Can a Portable Flash Visual Evoked Potential (VEP) Device Identify Chiasmal Decussation Anomalies in Albinism?
Abstract
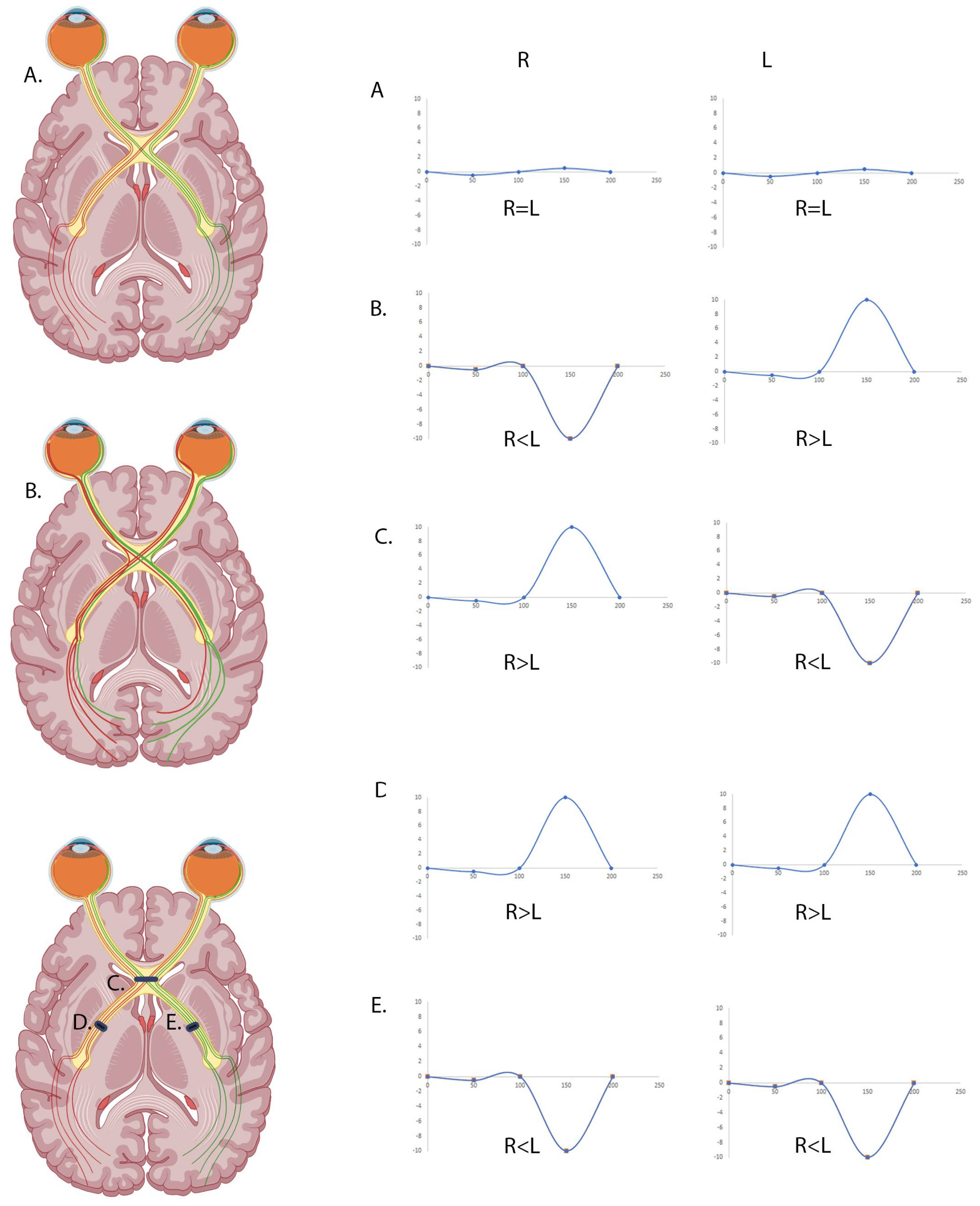
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Electrode Placement
2.3. VEP Recordings Using Standard Laboratory-Based EDT Techniques
2.4. VEP Recordings Using the RETeval®
2.5. Analysis
3. Results
3.1. Agreeability Between Standard Clinic VEPs and RETeval® VEP in Detecting Chiasmal Pathway Anomalies
3.2. Analysing the Full Epoch May Increase the Sensitivity of the RETeval® VEP to Identify Chiasmal Misrouting
3.3. The RETeval® Is Highly Sensitive and Specific at Detecting Chiasmal Misrouting
3.4. The RETeval® Score Is Not Age Dependent
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| VEP | Visual Evoke Potentials |
| ISCEV | International Society for Clinical Electrophysiology of Vision |
| OCA | Oculocutaneous Albinism |
References
- Grønskov, K.; Ek, J.; Brondum-Nielsen, K. Oculocutaneous albinism. Orphanet J. Rare Dis. 2007, 2, 43. [Google Scholar] [CrossRef] [PubMed]
- Brilliant, M.H. Albinism in Africa: A medical and social emergency. Int. Health 2015, 7, 223–225. [Google Scholar] [CrossRef] [PubMed]
- Hong, E.S.; Zeeb, H.; Repacholi, M.H. Albinism in Africa as a public health issue. BMC Public Health 2006, 6, 212. [Google Scholar] [CrossRef] [PubMed]
- Toyofuku, K.; Wada, I.; Spritz, R.A.; Hearing, V.J. The molecular basis of oculocutaneous albinism type 1 (OCA1): Sorting failure and degradation of mutant tyrosinases results in a lack of pigmentation. Biochem. J. 2001, 355, 259–269. [Google Scholar] [CrossRef]
- Thomas, M.G.; Kumar, A.; Mohammad, S.; Proudlock, F.A.; Engle, E.C.; Andrews, C.; Chan, W.M.; Thomas, S.; Gottlob, I. Structural grading of foveal hypoplasia using spectral-domain optical coherence tomography a predictor of visual acuity? Ophthalmology 2011, 118, 1653–1660. [Google Scholar] [CrossRef]
- Dorey, S.E.; Neveu, M.M.; Burton, L.C.; Sloper, J.J.; Holder, G.E. The clinical features of albinism and their correlation with visual evoked potentials. Br. J. Ophthalmol. 2003, 87, 767. [Google Scholar] [CrossRef]
- Kruijt, C.C.; de Wit, G.C.; Bergen, A.A.; Florijn, R.J.; Schalij-Delfos, N.E.; van Genderen, M.M. The Phenotypic Spectrum of Albinism. Ophthalmology 2018, 125, 1953–1960. [Google Scholar] [CrossRef]
- Lee, H.; Purohit, R.; Sheth, V.; Maconachie, G.; Tu, Z.; Thomas, M.G.; Pilat, A.; McLean, R.J.; Proudlock, F.A.; Gottlob, I. Retinal Development in Infants and Young Children with Albinism: Evidence for Plasticity in Early Childhood. Am. J. Ophthalmol. 2023, 245, 202–211. [Google Scholar] [CrossRef]
- Sanchez-Bretano, A.; Scott, J.A.; Newall, T.; Lynn, S.; Griffiths, H.; Salman, A.; Lotery, A.; Ratnayaka, J.A.; Self, J.E.; Lee, H. Oral human-equivalent L-DOPA/Carbidopa dosages administered during the postnatal critical period of neuroplasticity rescues retinal morphology and visual function in a mouse model of human albinism. Investig. Ophthalmol. Vis. Sci. 2019, 60, 1393. [Google Scholar]
- Liu, S.; Kuht, H.J.; Moon, E.H.; Maconachie, G.D.E.; Thomas, M.G. Current and emerging treatments for albinism. Surv. Ophthalmol. 2021, 66, 362–377. [Google Scholar] [CrossRef]
- Lin, S.; Sanchez-Bretaño, A.; Leslie, J.S.; Williams, K.B.; Lee, H.; Thomas, N.S.; Callaway, J.; Deline, J.; Ratnayaka, J.A.; Baralle, D.; et al. Evidence that the Ser192Tyr/Arg402Gln in cis Tyrosinase gene haplotype is a disease-causing allele in oculocutaneous albinism type 1B (OCA1B). NPJ Genom. Med. 2022, 7, 2. [Google Scholar] [CrossRef] [PubMed]
- Norman, C.S.; O’Gorman, L.; Gibson, J.; Pengelly, R.J.; Baralle, D.; Ratnayaka, J.A.; Griffiths, H.; Rose-Zerilli, M.; Ranger, M.; Bunyan, D.; et al. Identification of a functionally significant tri-allelic genotype in the Tyrosinase gene (TYR) causing hypomorphic oculocutaneous albinism (OCA1B). Sci. Rep. 2017, 7, 4415. [Google Scholar] [CrossRef] [PubMed]
- O’Gorman, L.; Norman, C.S.; Michaels, L.; Newall, T.; Crosby, A.H.; Mattocks, C.; Cree, A.J.; Lotery, A.J.; Baple, E.L.; Ratnayaka, J.A.; et al. A small gene sequencing panel realises a high diagnostic rate in patients with congenital nystagmus following basic phenotyping. Sci. Rep. 2019, 9, 13229. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, M.A.; Mossallam, E.; Allam, I.Y. The Role of the Flash Visual Evoked Potential in Evaluating Visual Function in Patients with Indirect Traumatic Optic Neuropathy. Clin. Ophthalmol. 2021, 15, 1349–1355. [Google Scholar] [CrossRef]
- Brecelj, J. Electrodiagnostics of chiasmal compressive lesions. Int. J. Psychophysiol. 1994, 16, 263–272. [Google Scholar] [CrossRef]
- Odom, J.V.; Bach, M.; Brigell, M.; Holder, G.E.; McCulloch, D.L.; Mizota, A.; Tormene, A.P.; International Society for Clinical Electrophysiology of Vision. ISCEV standard for clinical visual evoked potentials: (2016 update). Doc. Ophthalmol. 2016, 133, 1–9. [Google Scholar] [CrossRef]
- Kriss, A.; Russell-Eggitt, I. Electrophysiological assessment of visual pathway function in infants. Eye 1992, 6 Pt 2, 145–153. [Google Scholar] [CrossRef]
- Marmoy, O.R.; Moinuddin, M.; Thompson, D.A. An alternative electroretinography protocol for children: A study of diagnostic agreement and accuracy relative to ISCEV standard electroretinograms. Acta Ophthalmol. 2022, 100, 322–330. [Google Scholar] [CrossRef]
- Handley, S.E.; Šuštar, M.; Tekavčič Pompe, M. What can visual electrophysiology tell about possible visual-field defects in paediatric patients. Eye 2021, 35, 2354–2373. [Google Scholar] [CrossRef]
- Apkarian, P. A practical approach to albino diagnosis. VEP misrouting across the age span. Ophthalmic Paediatr. Genet. 1992, 13, 77–88. [Google Scholar] [CrossRef]
- Kriss, A.; Russell-Eggitt, I.; Taylor, D. Childhood albinism. Visual electrophysiological features. Ophthalmic Paediatr. Genet. 1990, 11, 185–192. [Google Scholar] [CrossRef] [PubMed]
- Kruijt, C.C.; de Wit, G.C.; Talsma, H.E.; Schalij-Delfos, N.E.; van Genderen, M.M. The Detection of Misrouting in Albinism: Evaluation of Different VEP Procedures in a Heterogeneous Cohort. Investig. Ophthalmol. Vis. Sci. 2019, 60, 3963–3969. [Google Scholar] [CrossRef] [PubMed]
- Neveu, M.M.; Jeffery, G.; Burton, L.C.; Sloper, J.J.; Holder, G.E. Age-related changes in the dynamics of human albino visual pathways. Eur. J. Neurosci. 2003, 18, 1939–1949. [Google Scholar] [CrossRef] [PubMed]
- Pott, J.W.R.; Jansonius, N.M.; Kooijman, A.C. Chiasmal coefficient of flash and pattern visual evoked potentials for detection of chiasmal misrouting in albinism. Doc. Ophthalmol. 2003, 106, 137–143. [Google Scholar] [CrossRef]
- Soong, F.; Levin, A.V.; Westall, C.A. Comparison of techniques for detecting visually evoked potential asymmetry in albinism. J. Am. Assoc. Pediatr. Ophthalmol. Strabismus 2000, 4, 302–310. [Google Scholar] [CrossRef]
- McCulloch, D.L.; Garcia-filion, P.; Matar, M.; Stewart, C.; Borchert, M.S. Repeated measurements of ERGs and VEPs using chloral hydrate sedation and propofol anesthesia in young children. Doc. Ophthalmol. 2021, 143, 141–153. [Google Scholar] [CrossRef]
- Kuba, M.; Kremláček, J.; Vít, F.; Kubová, Z.; Langrová, J.; Szanyi, J.; Chutná, M. VEP examination with new portable device. Doc. Ophthalmol. 2023, 146, 79–91. [Google Scholar] [CrossRef]
- You, J.Y.; Dorfman, A.L.; Gauvin, M.; Vatcher, D.; Polomeno, R.C.; Little, J.M.; Lachapelle, P. Comparing the RETeval(®) portable ERG device with more traditional tabletop ERG systems in normal subjects and selected retinopathies. Doc. Ophthalmol. 2022, 146, 137–150. [Google Scholar] [CrossRef]
- Carter, P.; Gordon-Reid, A.; Shawkat, F.; Self, J.E. Comparison of the handheld RETeval ERG system with a routine ERG system in healthy adults and in paediatric patients. Eye 2021, 35, 2180–2189. [Google Scholar] [CrossRef]
- Jansonius, N.M.; van der Vliet, T.M.; Cornelissen, F.W.; Pott, J.W.R.; Kooijman, A.C. A Girl Without a Chiasm: Electrophysiologic and MRI Evidence for the Absence of Crossing Optic Nerve Fibers in a Girl with a Congenital Nystagmus. J. Neuro-Ophthalmol. 2001, 21, 26–29. [Google Scholar] [CrossRef]
- Toga, A.W.; Thompson, P.M. Mapping brain asymmetry. Nat. Rev. Neurosci. 2003, 4, 37–48. [Google Scholar] [CrossRef]
- Mandrekar, J.N. Receiver Operating Characteristic Curve in Diagnostic Test Assessment. J. Thorac. Oncol. 2010, 5, 1315–1316. [Google Scholar] [CrossRef]



| Control | Albinism | |
|---|---|---|
| 0–7 | 15 | 20 |
| 8+ | 25 | 7 |
| Age (Years) | Iris Transillumination | Nystagmus | Pale Fundus | Foveal Hypoplasia | RETeval Pearson‘s Correlate | Genomics Variant 1 | Genomics Variant 2 | Genomic Diagnostic Summary | Albinism Diagnosis |
|---|---|---|---|---|---|---|---|---|---|
| 5 | No | No | Yes | Yes (Grade 3) | −0.6725 | TYR c.1217C>T p. (Pro406Leu) | TYR c.1036G>A p. (Gly346Arg) | OCA1 | proven |
| 1 | Yes | Yes | Yes | Yes (Grade 1/2) | −0.465 | OCA2 c.1327G>A, P.(Val443lle) | nil | Likely OCA2 with missing variant | likely |
| 0 | Yes | Yes | Yes | Yes (Grade 1/2) | −0.3107 | nil | nil | Clinical OCA but no variants on R39 panel | likely |
| 3 | Yes | Yes | Yes | No | −0.5299 | nil | Hom R402Q and Het S192Y | Likely OCA1b but segregation not complete | likely |
| 9 | No | No | Yes | Yes (Grade 3/4) | −0.09339 | TYR c.823G>T p.(Val275Phe) | het s192y and het r402Q | OCA1b | proven |
| 1 | Yes | Yes | Yes | Yes (Grade 3) | −0.4979 | nil | nil | Clinical OCA but no variants on R39 panel | likely |
| 1 | No | Yes | Yes | Yes | −0.5061 | nil | nil | Clinical OCA but no variants on R39 panel | likely |
| 1 | No | Yes | Yes | Yes | −0.6705 | OCA2 c.1255C>T p.(Arg419Trp) | nil | Likely OCA2 with missing variant | likely |
| 0 | Unknown | Yes | Yes | Yes | −0.7914 | nil | nil | Clinical OCA but no variants on R39 panel | likely |
| 7 | Yes | Yes | Yes | Yes (Grade 4) | −0.4442 | TYR c.229C>T p.(Arg77Trp) | TYR s192Y (hom) and R402Q (het) | Likely OCA1b but segregation not complete | likely |
| 1 | No | Yes | Yes | Yes (Grade 1/2) | −0.4607 | nil | nil | Clinical OCA but no variants on R39 panel | likely |
| 5 | Yes | Yes | Yes | Unknown | −0.7293 | Unknown | Unknown | Likely OCA1b but segregation not complete | likely |
| 4 | Yes | No | Yes | Yes | −0.1855 | TYR c.650G>A p.(Arg217Gln) | TYR s192Y (het) and R402Q (hom) | Likely OCA1b but segregation not complete | likely |
| 1 | Yes | No | Yes | Unknown | −0.4032 | nil | nil | Not done | possible |
| 2 | Yes | Yes | Unknown | Unknown | −0.01125 | nil | nil | Not done | possible |
| 26 | Yes | Yes | Yes | Yes (Grade 3/4) | −0.3472 | TYR c.1118C>A p.(Thr373Lys) | TYR s192Y (hom) and R402Q (het) | OCA1b | proven |
| 60 | No | Yes | No | Yes (Grade 1) | −0.2073 | TYR c.1118C>A p.(Thr373Lys) | TYR s192Y (het) and R402Q (het) | OCA1b | proven |
| 2 | No | No | Yes | Unknown | −0.6503 | nil | nil | Not done | possible |
| 41 | No | Yes | Yes | Yes (Grade 3) | −0.5923 | nil | nil | Not done | possible |
| 5 | Unknown | no | yes | Yes (Grade 3) | −0.442 | nil | nil | Not done | possible |
| 13 | Yes | No | Yes | mild | −0.3373 | OCA2 c.1025A>G p. (Tyr342Cys) | OCA2 c.1418T>A p.(lle473Asn) | OCA2 | proven |
| 14 | Yes | Yes | Yes | Yes | −0.1239 | OCA2 c.1025A>G p. (Tyr342Cys) | OCA2 c.1418T>A p.(lle473Asn) | OCA2 | proven |
| 0 | Yes | No | Yes | Yes (Grade 1) | −0.5789 | nil | nil | Clinical OCA but no variants on R39 panel | likely |
| 6 | Unknown | no | Yes | Yes Grade 3 | −0.4285 | TYR c.1118C>A p.(Thr373Lys) | nil | Likely OCA1 with missing variant | likely |
| 5 | Yes | Yes | yes | Yes Grade 3 | −0.2729 | OCA2 c.619_636del p.(Leu207_Leu212del) | OCA2 c.1103C>T p.(Ala368Val) | OCA2 | proven |
| 2 | Yes | Yes | Yes | Unknown | −0.06207 | nil | nil | Clinical OCA but no variants on R39 panel | likely |
| 0 | Unknown | Yes | Unknown | Unknown | −0.1491 | nil | nil | Not done | possible |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Keeling, E.; Carter, P.; Musa, A.M.; Shawkat, F.; Lee, H.; Self, J.E. Can a Portable Flash Visual Evoked Potential (VEP) Device Identify Chiasmal Decussation Anomalies in Albinism? Diagnostics 2025, 15, 1395. https://doi.org/10.3390/diagnostics15111395
Keeling E, Carter P, Musa AM, Shawkat F, Lee H, Self JE. Can a Portable Flash Visual Evoked Potential (VEP) Device Identify Chiasmal Decussation Anomalies in Albinism? Diagnostics. 2025; 15(11):1395. https://doi.org/10.3390/diagnostics15111395
Chicago/Turabian StyleKeeling, Eloise, Perry Carter, Abdi Malik Musa, Fatima Shawkat, Helena Lee, and Jay E. Self. 2025. "Can a Portable Flash Visual Evoked Potential (VEP) Device Identify Chiasmal Decussation Anomalies in Albinism?" Diagnostics 15, no. 11: 1395. https://doi.org/10.3390/diagnostics15111395
APA StyleKeeling, E., Carter, P., Musa, A. M., Shawkat, F., Lee, H., & Self, J. E. (2025). Can a Portable Flash Visual Evoked Potential (VEP) Device Identify Chiasmal Decussation Anomalies in Albinism? Diagnostics, 15(11), 1395. https://doi.org/10.3390/diagnostics15111395





