The Feasibility and Clinical Evaluation of an Immersive Augmented Reality Surgical Headset Integrated with Swept-Source Intraoperative Optical Coherence Tomography for Ophthalmic Surgery in the DISCOVER Study †
Abstract
1. Introduction
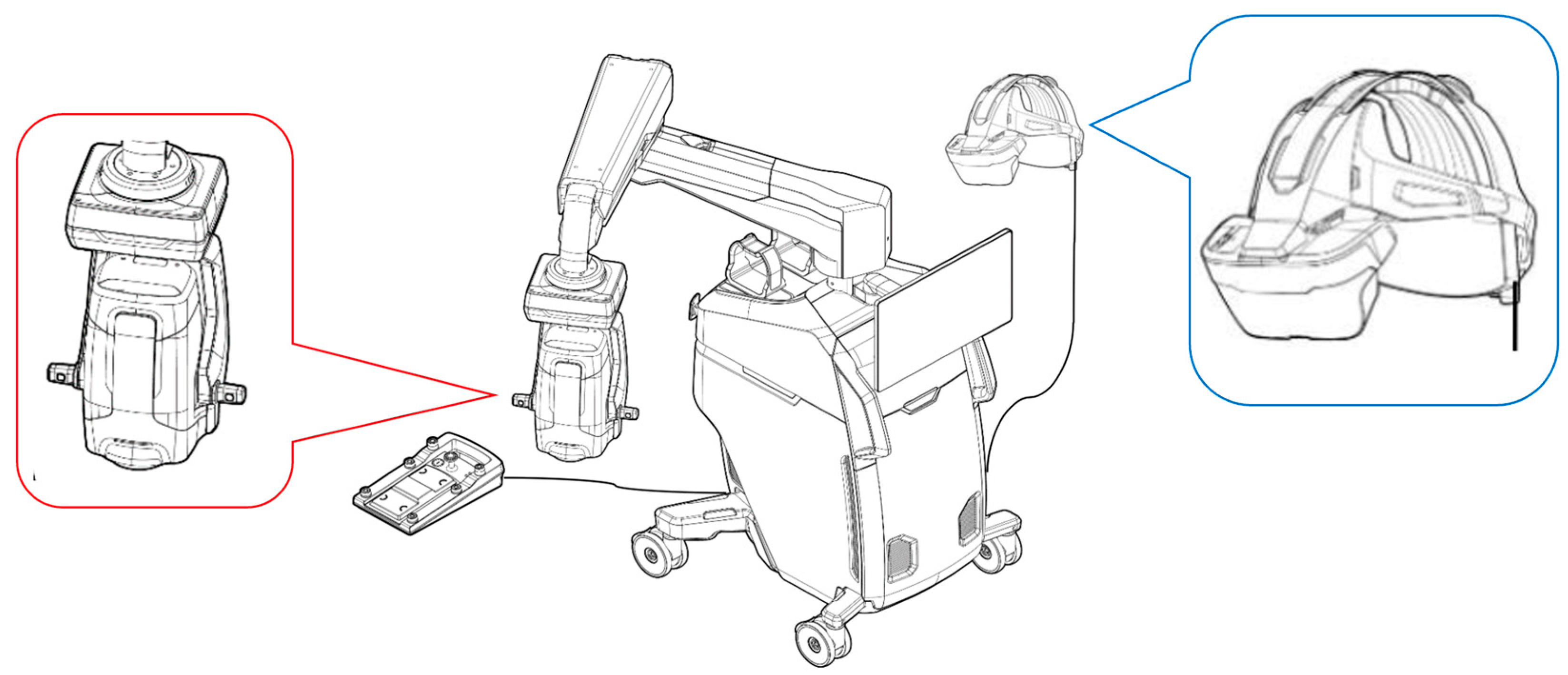
2. Materials and Methods
2.1. Subjects
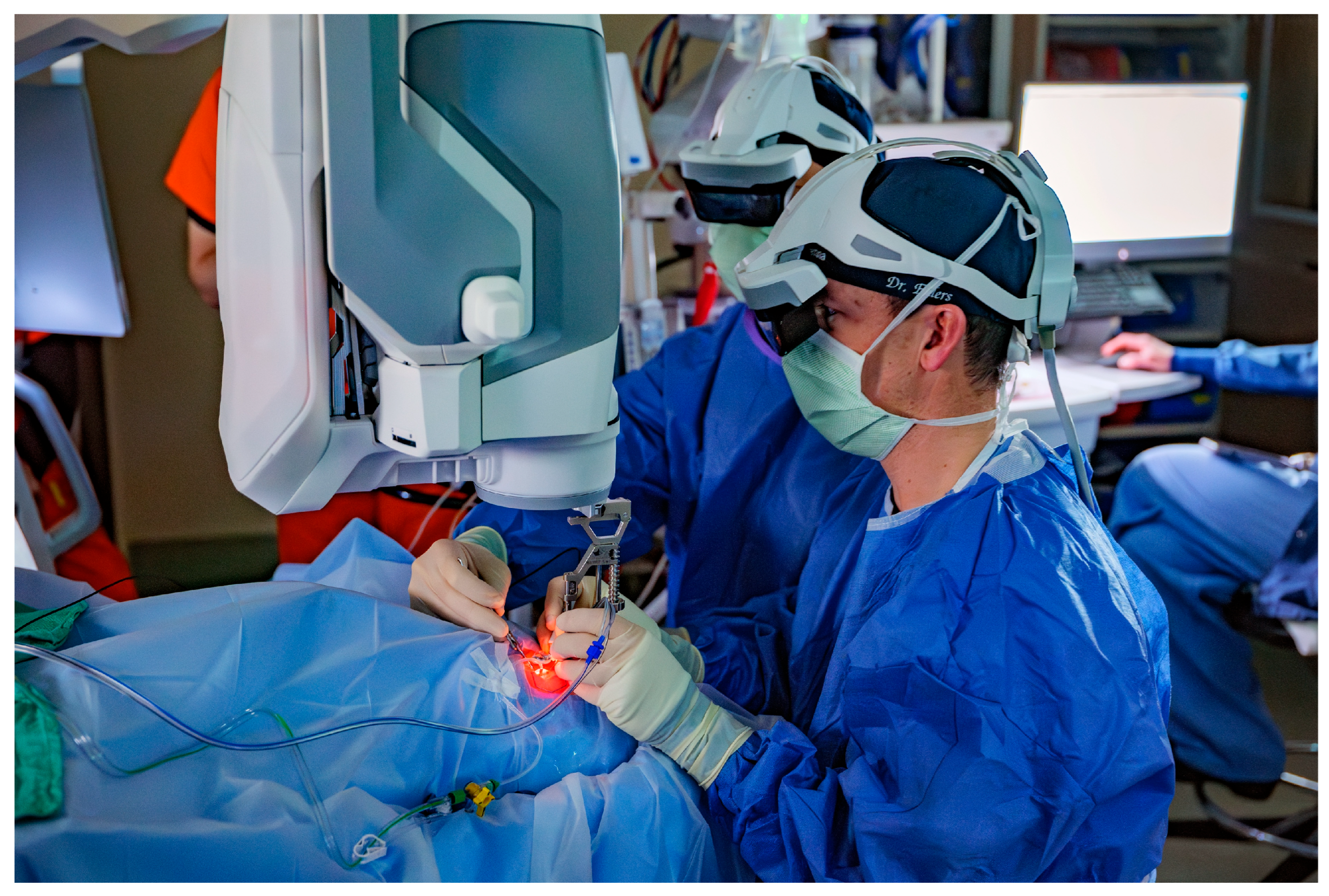
2.2. Surgical Proceadure
3. Results
3.1. Participants
3.2. Treatments
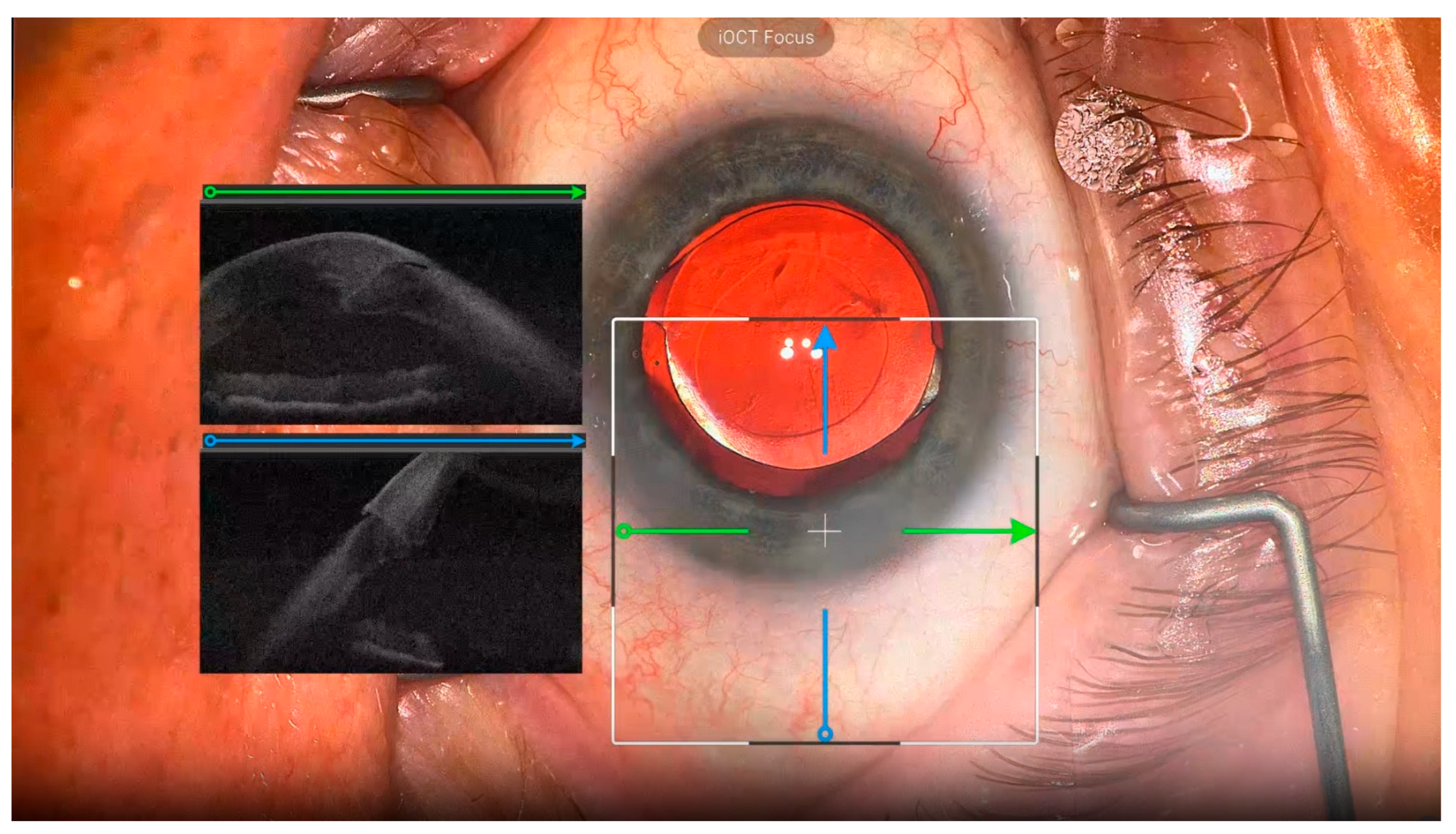
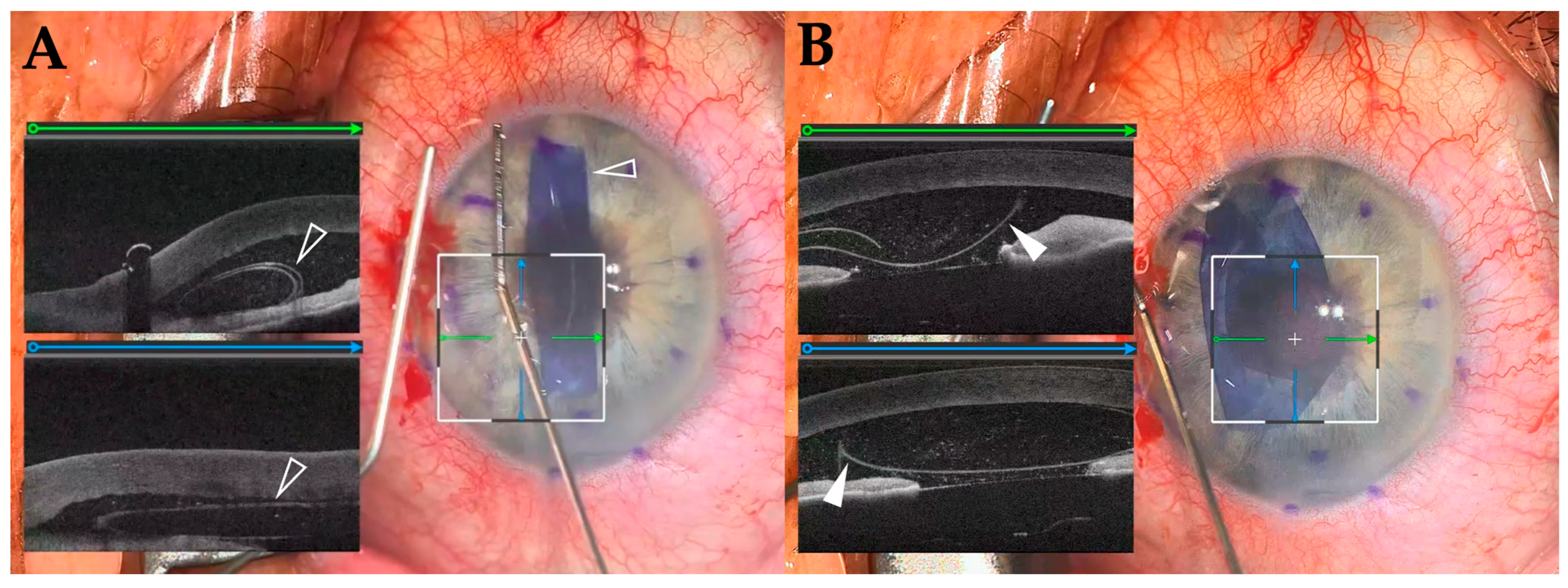
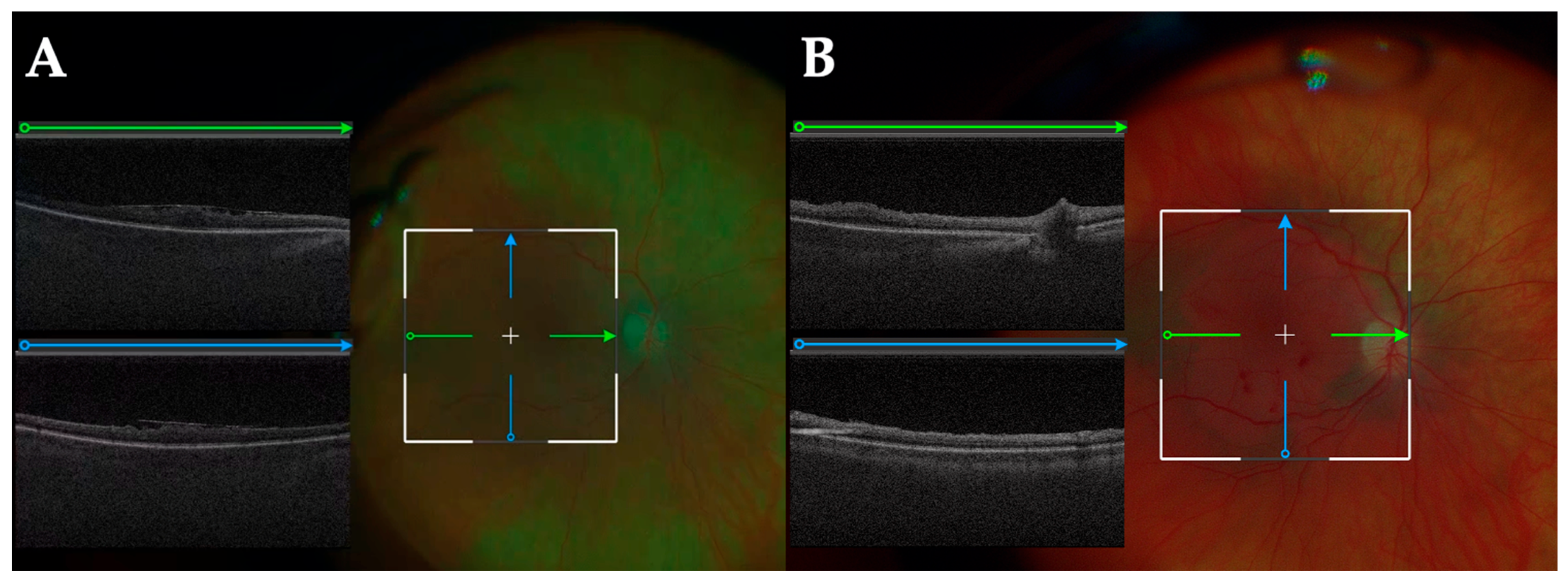
3.3. iOCT
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Keeler, R. The Evolution of the Ophthalmic Surgical Microscope. 2015. Available online: https://open.icm.edu.pl/handle/123456789/9977 (accessed on 15 December 2024).
- Srinivasan, S.; Tripathi, A.B.; Suryakumar, R. Evolution of operating microscopes and development of 3D visualization systems for intraocular surgery. J. Cataract. Refract. Surg. 2023, 49, 988–995. [Google Scholar] [CrossRef] [PubMed]
- Kantor, P.; Matonti, F.; Varenne, F.; Sentis, V.; Pagot-Mathis, V.; Fournié, P.; Soler, V. Use of the heads-up NGENUITY 3D Visualization System for vitreoretinal surgery: A retrospective evaluation of outcomes in a French tertiary center. Sci. Rep. 2021, 11, 10031. [Google Scholar] [CrossRef] [PubMed]
- Ge, X.; Liu, D.; Liu, Y.; Fan, F.; Wang, Y.; Zhang, Z.; Liu, H.; Li, S. Comparing the Randomized Trial Outcomes of 3D Low-Light Intensity-Assisted and Traditional Eyepiece-Assisted Pars Plana Vitrectomy for Rhegmatogenous Retinal Detachment. Ophthalmic Res. 2025, 68, 90–99. [Google Scholar] [CrossRef]
- Sorkin, N.; Levinger, E.; Achiron, A.; Gomel, N.; Cohen, S.; Rabina, G.; Schwartz, S.; Barak, A.; Loewenstein, A.; Varssano, D. Use of a three-dimensional head-mounted digital visualization platform in cataract surgery. Eye 2023, 37, 2905–2908. [Google Scholar] [CrossRef]
- Gomel, N.; Levinger, E.; Lankry, P.; Cohen, S.; Schwartz, S.; Barak, A.; Loewenstein, A.; Varssano, D.; Sorkin, N. Use of a Novel Three-dimensional Head-mounted Digital Visualization Platform in Corneal Endothelial Transplantation. Ophthalmol. Ther. 2023, 12, 625–631. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, S.; Gomel, N.; Loewenstein, A.; Barak, A. Use of a Novel Beyeonics One Three-dimensional Head-mounted Digital Visualization Platform in Vitreoretinal surgeries. Eur. J. Ophthalmol. 2024, 34, 880–883. [Google Scholar] [CrossRef]
- Muijzer, M.B.; Schellekens, P.; Beckers, H.J.M.; de Boer, J.H.; Imhof, S.M.; Wisse, R.P.L. Clinical applications for intraoperative optical coherence tomography: A systematic review. Eye 2022, 36, 379–391. [Google Scholar] [CrossRef]
- Ehlers, J.P.; Modi, Y.S.; Pecen, P.E.; Goshe, J.; Dupps, W.J.; Rachitskaya, A.; Sharma, S.; Yuan, A.; Singh, R.; Kaiser, P.K.; et al. The DISCOVER Study 3-Year Results: Feasibility and Usefulness of Microscope-Integrated Intraoperative OCT during Ophthalmic Surgery. Ophthalmology 2018, 125, 1014–1027. [Google Scholar] [CrossRef]
- Mizuno, M.; Matar, K.; Amine, R.; Indurkar, A.; Mamone, J.; Reese, J.; Talcott, K.E.; Goshe, J.M.; Dupps, W.J.; Sharma, S.; et al. Integration of a Novel Immersive Augmented Reality Surgical Headset and Intraoperative OCT for Ophthalmic Surgery in the DISCOVER Study. In In Proceedings of the Association for Research in Vision and Ophthalmology (ARVO) 2025, Salt Lake City, UT, USA, 4–8 May 2025. Abstract No. 1496-A0124. [Google Scholar]
- Franken, R.J.; Gupta, S.C.; Banis, J.C., Jr.; Thomas, S.V.; Derr, J.W.; Klein, S.A.; Kon, M.; Barker, J.H. Microsurgery without a microscope: Laboratory evaluation of a three-dimensional on-screen microsurgery system. Microsurgery 1995, 16, 746–751. [Google Scholar] [CrossRef]
- Kannan, N.B.; Jena, S.; Sen, S.; Kohli, P.; Ramasamy, K. A comparison of using digitally assisted vitreoretinal surgery during repair of rhegmatogenous retinal detachments to the conventional analog microscope: A prospective interventional study. Int. Ophthalmol. 2021, 41, 1689–1695. [Google Scholar] [CrossRef]
- Ding, N.; Wang, X.; Song, X. Digital versus slit-beam marking for toric intraocular lenses in cataract surgery. BMC Ophthalmol. 2022, 22, 323. [Google Scholar] [CrossRef]
- Nakajima, K.; Inoue, M.; Mizuno, M.; Koto, T.; Ishida, T.; Ozawa, H.; Oshika, T. Effects of image-sharpening algorithm on surgical field visibility during 3D heads-up surgery for vitreoretinal diseases. Sci. Rep. 2023, 13, 2758. [Google Scholar] [CrossRef] [PubMed]
- Charles, S. Illumination and phototoxicity issues in vitreoretinal surgery. Retina 2008, 28, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Rosenberg, E.D.; Nuzbrokh, Y.; Sippel, K.C. Efficacy of 3D digital visualization in minimizing coaxial illumination and phototoxic potential in cataract surgery: Pilot study. J. Cataract. Refract. Surg. 2021, 47, 291–296. [Google Scholar] [CrossRef] [PubMed]
- Tan, N.E.; Wortz, B.T.; Rosenberg, E.D.; Radcliffe, N.M.; Gupta, P.K. Impact of Heads-Up Display Use on Ophthalmologist Productivity, Wellness, and Musculoskeletal Symptoms: A Survey Study. J. Curr. Ophthalmol. 2022, 34, 305–311. [Google Scholar] [CrossRef]
- Weinstock, R.J.; Ainslie-Garcia, M.H.; Ferko, N.C.; Qadeer, R.A.; Morris, L.P.; Cheng, H.; Ehlers, J.P. Comparative Assessment of Ergonomic Experience with Heads-Up Display and Conventional Surgical Microscope in the Operating Room. Clin. Ophthalmol. 2021, 15, 347–356. [Google Scholar] [CrossRef]
- Posarelli, C.; Sartini, F.; Casini, G.; Passani, A.; Toro, M.D.; Vella, G.; Figus, M. What Is the Impact of Intraoperative Microscope-Integrated OCT in Ophthalmic Surgery? Relevant Applications and Outcomes. A Systematic Review. J. Clin. Med. 2020, 9, 1682. [Google Scholar] [CrossRef]
- Ehlers, J.P.; Srivastava, S.K.; Feiler, D.; Noonan, A.I.; Rollins, A.M.; Tao, Y.K. Integrative advances for OCT-guided ophthalmic surgery and intraoperative OCT: Microscope integration, surgical instrumentation, and heads-up display surgeon feedback. PLoS ONE 2014, 9, e105224. [Google Scholar] [CrossRef]
- Lee, J.; Wijesinghe, R.E.; Jeon, D.; Kim, P.; Choung, Y.H.; Jang, J.H.; Jeon, M.; Kim, J. Clinical Utility of Intraoperative Tympanomastoidectomy Assessment Using a Surgical Microscope Integrated with an Optical Coherence Tomography. Sci. Rep. 2018, 8, 17432. [Google Scholar] [CrossRef]
- Lang, S.J.; Heinzelmann, S.; Böhringer, D.; Reinhard, T.; Maier, P. Indications for intraoperative anterior segment optical coherence tomography in corneal surgery. Int. Ophthalmol. 2020, 40, 2617–2625. [Google Scholar] [CrossRef]
- Todorich, B.; Shieh, C.; DeSouza, P.J.; Carrasco-Zevallos, O.M.; Cunefare, D.L.; Stinnett, S.S.; Izatt, J.A.; Farsiu, S.; Mruthyunjaya, P.; Kuo, A.N.; et al. Impact of Microscope-Integrated OCT on Ophthalmology Resident Performance of Anterior Segment Surgical Maneuvers in Model Eyes. Invest. Ophthalmol. Vis. Sci. 2016, 57, Oct146–Oct153. [Google Scholar] [CrossRef] [PubMed]
- Moramarco, A.; di Geronimo, N.; Airaldi, M.; Gardini, L.; Semeraro, F.; Iannetta, D.; Romano, V.; Fontana, L. Intraoperative OCT for Lamellar Corneal Surgery: A User Guide. J. Clin. Med. 2023, 12, 3048. [Google Scholar] [CrossRef] [PubMed]
- Ehlers, J.P.; Uchida, A.; Srivastava, S.K.; Hu, M. Predictive model for macular hole closure speed: Insights from intraoperative optical coherence tomography. Transl. Vis. Sci. Technol. 2019, 8, 18. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mizuno, M.; Matar, K.; Amine, R.; Talcott, K.E.; Goshe, J.M.; Dupps, W.J.; Sharma, S.; Indurkar, A.; Mamone, J.; Reese, J.; et al. The Feasibility and Clinical Evaluation of an Immersive Augmented Reality Surgical Headset Integrated with Swept-Source Intraoperative Optical Coherence Tomography for Ophthalmic Surgery in the DISCOVER Study. Diagnostics 2025, 15, 1394. https://doi.org/10.3390/diagnostics15111394
Mizuno M, Matar K, Amine R, Talcott KE, Goshe JM, Dupps WJ, Sharma S, Indurkar A, Mamone J, Reese J, et al. The Feasibility and Clinical Evaluation of an Immersive Augmented Reality Surgical Headset Integrated with Swept-Source Intraoperative Optical Coherence Tomography for Ophthalmic Surgery in the DISCOVER Study. Diagnostics. 2025; 15(11):1394. https://doi.org/10.3390/diagnostics15111394
Chicago/Turabian StyleMizuno, Masaharu, Karen Matar, Reem Amine, Katherine E. Talcott, Jeffrey M. Goshe, William J. Dupps, Sumit Sharma, Asmita Indurkar, John Mamone, Jamie Reese, and et al. 2025. "The Feasibility and Clinical Evaluation of an Immersive Augmented Reality Surgical Headset Integrated with Swept-Source Intraoperative Optical Coherence Tomography for Ophthalmic Surgery in the DISCOVER Study" Diagnostics 15, no. 11: 1394. https://doi.org/10.3390/diagnostics15111394
APA StyleMizuno, M., Matar, K., Amine, R., Talcott, K. E., Goshe, J. M., Dupps, W. J., Sharma, S., Indurkar, A., Mamone, J., Reese, J., Srivastava, S. K., & Ehlers, J. P. (2025). The Feasibility and Clinical Evaluation of an Immersive Augmented Reality Surgical Headset Integrated with Swept-Source Intraoperative Optical Coherence Tomography for Ophthalmic Surgery in the DISCOVER Study. Diagnostics, 15(11), 1394. https://doi.org/10.3390/diagnostics15111394







