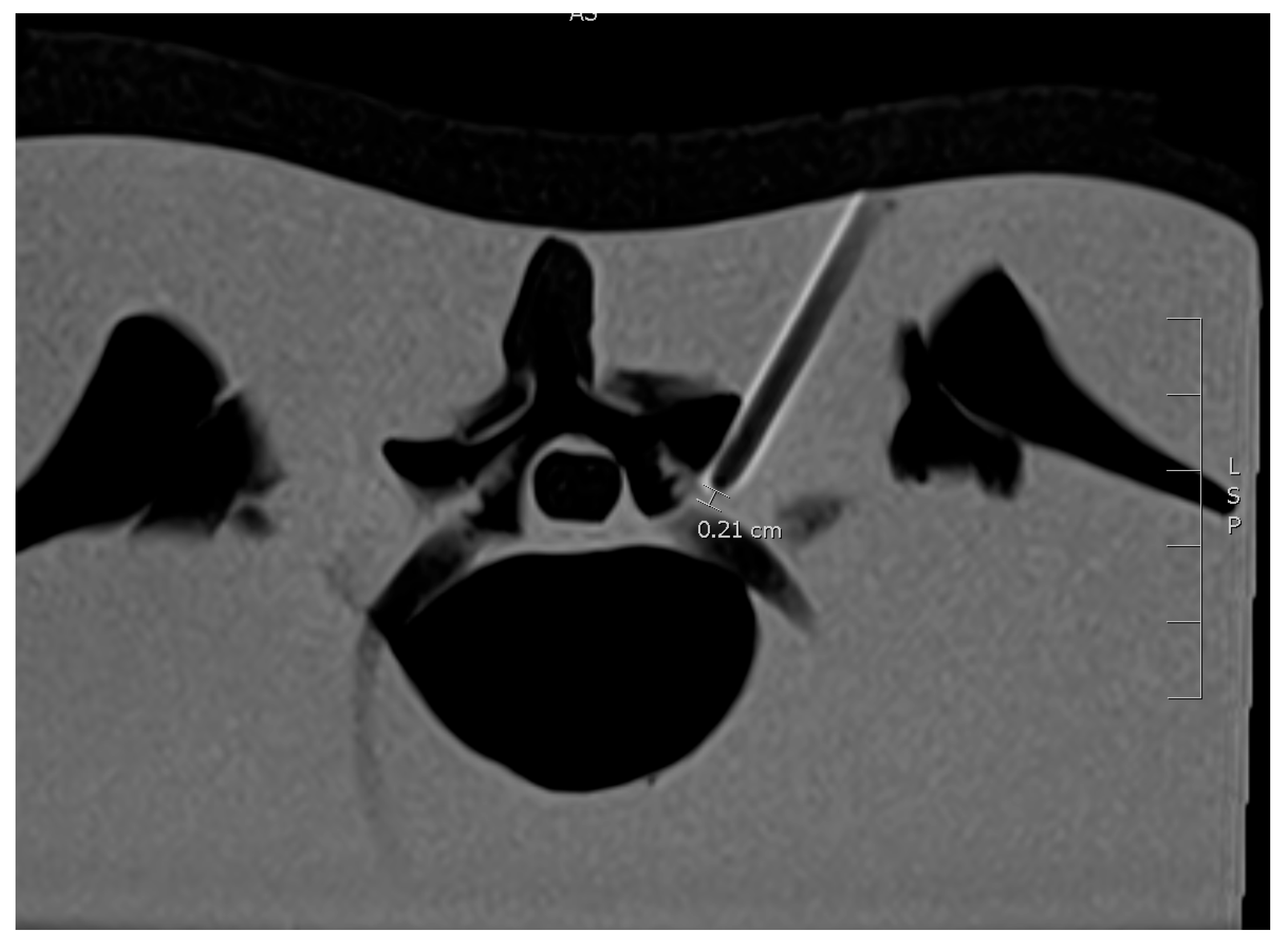
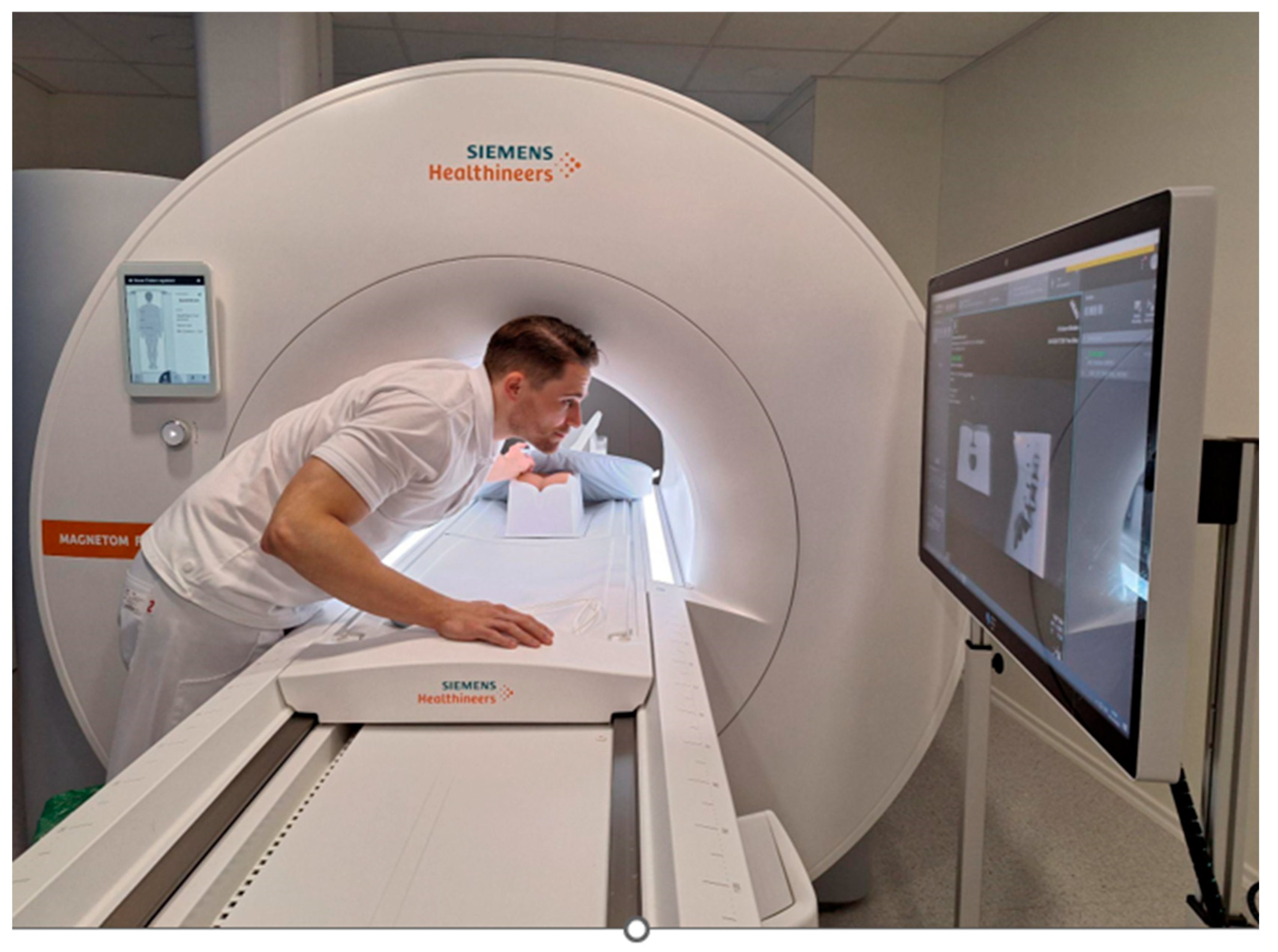
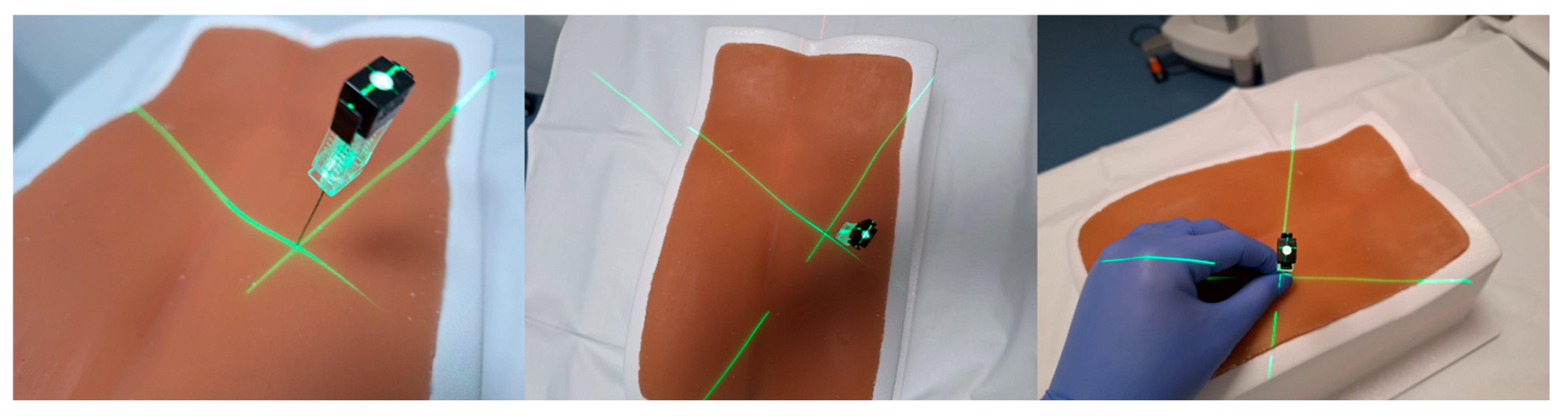
2.3. MRI Needle Guidance
2.4. 3D Laser MDCT Guidance System
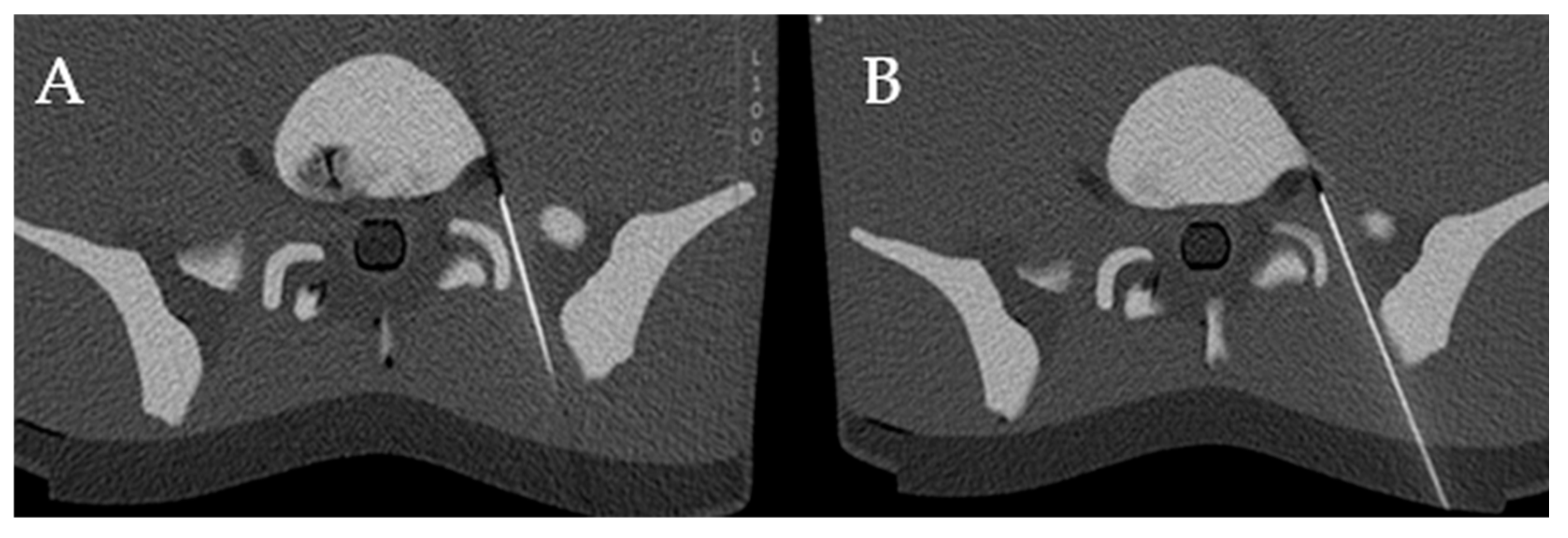
2.5. Conventional Needle Guidance
3. Statistic
4. Results
4.1. Technical Success
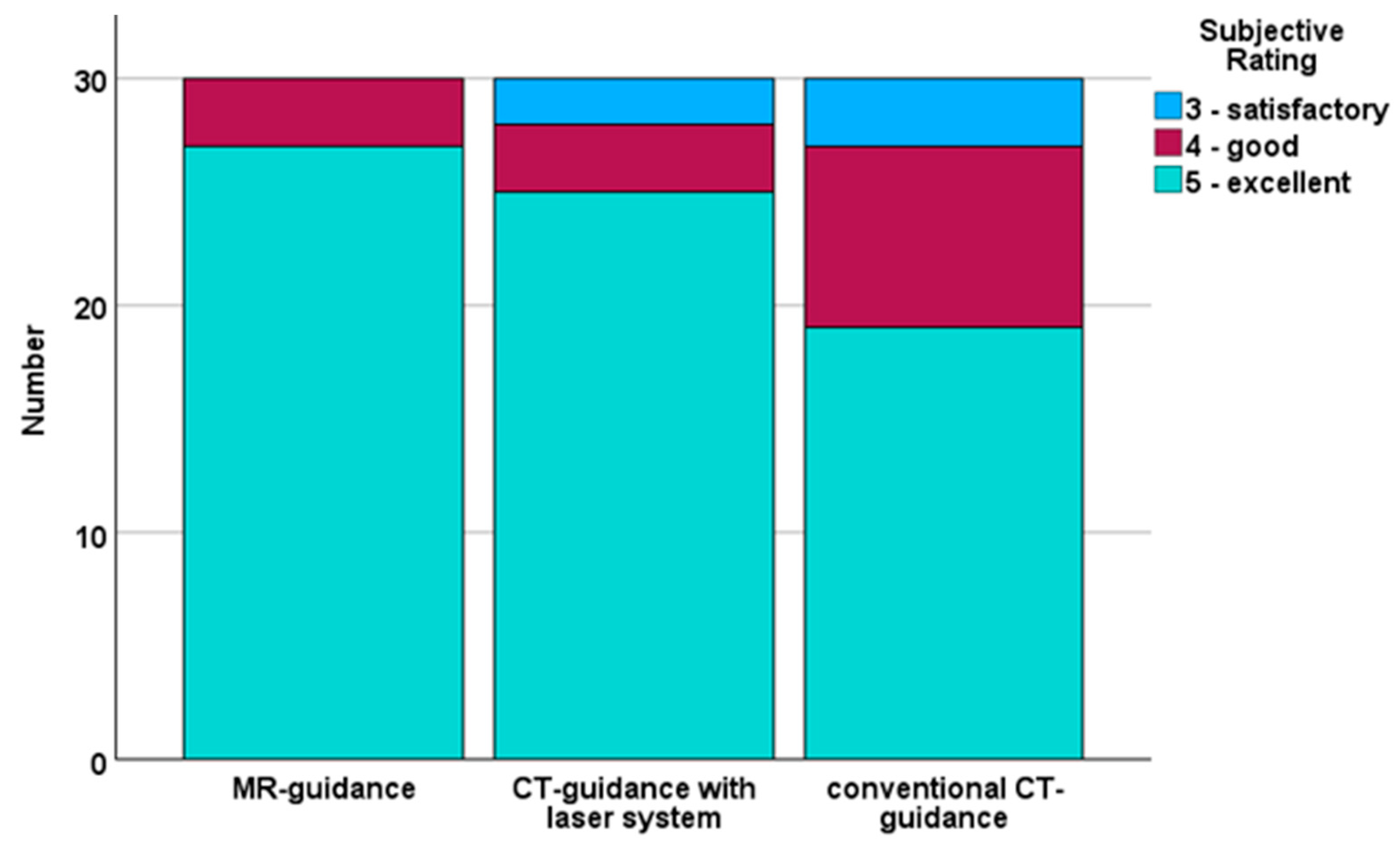
4.2. Subjective Evaluation
4.3. Distance from Needle Tip to the Nerve Root
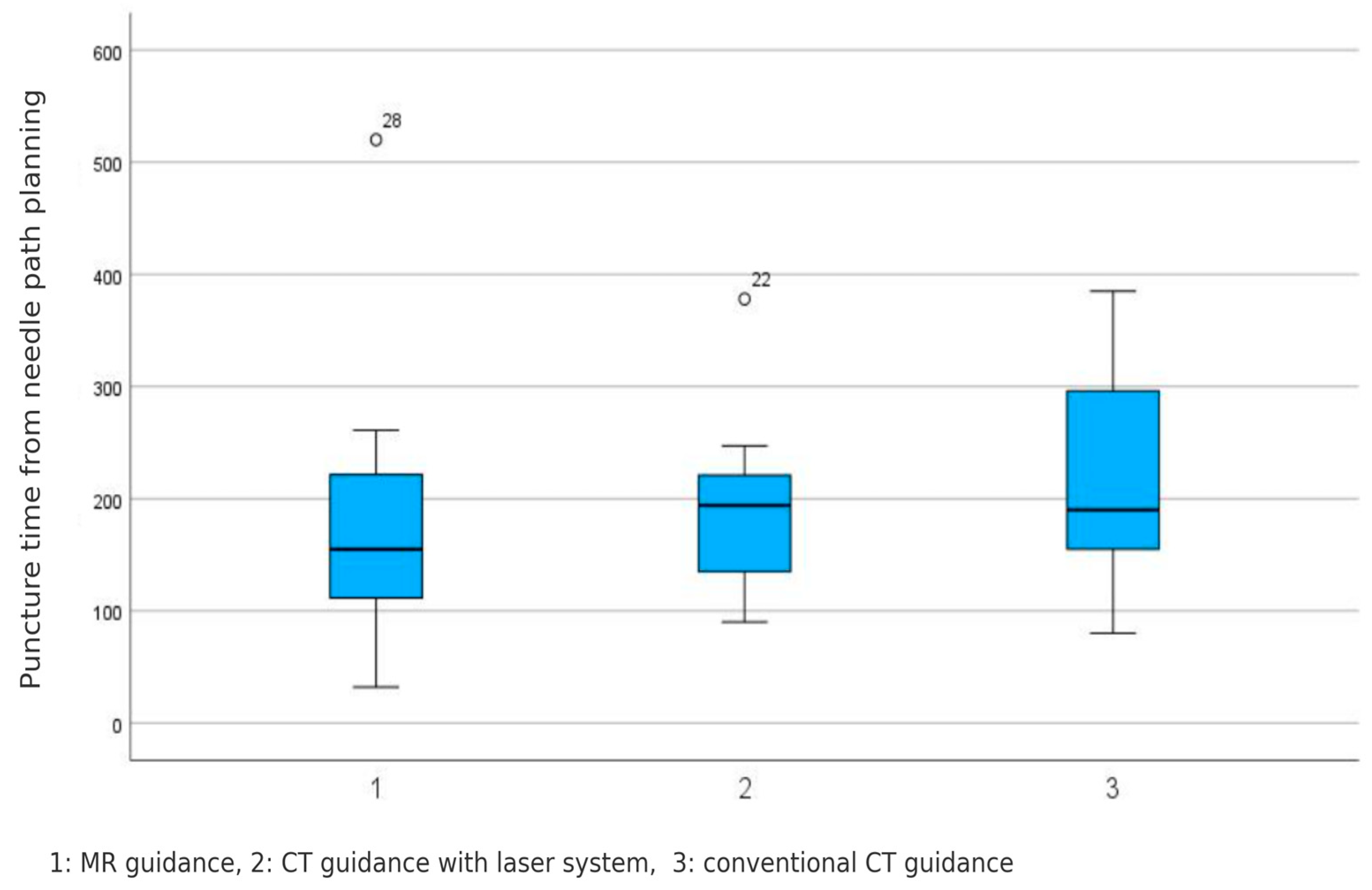
4.4. Puncture Time
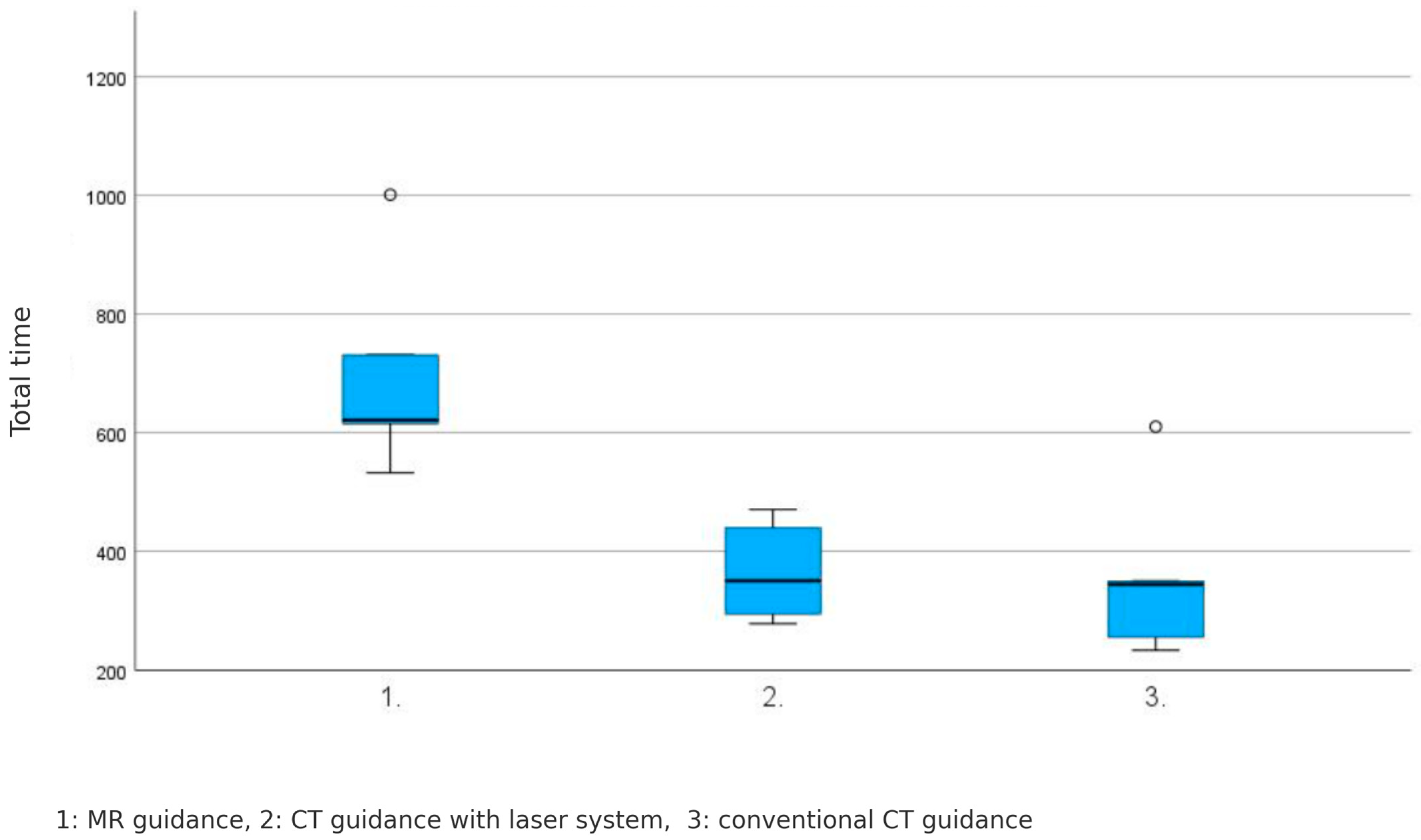
4.5. Total Procedure Time
5. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fatoye, F.; Gebrye, T.; Ryan, C.G.; Useh, U.; Mbada, C. Global and regional estimates of clinical and economic burden of low back pain in high-income countries: A systematic review and meta-analysis. Front. Public Health 2023, 11, 1098100. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sharma, S.; McAuley, J.H. Low Back Pain in Low- and Middle-Income Countries, Part 1: The Problem. J. Orthop. Sports Phys. Ther. 2022, 52, 233–235. [Google Scholar] [CrossRef] [PubMed]
- Dydyk, A.M.; Ngnitewe Massa, R.; Mesfin, F.B. Disc Herniation. [Updated 2023 Jan 16]. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2025. Available online: https://www.ncbi.nlm.nih.gov/books/NBK441822/ (accessed on 29 January 2025).
- Lee, J.S.; Lee, S.B.; Kang, K.Y.; Oh, S.H.; Chae, D.S. Review of Recent Treatment Strategies for Lumbar Disc Herniation (LDH) Focusing on Nonsurgical and Regenerative Therapies. J. Clin. Med. 2025, 14, 1196. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Guyot, J.P. Lumbar Selective Nerve Root Block: Comparative Study Using Two Pharmacological Formulae. Glob. Spine J. 2018, 8, 374–377. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sekhadia, M. Transforaminal Epidural Steroid Injections and Selective Nerve Root Blocks for the Treatment of Pain in the Rehabilitation Patient. In Comprehensive Pain Management in the Rehabilitation Patient; Alexios Carayannopoulos DO, M.P.H., Ed.; Springer: Cham, Switzerland, 2017. [Google Scholar] [CrossRef]
- Song, S.H.; Ryu, G.H.; Park, J.W.; Lee, H.J.; Nam, K.Y.; Kim, H.; Kim, S.Y.; Kwon, B.S. The Effect and Safety of Steroid Injection in Lumbar Spinal Stenosis: With or Without Local Anesthetics. Ann. Rehabil. Med. 2016, 40, 14–20. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- CT Fluoroscopy. Available online: https://radiopaedia.org/articles/ct-fluoroscopy-1 (accessed on 14 April 2025).
- Kimura, R.; Yamamoto, N.; Watanabe, J.; Ono, Y.; Hongo, M.; Miyakoshi, N. Comparative efficacy of ultrasound guidance and fluoroscopy or computed tomography guidance in spinal nerve injections: A systematic review and meta-analysis. Eur. Spine J 2023, 32, 4101–4110. [Google Scholar] [CrossRef]
- Wang, D. Image Guidance Technologies for Interventional Pain Procedures: Ultrasound, Fluoroscopy, and CT. Curr. Pain Headache Rep. 2018, 22, 6. [Google Scholar] [CrossRef] [PubMed]
- Ehsanian, R.; Schneider, B.J.; Kennedy, D.J.; Koshkin, E. Ultrasound-guided cervical selective nerve root injections: A narrative review of literature. Reg. Anesth. Pain Med. 2021, 46, 416–421. [Google Scholar] [CrossRef] [PubMed]
- Filippiadis, D.K.; Kelekis, A. A review of percutaneous techniques for low back pain and neuralgia: Current trends in epidural infiltrations, intervertebral disk and facet joint therapie. Br. J. Radiol. 2016, 89, 20150357. [Google Scholar] [CrossRef]
- Reuschel, V.; Scherlach, C.; Pfeifle, C.; Krause, M.; Struck, M.F.; Hoffmann, K.T.; Schob, S. Treatment Effect of CT-Guided Periradicular Injections in Context of Different Contrast Agent Distribution Patterns. Diagnostics 2022, 12, 787. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Streitparth, F.; De Bucourt, M.; Hartwig, T.; Leidenberger, T.; Rump, J.; Walter, T.; Maurer, M.; Renz, D.; Stelter, L.; Wiener, E.; et al. Real-time MR-guided lumbosacral periradicular injection therapy using an open 1.0-T MRI system: An outcome study. Investig. Radiol. 2013, 48, 471–476. [Google Scholar] [CrossRef] [PubMed]
- Fritz, J.; Chhabra, A.; Wang, K.C.; Carrino, J.A. Magnetic resonance neurography-guided nerve blocks for the diagnosis and treatment of chronic pelvic pain syndrome. Neuroimaging Clin. N. Am. 2014, 24, 211–234. [Google Scholar] [CrossRef] [PubMed]
- Wong, O.; Zhang, G.; Matthews, H.; Skalski, M.; Asadi, H.; Lalloo, S.; Kurda, D. Image-guided spinal injection for pain management. J. Med. Imaging Radiat. Oncol. 2022, 66, 79–91. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Patel, N.A.; Hagemeister, J.; Yan, J.; Wu, D.; Sharma, K.; Cleary, K.; Iordachita, I. Body-mounted robotic assistant for MRI-guided low back pain injection. Int. J. CARS 2020, 15, 321–331. [Google Scholar] [CrossRef]
- Dalili, D.; Isaac, A.; Fritz, J. MRI-guided sacroiliac joint injections in children and adults: Current practice and future developments. Skelet. Radiol. 2023, 52, 951–965. [Google Scholar] [CrossRef]
- Palmer, W.E. Spinal Injections for Pain Management. Radiology 2016, 281, 669–688. [Google Scholar] [CrossRef] [PubMed]
- Timpone, V.M.; Hirsch, J.A.; Gilligan, C.J.; Chandra, R.V. Computed tomography guidance for spinal intervention: Basics of technique, pearls, and avoiding pitfalls. Pain Physician 2013, 16, 369–377. [Google Scholar] [CrossRef] [PubMed]
- Ökçesiz, İ.; Dönmez, H.; Herdem, N.; Sarilar, A.Ç. Computed Tomography-Guided Lumbar Puncture: Advantages and Disadvantages. J. Comput. Assist. Tomogr. 2022, 46, 269–273. [Google Scholar] [CrossRef] [PubMed]
- Maurer, M.H.; Froeling, V.; Röttgen, R.; Bretschneider, T.; Hartwig, T.; Disch, A.C.; de Bucourt, M.; Hamm, B.; Streitparth, F. MRI-guided and CT-guided cervical nerve root infiltration therapy: A cost comparison. Rofo. 2014, 186, 559–566. [Google Scholar] [CrossRef] [PubMed]
- Scheffler, M.; Guillemin, P.C.; Lorton, O.; Maturana, E.; Lauper, N.; Dominguez, D.E.; Terraz, S.; Poletti, P.-A.; Salomir, R.; Boudabbous, S. Magnetic resonance imaging-guided lumbar nerve root infiltrations: Optimization of an in-house protocol. BMC Med. Imaging 2021, 21, 110. [Google Scholar] [CrossRef]
- Guillemin, P.C.; Salomir, R.; Lauper, N.; Lorton, O.; Maturana, E.; Stöckli, A.; Poletti, P.-A.; Dominguez, D.E.; Boudabbous, S.; Scheffler, M. Clinical outcomes of 3T magnetic resonance imaging-guided lumbar and sacral foraminal injections. Neuroradiology 2023, 65, 1793–1802. [Google Scholar] [CrossRef] [PubMed]
- Sequeiros, R.; Ojala, R.O.; Klemola, R.; Vaara, T.J.; Jyrkinen, L.; Tervonen, O.A. MRI-guided periradicular nerve root infiltration therapy in low-field (0.23-T) MRI system using optical instrument tracking. Eur. Radiol. 2002, 12, 1331–1337. [Google Scholar] [CrossRef] [PubMed]
- Fritz, J.; Thomas, C.; Clasen, S.; Claussen, C.D.; Lewin, J.S.; Pereira, P.L. Freehand real-time MRI-guided lumbar spinal injection procedures at 1.5 T: Feasibility, accuracy, and safety. AJR Am. J. Roentgenol. 2009, 192, W161–W167. [Google Scholar] [CrossRef] [PubMed]
- Lopez Schmidt, I.; Haag, N.; Shahzadi, I.; Frohwein, L.J.; Schneider, C.; Niehoff, J.H.; Kroeger, J.R.; Borggrefe, J.; Moenninghoff, C. Diagnostic Image Quality of a Low-Field (0.55T) Knee MRI Protocol Using Deep Learning Image Reconstruction Compared with a Standard (1.5T) Knee MRI Protocol. J. Clin. Med. 2023, 12, 1916. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hori, M.; Hagiwara, A.; Goto, M.; Wada, A.; Aoki, S. Low-field magnetic resonance imaging: Its history and renaissance. Investig. Radiol. 2021, 56, 669. [Google Scholar] [CrossRef]
- Runge, V.M.; Heverhagen, J.T. Advocating the development of next-generation, advanced-design low-field magnetic resonancesystems. Investig. Radiol. 2020, 55, 747–753. [Google Scholar] [CrossRef]
- Campbell-Washburn, A.E.; Ramasawmy, R.; Restivo, M.C.; Bhattacharya, I.; Basar, B.; Herzka, D.A.; Hansen, M.S.; Rogers, T.; Bandettini, W.P.; McGuirt, D.R.; et al. Opportunities in interventional and diagnostic imaging by using high-performancelow-field-strength MRI. Radiology 2019, 293, 384–393. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saeed, S.; Boriesosdick, J.; Michael, A.; Haag, N.P.; Schreck, J.; Schoenbeck, D.; Woeltjen, M.M.; Niehoff, J.H.; Moenninghoff, C.; Borggrefe, J.; et al. Real-Time MR-Guided Lumbosacral Periradicular Injection Therapy Using a 0.55 T MRI System: A Phantom Study. Diagnostics 2025, 15, 1413. https://doi.org/10.3390/diagnostics15111413
Saeed S, Boriesosdick J, Michael A, Haag NP, Schreck J, Schoenbeck D, Woeltjen MM, Niehoff JH, Moenninghoff C, Borggrefe J, et al. Real-Time MR-Guided Lumbosacral Periradicular Injection Therapy Using a 0.55 T MRI System: A Phantom Study. Diagnostics. 2025; 15(11):1413. https://doi.org/10.3390/diagnostics15111413
Chicago/Turabian StyleSaeed, Saher, Jan Boriesosdick, Arwed Michael, Nina Pauline Haag, Julian Schreck, Denise Schoenbeck, Matthias Michael Woeltjen, Julius Henning Niehoff, Christoph Moenninghoff, Jan Borggrefe, and et al. 2025. "Real-Time MR-Guided Lumbosacral Periradicular Injection Therapy Using a 0.55 T MRI System: A Phantom Study" Diagnostics 15, no. 11: 1413. https://doi.org/10.3390/diagnostics15111413
APA StyleSaeed, S., Boriesosdick, J., Michael, A., Haag, N. P., Schreck, J., Schoenbeck, D., Woeltjen, M. M., Niehoff, J. H., Moenninghoff, C., Borggrefe, J., & Kroeger, J. R. (2025). Real-Time MR-Guided Lumbosacral Periradicular Injection Therapy Using a 0.55 T MRI System: A Phantom Study. Diagnostics, 15(11), 1413. https://doi.org/10.3390/diagnostics15111413





