The Vascular Architecture of Macular Neovascularization in Age-Related Macular Degeneration as a Predictor of Therapy Requirements: A 3-Year Longitudinal Analysis
Abstract
1. Background
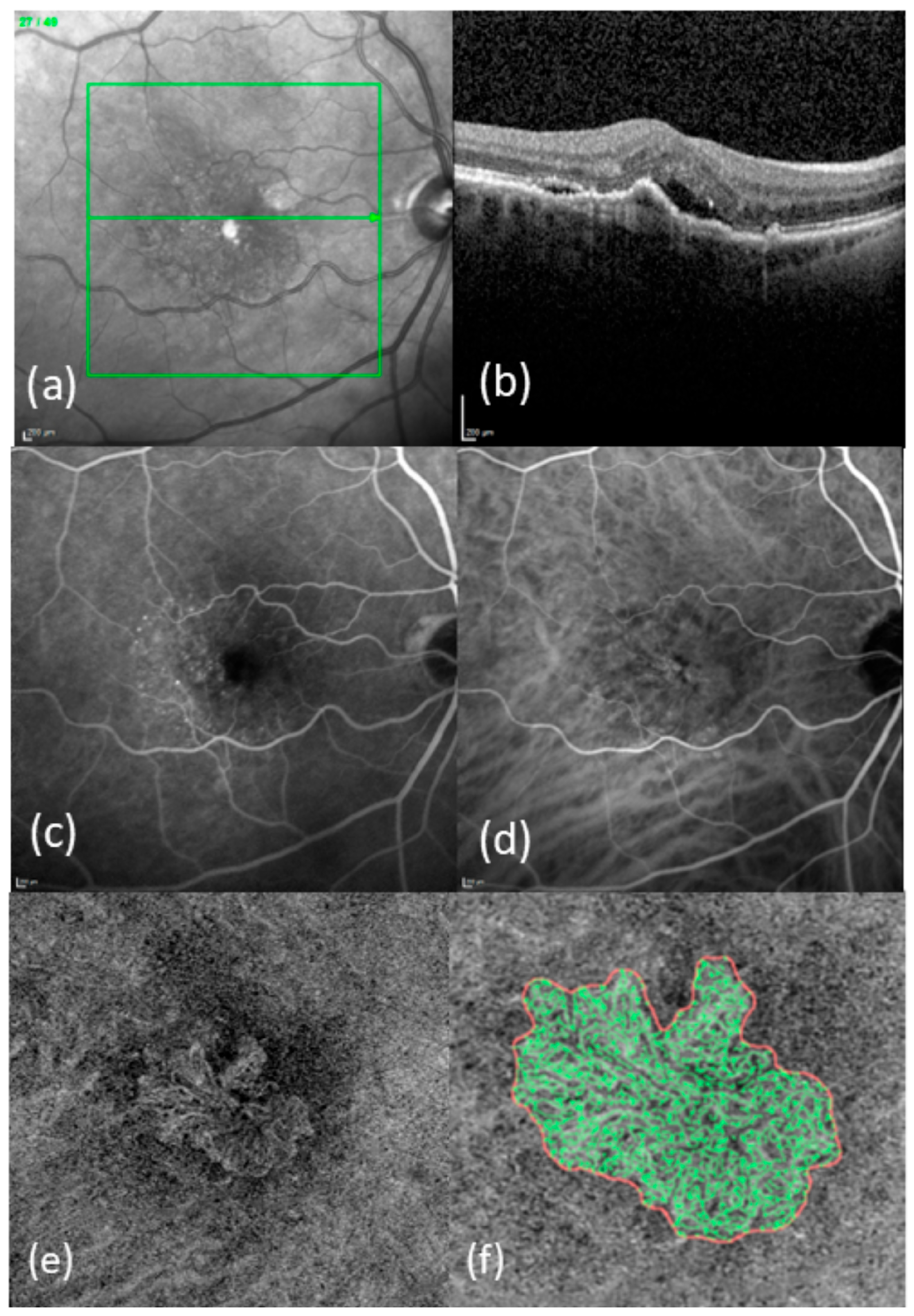
2. Methods
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bressler, N.M. Age-Related Macular Degeneration Is the Leading Cause of Blindness. JAMA 2004, 291, 1900–1901. [Google Scholar] [CrossRef]
- Pfau, M.; von der Emde, L.; de Sisternes, L.; Hallak, J.A.; Leng, T.; Schmitz-Valckenberg, S.; Holz, F.G.; Fleckenstein, M.; Rubin, D.L. Progression of Photoreceptor Degeneration in Geographic Atrophy Secondary to Age-related Macular Degeneration. JAMA Ophthalmol. 2020, 138, 1026–1034. [Google Scholar] [CrossRef]
- Ferris, F.L.; Fine, S.L.; Hyman, L. Age-related macular degeneration and blindness due to neovascular maculopathy. Arch. Ophthalmol. Chic. Ill 1960 1984, 102, 1640–1642. [Google Scholar] [CrossRef] [PubMed]
- Spaide, R.F.; Jaffe, G.J.; Sarraf, D.; Freund, K.B.; Sadda, S.R.; Staurenghi, G.; Waheed, N.K.; Chakravarthy, U.; Rosenfeld, P.J.; Holz, F.G.; et al. Consensus Nomenclature for Reporting Neovascular Age-Related Macular Degeneration Data: Consensus on Neovascular Age-Related Macular Degeneration Nomenclature Study Group. Ophthalmology 2020, 127, 616–636. [Google Scholar] [CrossRef]
- Deutsche Ophthalmologische Gesellschaft (DOG); Retinologische Gesellschaft e. V. (RG); Berufsverband der Augenärzte Deutschlands e. V. (BVA). Stellungnahme der DOG, der RG und des BVA zur Anti-VEGF-Therapie bei der neovaskulären altersabhängigen Makuladegeneration: Stand Februar 2020. Ophthalmologe 2020, 117, 746–754. [Google Scholar] [CrossRef] [PubMed]
- de Carlo, T.E.; Romano, A.; Waheed, N.K.; Duker, J.S. A review of optical coherence tomography angiography (OCTA). Int. J. Retina Vitr. 2015, 1, 5. [Google Scholar] [CrossRef] [PubMed]
- Meleppat, R.K.; Fortenbach, C.R.; Jian, Y.; Martinez, E.S.; Wagner, K.; Modjtahedi, B.S.; Motta, M.J.; Ramamurthy, D.L.; Schwab, I.R.; Zawadzki, R.J. In Vivo Imaging of Retinal and Choroidal Morphology and Vascular Plexuses of Vertebrates Using Swept-Source Optical Coherence Tomography. Transl. Vis. Sci. Technol. 2022, 11, 11. [Google Scholar] [CrossRef]
- Coscas, G.; Lupidi, M.; Coscas, F. Image Analysis of Optical Coherence Tomography Angiography. OCT Angiogr. Retin. Macular Dis. 2016, 56, 30–36. [Google Scholar] [CrossRef]
- Spaide, R.F.; Fujimoto, J.G.; Waheed, N.K. Optical Coherence Tomography Angiography. RETINA 2015, 35, 2161–2162. [Google Scholar] [CrossRef]
- Nakano, Y.; Kataoka, K.; Takeuchi, J.; Fujita, A.; Kaneko, H.; Shimizu, H.; Ito, Y.; Terasaki, H. Vascular maturity of type 1 and type 2 choroidal neovascularization evaluated by optical coherence tomography angiography. PLoS ONE 2019, 14, e0216304. [Google Scholar] [CrossRef]
- Faatz, H.; Rothaus, K.; Ziegler, M.; Book, M.; Heimes-Bussmann, B.; Pauleikhoff, D.; Lommatzsch, A. Vascular Analysis of Type 1, 2, and 3 Macular Neovascularization in Age-Related Macular Degeneration Using Swept-Source Optical Coherence Tomography Angiography Shows New Insights into Differences of Pathologic Vasculature and May Lead to a More Personalized Understanding. Biomedicines 2022, 10, 694. [Google Scholar] [CrossRef] [PubMed]
- Faatz, H.; Rothaus, K.; Ziegler, M.; Book, M.; Spital, G.; Heimes-Bussmann, B.; Pauleikhoff, D.; Lommatzsch, A. Correlation of retinal alterations with vascular structure of macular neovascularisation in swept-source optical coherence tomography angiography in age-related macular degeneration. Int. Ophthalmol. 2022, 42, 1553–1562. [Google Scholar] [CrossRef]
- Faatz, H.; Rothaus, K.; Ziegler, M.; Book, M.; Spital, G.; Lange, C.; Lommatzsch, A. The Architecture of Macular Neovascularizations Predicts Treatment Responses to Anti-VEGF Therapy in Neovascular AMD. Diagnostics 2022, 12, 2807. [Google Scholar] [CrossRef]
- Faatz, H.; Gunnemann, M.-L.; Rothaus, K.; Book, M.; Gutfleisch, M.; Lommatzsch, A.; Pauleikhoff, D. Influence of CNV vascular morphology in exudative age-related macular degeneration on development of visual acuity and need for anti-VEGF therapy after 1 year. Ophthalmol. Z. Dtsch. Ophthalmol. Ges. 2021, 118, 154–161. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhang, A.; Lee, C.S.; Lee, A.Y.; Rezaei, K.A.; Roisman, L.; Miller, A.; Zheng, F.; Gregori, G.; Durbin, M.K.; et al. Projection artifact removal improves visualization and quantitation of macular neovascularization imaged by optical coherence tomography angiography. Ophthalmol. Retina 2017, 1, 124–136. [Google Scholar] [CrossRef]
- Rothaus, K.; Jiang, X. Multi-scale Midline Extraction Using Creaseness. In Pattern Recognition and Image Analysis; Springer: Berlin/Heidelberg, Germany, 2005; pp. 502–511. [Google Scholar]
- Faatz, H.; Farecki, M.-L.; Rothaus, K.; Gunnemann, F.; Gutfleisch, M.; Lommatzsch, A.; Pauleikhoff, D. Optical coherence tomography angiography of types 1 and 2 choroidal neovascularization in age-related macular degeneration during anti-VEGF therapy: Evaluation of a new quantitative method. Eye 2019, 33, 1466–1471. [Google Scholar] [CrossRef] [PubMed]
- Al-Sheikh, M.; Iafe, N.A.; Phasukkijwatana, N.; Sadda, S.R.; Sarraf, D. Biomarkers of Neovascular Activity in Age-Related Macular Degeneration Using Oct Angiography. Retina Phila. Pa 2017, 38, 220–230. [Google Scholar] [CrossRef]
- Faatz, H.; Farecki, M.-L.; Rothaus, K.; Gutfleisch, M.; Pauleikhoff, D.; Lommatzsch, A. Changes in the OCT angiographic appearance of type 1 and type 2 CNV in exudative AMD during anti-VEGF treatment. BMJ Open Ophthalmol. 2019, 4, e000369. [Google Scholar] [CrossRef]
- Bogunović, H.; Waldstein, S.M.; Schlegl, T.; Langs, G.; Sadeghipour, A.; Liu, X.; Gerendas, B.S.; Osborne, A.; Schmidt-Erfurth, U. Prediction of Anti-VEGF Treatment Requirements in Neovascular AMD Using a Machine Learning Approach. Invest. Ophthalmol. Vis. Sci. 2017, 58, 3240–3248. [Google Scholar] [CrossRef]
- Waldstein, S.M.; Wright, J.; Warburton, J.; Margaron, P.; Simader, C.; Schmidt-Erfurth, U. Predictive Value of Retinal Morphology for Visual Acuity Outcomes of Different Ranibizumab Treatment Regimens for Neovascular AMD. Ophthalmology 2016, 123, 60–69. [Google Scholar] [CrossRef]
- Souedan, V.; Souied, E.H.; Caillaux, V.; Miere, A.; Ameen, A.E.; Blanco-Garavito, R. Sensitivity and specificity of optical coherence tomography angiography (OCT-A) for detection of choroidal neovascularization in real-life practice and varying retinal expertise level. Int. Ophthalmol. 2017, 38, 1051–1060. [Google Scholar] [CrossRef]
- Gong, J.; Yu, S.; Gong, Y.; Wang, F.; Sun, X. The Diagnostic Accuracy of Optical Coherence Tomography Angiography for Neovascular Age-Related Macular Degeneration: A Comparison with Fundus Fluorescein Angiography. J. Ophthalmol. 2016, 2016, 7521478. [Google Scholar] [CrossRef] [PubMed]
- El Ameen, A.; Cohen, S.Y.; Semoun, O.; Miere, A.; Srour, M.; Quaranta-El Maftouhi, M.; Oubraham, H.; Blanco-Garavito, R.; Querques, G.; Souied, E.H. Type 2 Neovascularization Secondary to Age-Related Macular Degeneration Imaged by Optical Coherence Tomography Angiography. Retina Phila. Pa 2015, 35, 2212–2218. [Google Scholar] [CrossRef] [PubMed]
- Pilotto, E.; Frizziero, L.; Daniele, A.R.; Convento, E.; Longhin, E.; Guidolin, F.; Parrozzani, R.; Cavarzeran, F.; Midena, E. Early OCT angiography changes of type 1 CNV in exudative AMD treated with anti-VEGF. Br. J. Ophthalmol. 2019, 103, 67–71. [Google Scholar] [CrossRef] [PubMed]
- Spaide, R.F. Optical Coherence Tomography Angiography Signs of Vascular Abnormalization With Antiangiogenic Therapy for Choroidal Neovascularization. Am. J. Ophthalmol. 2015, 160, 6–16. [Google Scholar] [CrossRef]
- Heinke, A.; Zhang, H.; Broniarek, K.; Michalska-Małecka, K.; Elsner, W.; Galang, C.M.B.; Deussen, D.N.; Warter, A.; Kalaw, F.; Nagel, I.; et al. Cross-instrument optical coherence tomography-angiography (OCTA)-based prediction of age-related macular degeneration (AMD) disease activity using artificial intelligence. Sci. Rep. 2024, 14, 27085. [Google Scholar] [CrossRef]
- Jin, K.; Yan, Y.; Chen, M.; Wang, J.; Pan, X.; Liu, X.; Liu, M.; Lou, L.; Wang, Y.; Ye, J. Multimodal deep learning with feature level fusion for identification of choroidal neovascularization activity in age-related macular degeneration. Acta Ophthalmol. 2021, 100, e512–e520. [Google Scholar] [CrossRef]
- Schranz, M.; Bogunovic, H.; Deak, G.; Sadeghipour, A.; Reiter, G.S.; Schmidt-Erfurth, U. Linking disease activity with optical coherence tomography angiography in neovascular age related macular degeneration using artificial intelligence. Sci. Rep. 2024, 14, 19278. [Google Scholar] [CrossRef]
- Coscas, G.; Lupidi, M.; Coscas, F.; Français, C.; Cagini, C.; Souied, E.H. Optical coherence tomography angiography during follow-up: Qualitative and quantitative analysis of mixed type I and II choroidal neovascularization after vascular endothelial growth factor trap therapy. Ophthalmic Res. 2015, 54, 57–63. [Google Scholar] [CrossRef]
- McClintic, S.M.; Gao, S.; Wang, J.; Hagag, A.; Lauer, A.K.; Flaxel, C.J.; Bhavsar, K.; Hwang, T.S.; Huang, D.; Jia, Y.; et al. Quantitative Evaluation of Choroidal Neovascularization under Pro Re Nata Anti-Vascular Endothelial Growth Factor Therapy with OCT Angiography. Ophthalmol. Retina 2018, 2, 931–941. [Google Scholar] [CrossRef]
- Torrecillas-Picazo, R.; Cerdà-Ibáñez, M.; Almor Palacios, I.; Hervás Hernandis, J.M.; Ramón-Cosín, R.; Ruiz Del Rio, N.; Duch-Samper, A. Analysis and follow-up of type 1 choroidal neovascularisation with optical coherence tomography-angiography after antiangiogenic treatment. Arch. Soc. Espanola Oftalmol. 2017, 92, 265–272. [Google Scholar] [CrossRef] [PubMed]
- Choi, W.; Moult, E.M.; Waheed, N.K.; Adhi, M.; Lee, B.; Lu, C.D.; de Carlo, T.E.; Jayaraman, V.; Rosenfeld, P.J.; Duker, J.S.; et al. Ultrahigh-Speed, Swept-Source Optical Coherence Tomography Angiography in Nonexudative Age-Related Macular Degeneration with Geographic Atrophy. Ophthalmology 2015, 122, 2532–2544. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grün, M.; Rothaus, K.; Ziegler, M.; Lange, C.; Lommatzsch, A.; Faatz, H. The Vascular Architecture of Macular Neovascularization in Age-Related Macular Degeneration as a Predictor of Therapy Requirements: A 3-Year Longitudinal Analysis. Diagnostics 2025, 15, 982. https://doi.org/10.3390/diagnostics15080982
Grün M, Rothaus K, Ziegler M, Lange C, Lommatzsch A, Faatz H. The Vascular Architecture of Macular Neovascularization in Age-Related Macular Degeneration as a Predictor of Therapy Requirements: A 3-Year Longitudinal Analysis. Diagnostics. 2025; 15(8):982. https://doi.org/10.3390/diagnostics15080982
Chicago/Turabian StyleGrün, Michael, Kai Rothaus, Martin Ziegler, Clemens Lange, Albrecht Lommatzsch, and Henrik Faatz. 2025. "The Vascular Architecture of Macular Neovascularization in Age-Related Macular Degeneration as a Predictor of Therapy Requirements: A 3-Year Longitudinal Analysis" Diagnostics 15, no. 8: 982. https://doi.org/10.3390/diagnostics15080982
APA StyleGrün, M., Rothaus, K., Ziegler, M., Lange, C., Lommatzsch, A., & Faatz, H. (2025). The Vascular Architecture of Macular Neovascularization in Age-Related Macular Degeneration as a Predictor of Therapy Requirements: A 3-Year Longitudinal Analysis. Diagnostics, 15(8), 982. https://doi.org/10.3390/diagnostics15080982






