Integrating Radiogenomics and Machine Learning in Musculoskeletal Oncology Care
Abstract
1. Introduction
2. Historical Context and Evolution of Radiogenomics in Oncology
3. Technical Evolution and Methodological Advances
4. Current Applications in Musculoskeletal Cancer Diagnosis and Risk Stratification
5. Unique Diagnostic Challenges for Bony Tumors
6. Radiomic Feature Extraction
- First-order features such as mean intensity, skewness, and kurtosis are derived from histogram analysis of voxel intensity distributions. For example, kurtosis quantifies the peakedness of intensity distributions, where higher values may indicate regions of dense cellularity. A tumor with high intensity kurtosis may exhibit regions of dense cellularity. PyRadiomics provides the formula for kurtosis as where is the fourth central moment. Diffusion kurtosis imaging studies demonstrate that kurtosis metrics strongly correlate with glioma cellularity and proliferation indices [93,94].
- Second-order and higher-order texture features are derived from the gray-level co-occurrence matrix (GLCM) and gray-level run-length matrix (GLRLM). GLCM features include contrast (highlighting local intensity variation, with higher values in rough/textured regions), entropy (indicating randomness in intensity distributions, where higher values indicate heterogeneity), and homogeneity (measuring uniformity, with lower values found in heterogeneous tumors) [86]. GLRLM features quantify runs of similar intensity levels, such as short-run emphasis (highlights fine textures, reflecting necrosis of fibrosis) [86] or long-run low gray-level emphasis (captures extended regions of low intensity, associated with cystic components or edema) [86,97].
7. Advanced Imaging Modalities for Feature Extraction
8. Quantitative Feature Analysis and Standardization
9. Standardized Workflow and Quality Control
10. Genomic and Transcriptomic Integration with Imaging Features
11. Correlation of Imaging Phenotypes with Genomic Signatures
12. Inflammatory Cytokine Profiles and Their Predictive Value
13. Machine Learning Algorithms in Musculoskeletal Tumor Classification
14. Classical Machine Learning Algorithms
15. Deep Learning Models
16. Unsupervised Learning for Pattern Discovery
17. Translational Barriers and Technical Challenges
18. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Grimer, R.J.; Briggs, T.W. Earlier Diagnosis of Bone and Soft-Tissue Tumours. J. Bone Jt. Surg. Br. 2010, 92, 1489–1492. [Google Scholar] [CrossRef] [PubMed]
- Rechl, H.; Kirchhoff, C.; Wörtler, K.; Lenze, U.; Töpfer, A.; von Eisenhart-Rothe, R. Diagnosis of Malignant Bone and Soft Tissue Tumors. Orthopäde 2011, 40, 931–941. [Google Scholar] [CrossRef] [PubMed]
- Clark, M.A.; Thomas, J.M. Delay in Referral to a Specialist Soft-Tissue Sarcoma Unit. Eur. J. Surg. Oncol. 2005, 31, 443–448. [Google Scholar] [CrossRef] [PubMed]
- Shui, L.; Ren, H.; Yang, X.; Li, J.; Chen, Z.; Yi, C.; Zhu, H.; Shui, P. The Era of Radiogenomics in Precision Medicine: An Emerging Approach to Support Diagnosis, Treatment Decisions, and Prognostication in Oncology. Front. Oncol. 2021, 10, 570465. [Google Scholar] [CrossRef] [PubMed]
- Trivizakis, E.; Papadakis, G.Z.; Souglakos, I.; Papanikolaou, N.; Koumakis, L.; Spandidos, D.A.; Tsatsakis, A.; Karantanas, A.H.; Marias, K. Artificial Intelligence Radiogenomics for Advancing Precision and Effectiveness in Oncologic Care (Review). Int. J. Oncol. 2020, 57, 43–53. [Google Scholar] [CrossRef] [PubMed]
- Ferro, M.; de Cobelli, O.; Vartolomei, M.D.; Lucarelli, G.; Crocetto, F.; Barone, B.; Sciarra, A.; Del Giudice, F.; Muto, M.; Maggi, M.; et al. Prostate Cancer Radiogenomics—From Imaging to Molecular Characterization. Int. J. Mol. Sci. 2021, 22, 9971. [Google Scholar] [CrossRef] [PubMed]
- Kickingereder, P.; Bonekamp, D.; Nowosielski, M.; Kratz, A.; Sill, M.; Burth, S.; Wick, A.; Eidel, O.; Schlemmer, H.; Radbruch, A. Radiogenomics of Glioblastoma: Machine Learning-Based Classification of Molecular Characteristics by Using Multiparametric and Multiregional MR Imaging Features. Radiology 2016, 281, 907–918. [Google Scholar] [CrossRef] [PubMed]
- Hinterwimmer, F.; Guenther, M.; Consalvo, S.; Neumann, J.; Gersing, A.; Wörtler, K.; von Eisenhart-Rothe, R.; Burgkart, R.; Rueckert, D. Applications of Machine Learning for Imaging-Driven Diagnosis of Musculoskeletal Malignancies—A Scoping Review. BMC Musculoskelet. Disord. 2024, 25, 822. [Google Scholar] [CrossRef] [PubMed]
- Breden, S.; Hinterwimmer, F.; Consalvo, S.; Neumann, J.; Knebel, C.; von Eisenhart-Rothe, R.; Burgkart, R.H.; Lenze, U. Deep Learning-Based Detection of Bone Tumors Around the Knee in X-Rays of Children. J. Clin. Med. 2023, 12, 5960. [Google Scholar] [CrossRef] [PubMed]
- Cuocolo, R.; Caruso, M.; Perillo, T.; Ugga, L.; Petretta, M. Machine Learning in Oncology: A Clinical Appraisal. Cancer Lett. 2020, 481, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Martin-Gonzalez, P.; Crispin-Ortuzar, M.; Rundo, L.; Delgado-Ortet, M.; Reinius, M.; Brenton, J.D.; Jimenez-Linan, M.; Sala, E. Integrative Radiogenomics for Virtual Biopsy and Treatment Monitoring in Ovarian Cancer. Insights Imaging 2020, 11, 94. [Google Scholar] [CrossRef] [PubMed]
- Thomford, N.E.; Bope, C.D.; Agamah, F.E.; Dzobo, K.; Owusu Ateko, R.; Chimusa, E.; Mazandu, G.K.; Ntumba, S.B.; Dandara, C.; Wonkam, A. Implementing Artificial Intelligence and Digital Health in Resource-Limited Settings? Top 10 Lessons We Learned in Congenital Heart Defects and Cardiology. OMICS 2020, 24, 264–277. [Google Scholar] [CrossRef] [PubMed]
- Anagnostopoulos, A.K.; Gaitanis, A.; Gkiozos, I.; Athanasiadis, E.I.; Chatziioannou, S.N.; Syrigos, K.N.; Thanos, D.; Chatziioannou, A.N.; Papanikolaou, N. Radiomics/Radiogenomics in Lung Cancer: Basic Principles and Initial Clinical Results. Cancers 2022, 14, 1657. [Google Scholar] [CrossRef] [PubMed]
- Kelly, C.J.; Karthikesalingam, A.; Suleyman, M.; Corrado, G.; King, D. Key Challenges for Delivering Clinical Impact with Artificial Intelligence. BMC Med. 2019, 17, 195. [Google Scholar] [CrossRef] [PubMed]
- Tseng, H.H.; Wei, L.; Cui, S.; Luo, Y.; Ten Haken, R.K.; El Naqa, I. Machine Learning and Imaging Informatics in Oncology. Oncology 2020, 98, 344–362. [Google Scholar] [CrossRef] [PubMed]
- Saxena, S.; Jena, B.; Gupta, N.; Das, S.; Sarmah, D.; Bhattacharya, P.; Nath, T.; Paul, S.; Fouda, M.M.; Kalra, M.; et al. Role of Artificial Intelligence in Radiogenomics for Cancers in the Era of Precision Medicine. Cancers 2022, 14, 2860. [Google Scholar] [CrossRef] [PubMed]
- Rudie, J.D.; Rauschecker, A.M.; Bryan, R.N.; Davatzikos, C.; Mohan, S. Emerging Applications of Artificial Intelligence in Neuro-Oncology. Radiology 2019, 290, 607–618. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.T.; Chen, Z.; Ye, N.; Mambetsariev, I.; Fricke, J.; Daniel, E.; Wang, G.; Wong, C.W.; Rockne, R.C.; Colen, R.R.; et al. Differentiating Peripherally-Located Small Cell Lung Cancer from Non-Small Cell Lung Cancer Using a CT Radiomic Approach. Front. Oncol. 2020, 10, 593. [Google Scholar] [CrossRef] [PubMed]
- Mun, S.K.; Wong, K.H.; Lo, S.B.; Li, Y.; Bayarsaikhan, S. Artificial Intelligence for the Future Radiology Diagnostic Service. Front. Mol. Biosci. 2020, 7, 614258. [Google Scholar] [CrossRef] [PubMed]
- Shiri, I.; Maleki, H.; Hajianfar, G.; Abdollahi, H.; Ashrafinia, S.; Hatt, M.; Zaidi, H.; Oveisi, M.; Rahmim, A. Next-Generation Radiogenomics Sequencing for Prediction of EGFR and KRAS Mutation Status in NSCLC Patients Using Multimodal Imaging and Machine Learning Algorithms. Mol. Imaging Biol. 2020, 22, 1132–1148. [Google Scholar] [CrossRef] [PubMed]
- Tu, S.J.; Wang, C.W.; Pan, K.T.; Wu, Y.C.; Wu, C.T. Localized Thin-Section CT with Radiomics Feature Extraction and Machine Learning to Classify Early-Detected Pulmonary Nodules from Lung Cancer Screening. Phys. Med. Biol. 2018, 63, 065005. [Google Scholar] [CrossRef] [PubMed]
- Ferreira Junior, J.R.; Koenigkam-Santos, M.; Cipriano, F.E.G.; Fabro, A.T.; Azevedo-Marques, P.M. Radiomics-Based Features for Pattern Recognition of Lung Cancer Histopathology and Metastases. Comput. Methods Programs Biomed. 2018, 159, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Bourbonne, V.; Vallieres, M.; Lucia, F.; Doucet, L.; Visvikis, D.; Tixier, F.; Le Prise, E.; Hatt, M.; Schick, U. MRI-Derived Radiomics to Guide Post-Operative Management for High-Risk Prostate Cancer Patients. Cancers 2022, 14, 1418. [Google Scholar] [CrossRef] [PubMed]
- Bourbonne, V.; Fournier, G.; Vallières, M.; Lucia, F.; Doucet, L.; Tissot, V.; Cuvelier, C.; Hue, S.; Le Prisé, E.; Blais, E.; et al. External Validation of a Radiomic Signature to Predict p16 Status and Outcome in Oropharyngeal Cancers. Radiother. Oncol. 2020, 152, 20–26. [Google Scholar] [CrossRef] [PubMed]
- Grochans, S.; Cybulska, A.M.; Simińska, D.; Korbecki, J.; Kojder, K.; Chlubek, D.; Baranowska-Bosiacka, I. Epidemiology of Glioblastoma Multiforme—Literature Review. Cancers 2022, 14, 2412. [Google Scholar] [CrossRef] [PubMed]
- Fanizzi, A.; Catino, A.; Bove, S.; Comes, M.C.; Montrone, M.; Sicolo, A.; Signorile, R.; Perrotti, P.; Pizzutilo, P.; Galetta, D.; et al. Transfer Learning Approach in Pre-Treatment CT Images to Predict Therapeutic Response in Advanced Malignant Pleural Mesothelioma. Front. Oncol. 2024, 14, 1432188. [Google Scholar] [CrossRef] [PubMed]
- Lu, I.N.; Dobersalske, C.; Rauschenbach, L.; Teuber-Hanselmann, S.; Steinbach, A.; Ullrich, V.; Prasad, S.; Blau, T.; Kebir, S.; Siveke, J.T. Tumor-Associated Hematopoietic Stem and Progenitor Cells Positively Linked to Glioblastoma Progression. Nat. Commun. 2021, 12, 3895. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Park, J.W.; Lee, J.H. Genetic Architectures and Cell-of-Origin in Glioblastoma. Front. Oncol. 2021, 10, 615400. [Google Scholar] [CrossRef] [PubMed]
- Melhem, J.M.; Detsky, J.; Lim-Fat, M.J.; Perry, J.R. Updates in IDH-Wildtype Glioblastoma. Neurotherapeutics 2022, 19, 1705–1723. [Google Scholar] [CrossRef] [PubMed]
- Tykocki, T.; Eltayeb, M. Ten-Year Survival in Glioblastoma. A Systematic Review. J. Clin. Neurosci. 2018, 54, 7–13. [Google Scholar] [CrossRef] [PubMed]
- Jena, B.; Saxena, S.; Nayak, G.K.; Balestrieri, A.; Gupta, N.; Khanna, N.N.; Laird, J.R.; Kalra, M.K.; Fouda, M.M.; Saba, L.; et al. Brain Tumor Characterization Using Radiogenomics in Artificial Intelligence Framework. Cancers 2022, 14, 4052. [Google Scholar] [CrossRef] [PubMed]
- Wikimedia Commons. File:Cancer Biomarker Figure.png. Published 30 January 2024. Available online: https://commons.wikimedia.org/w/index.php?title=File:Cancer_biomarker_figure.png&oldid=847271038 (accessed on 20 May 2025).
- Vogrin, M.; Trojner, T.; Kelc, R. Artificial Intelligence in Musculoskeletal Oncological Radiology. Radiol. Oncol. 2020, 55, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, I.; Crawley, A.; Bhethanabotla, M.; Daldrup-Link, H.E.; Rubin, D.L. Transfer Learning on Fused Multiparametric MR Images for Classifying Histopathological Subtypes of Rhabdomyosarcoma. Comput. Med. Imaging Graph. 2018, 65, 167–175. [Google Scholar] [CrossRef] [PubMed]
- Haubold, J.; Hosch, R.; Parmar, V.; Glas, M.; Guberina, N.; Catalano, O.A.; Pierscianek, D.; Wrede, K.; Deuschl, C.; Forsting, M.; et al. Fully Automated MR Based Virtual Biopsy of Cerebral Gliomas. Cancers 2023, 15, 618. [Google Scholar] [CrossRef] [PubMed]
- Ma, F.; Shao, X.; Zhang, Y.; Li, J.; Li, Q.; Sun, H.; Wang, T.; Liu, H.; Zhao, F.; Chen, L.; et al. An Arterial Spin Labeling-Based Radiomics Signature and Machine Learning for the Prediction and Detection of Various Stages of Kidney Damage Due to Diabetes. Front. Endocrinol. 2024, 15, 1333881. [Google Scholar] [CrossRef] [PubMed]
- Feng, Z.; Kong, D.; Jin, W.; He, K.; Zhao, J.; Liu, B.; Xu, H.; Yu, X.; Feng, S. Rapid Detection of Isocitrate Dehydrogenase 1 Mutation Status in Glioma Based on Crispr-Cas12a. Sci. Rep. 2023, 13, 5748. [Google Scholar] [CrossRef] [PubMed]
- Nagy, M.; Radakovich, N.; Nazha, A. Machine Learning in Oncology: What Should Clinicians Know? JCO Clin. Cancer Inform. 2020, 4, 799–810. [Google Scholar] [CrossRef] [PubMed]
- D’Angelo, T.; Caudo, D.; Blandino, A.; Albrecht, M.H.; Vogl, T.J.; Gruenewald, L.D.; Gaeta, M.; Yel, I.; Koch, V.; Martin, S.S.; et al. Artificial Intelligence, Machine Learning and Deep Learning in Musculoskeletal Imaging: Current Applications. J. Clin. Ultrasound 2022, 50, 1414–1431. [Google Scholar] [CrossRef] [PubMed]
- Scapicchio, C.; Gabelloni, M.; Barucci, A.; Cioni, D.; Saba, L.; Neri, E. A Deep Look into Radiomics. Radiol. Med. 2021, 126, 1296–1311. [Google Scholar] [CrossRef] [PubMed]
- Pitarch, C.; Ungan, G.; Julià-Sapé, M.; Vellido, A. Advances in the Use of Deep Learning for the Analysis of Magnetic Resonance Image in Neuro-Oncology. Cancers 2024, 16, 300. [Google Scholar] [CrossRef] [PubMed]
- Aneja, S.; Chang, E.; Omuro, A. Applications of Artificial Intelligence in Neuro-Oncology. Curr. Opin. Neurol. 2019, 32, 850–856. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, H.; Nakayama, K.I. Artificial Intelligence in Oncology. Cancer Sci. 2020, 111, 1452–1460. [Google Scholar] [CrossRef] [PubMed]
- Kawaguchi, R.K.; Takahashi, M.; Miyake, M.; Kinoshita, M.; Takahashi, S.; Ichimura, K.; Hamamoto, R.; Narita, Y.; Sese, J. Assessing Versatile Machine Learning Models for Glioma Radiogenomic Studies across Hospitals. Cancers 2021, 13, 3611. [Google Scholar] [CrossRef] [PubMed]
- Paladugu, P.S.; Ong, J.; Nelson, N.; Kamran, S.A.; Waisberg, E.; Zaman, N.; Kumar, R.; Dias, R.D.; Lee, A.G.; Tavakkoli, A. Generative Adversarial Networks in Medicine: Important Considerations for this Emerging Innovation in Artificial Intelligence. Ann. Biomed. Eng. 2023, 51, 2130–2142. [Google Scholar] [CrossRef] [PubMed]
- Bukowski, M.; Farkas, R.; Beyan, O.; Moll, J.; Hahn, H.; Kiessling, F.; Schmitz-Rode, T. Implementation of eHealth and AI Integrated Diagnostics with Multidisciplinary Digitized Data: Are We Ready from an International Perspective? Eur. Radiol. 2020, 30, 5510–5524. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.; Park, H.; Yang, H.J.; Lee, S.; Lee, K.Y.; Kim, T.S.; Shin, J.; Nam, D.H. Cancer Drug Response Profile Scan (CDRscan): A Deep Learning Model That Predicts Drug Effectiveness from Cancer Genomic Signature. Sci. Rep. 2018, 8, 8857. [Google Scholar] [CrossRef] [PubMed]
- Ciriello, G.; Miller, M.L.; Aksoy, B.A.; Senbabaoglu, Y.; Schultz, N.; Sander, C. Emerging Landscape of Oncogenic Signatures across Human Cancers. Nat. Genet. 2013, 45, 1127–1133. [Google Scholar] [CrossRef] [PubMed]
- Cruz, J.A.; Wishart, D.S. Applications of Machine Learning in Cancer Prediction and Prognosis. Cancer Inform. 2006, 2, 59–77. [Google Scholar] [CrossRef] [PubMed]
- “File:Flowchart of the Standard Radiomics Model.png.” Wikimedia Commons. 9 October 2024. Available online: https://commons.wikimedia.org/w/index.php?title=File:Flowchart_of_the_standard_radiomics_model.png&oldid=935994688 (accessed on 20 May 2025).
- Cancer Pharmacogenomics.png. Wikimedia Commons. Published 30 January 2024. Available online: https://commons.wikimedia.org/w/index.php?title=File:Cancer_pharmacogenomics.png&oldid=847271761 (accessed on 20 May 2025).
- The First and Second Phases of the NIH Human Microbiome Project.png. Wikimedia Commons. Published 8 November 2024. Available online: https://commons.wikimedia.org/w/index.php?title=File:The_first_and_second_phases_of_the_NIH_Human_Microbiome_Project.png&oldid=954301671 (accessed on 20 May 2025).
- MultiLayerNeuralNetworkBigger english.png. Wikimedia Commons. Published 15 July 2024. Available online: https://commons.wikimedia.org/w/index.php?title=File:MultiLayerNeuralNetworkBigger_english.png&oldid=898886601 (accessed on 20 May 2025).
- Liu, Z.; Duan, T.; Zhang, Y.; Weng, S.; Xu, H.; Ren, Y.; Zhang, Z.; Han, X. Radiogenomics: A key component of precision cancer medicine. Br. J. Cancer 2023, 129, 741–753. [Google Scholar] [CrossRef]
- Singh, G.; Singh, A.; Bae, J.; Manjila, S.; Spektor, V.; Prasanna, P.; Lignelli, A. New Frontiers in Domain-Inspired Radiomics and Radiogenomics: Increasing Role of Molecular Diagnostics in CNS Tumor Classification and Grading Following WHO CNS-5 Updates. Cancer Imaging 2024, 24, 133. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Hao, L.; Qi, M.; Xu, Q.; Zhang, N.; Feng, H.; Shi, G. Radiomics Nomogram for Preoperative Differentiation of Pulmonary Mucinous Adenocarcinoma from Tuberculoma in Solitary Pulmonary Solid Nodules. BMC Cancer 2023, 23, 261. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Li, Y.; Cheng, Q.; Van Valkenburgh, J.; Sun, X.; Zheng, C.; Zhang, R.; Yuan, R. EGFR Mutation Status and Subtypes Predicted by CT-Based 3D Radiomic Features in Lung Adenocarcinoma. OncoTargets Ther. 2022, 15, 597–608. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Gao, C.; Shao, Y.; Lou, X.; Hua, M.; Lin, J.; Wu, L.; Gao, C. The Prognostic Value of Radiogenomics Using CT in Patients with Lung Cancer: A Systematic Review. Insights Imaging 2024, 15, 259. [Google Scholar] [CrossRef] [PubMed]
- Miles, R.C.; Lehman, C.D.; Mercaldo, S.F.; Tamimi, R.M.; Hong, D.; Baker, J.; McCarthy, A.M. A Radiomics Model for Predicting the Response to Bevacizumab in Brain Necrosis after Nasopharyngeal Carcinoma Radiotherapy. Clin. Transl. Radiat. Oncol. 2023, 39, 100586. [Google Scholar] [CrossRef] [PubMed]
- Spraker, M.B.; Wootton, L.S.; Hippe, D.S.; Ball, K.C.; Peeken, J.C.; Macomber, M.W.; Chapman, T.R.; Hoff, M.N.; Kim, E.Y.; Pollack, S.M.; et al. MRI Radiomic Features Are Independently Associated with Overall Survival in Soft Tissue Sarcoma. Adv. Radiat. Oncol. 2019, 4, 413–421. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Xu, L.; Yang, L.; Hong, J.; Zhang, Z.; Xu, Z.; Zhou, Y.; Liu, Z.; Xu, X.; Wu, D.; et al. Survival Prediction in High-Grade Osteosarcoma Using Radiomics of Diagnostic Computed Tomography. EBioMedicine 2018, 34, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Ngiam, K.Y.; Khor, I.W. Big Data and Machine Learning Algorithms for Health-Care Delivery. Lancet Oncol. 2019, 20, e262–e273. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.C.; Swisher, C.L.; Chung, C.; Jaffray, D.; Sidey-Gibbons, C. On the Importance of Interpretable Machine Learning Predictions to Inform Clinical Decision Making in Oncology. Front. Oncol. 2023, 13, 1129380. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.A.; Li, Y.; Zeng, T. Deep Learning of Radiology-Genomics Integration for Computational Oncology: A Mini Review. Comput. Struct. Biotechnol. J. 2024, 23, 2708–2716. [Google Scholar] [CrossRef] [PubMed]
- Shams, A. Leveraging State-of-the-Art AI Algorithms in Personalized Oncology: From Transcriptomics to Treatment. Diagnostics 2024, 14, 2174. [Google Scholar] [CrossRef] [PubMed]
- Kalidindi, S. The Role of Artificial Intelligence in the Diagnosis of Melanoma. Cureus 2024, 16, e69818. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Song, Y.; Tian, X.; Hua, Y.; Zhang, R.; Wu, J. Survey on Deep Learning for Pulmonary Medical Imaging. Front. Med. 2020, 14, 450–469. [Google Scholar] [CrossRef] [PubMed]
- Paranavithana, I.R.; Stirling, D.; Ros, M.; Field, M. Systematic Review of Tumor Segmentation Strategies for Bone Metastases. Cancers 2023, 15, 1750. [Google Scholar] [CrossRef] [PubMed]
- Bach Cuadra, M.; Favre, J.; Omoumi, P. Quantification in Musculoskeletal Imaging Using Computational Analysis and Machine Learning: Segmentation and Radiomics. Semin. Musculoskelet. Radiol. 2020, 24, 50–64. [Google Scholar] [CrossRef] [PubMed]
- Debs, P.; Fayad, L.M. The Promise and Limitations of Artificial Intelligence in Musculoskeletal Imaging. Front. Radiol. 2023, 3, 1242902. [Google Scholar] [CrossRef] [PubMed]
- Montin, E.; Kijowski, R.; Youm, T.; Lattanzi, R. A Radiomics Approach to the Diagnosis of Femoroacetabular Impingement. Front. Radiol. 2023, 3, 1151258. [Google Scholar] [CrossRef] [PubMed]
- Gohla, G.; Kraus, M.S.; Peyker, I.; Springer, F.; Keller, G. Diagnostic Accuracy of 128-Slice Single-Source CT for the Detection of Dislocated Bucket Handle Meniscal Tears in the Setting of an Acute Knee Trauma—Correlation with MRI and Arthroscopy. Diagnostics 2023, 13, 1295. [Google Scholar] [CrossRef] [PubMed]
- Huber, F.A.; Guggenberger, R. AI MSK Clinical Applications: Spine Imaging. Skeletal Radiol. 2022, 51, 279–291. [Google Scholar] [CrossRef] [PubMed]
- Razavian, N.; Knoll, F.; Geras, K.J. Artificial Intelligence in Musculoskeletal Imaging: A Paradigm Shift. Semin. Musculoskelet. Radiol. 2020, 24, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.J.; Moorin, R.; Taylor, K.; Newton, J.; Smith, S. Collecting Routine and Timely Cancer Stage at Diagnosis by Implementing a Cancer Staging Tiered Framework: The Western Australian Cancer Registry Experience. BMC Health Serv. Res. 2024, 24, 770. [Google Scholar] [CrossRef] [PubMed]
- Feuerecker, B.; Heimer, M.M.; Geyer, T.; Fabritius, M.P.; Gu, S.; Schachtner, B.; Beyer, L.; Ricke, J.; Gatidis, S.; Ingrisch, M.; et al. Artificial Intelligence in Oncological Hybrid Imaging. Rofo 2023, 195, 105–114. [Google Scholar] [CrossRef] [PubMed]
- D’Amore, B.; Smolinski-Zhao, S.; Daye, D.; Uppot, R.N. Role of Machine Learning and Artificial Intelligence in Interventional Oncology. Curr. Oncol. Rep. 2021, 23, 70. [Google Scholar] [CrossRef] [PubMed]
- Di, J.; Hickey, C.; Bumgardner, C.; Yousif, M.; Zapata, M.; Bocklage, T.; Balzer, B.; Bui, M.M.; Gardner, J.M.; Pantanowitz, L.; et al. Utility of artificial intelligence in a binary classification of soft tissue tumors. J. Pathol Inform. 2024, 15, 100368. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Salehi, M.A.; Mohammadi, S.; Harandi, H.; Zakavi, S.S.; Jahanshahi, A.; Shahrabi Farahani, M.; Wu, J.S. Diagnostic Performance of Artificial Intelligence in Detection of Primary Malignant Bone Tumors: A Meta-Analysis. J. Imaging Inform. Med. 2024, 37, 766–777. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ong, W.; Zhu, L.; Tan, Y.L.; Teo, E.C.; Tan, J.H.; Kumar, N.; Vellayappan, B.A.; Ooi, B.C.; Quek, S.T.; Makmur, A.; et al. Application of Machine Learning for Differentiating Bone Malignancy on Imaging: A Systematic Review. Cancers 2023, 15, 1837. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Choi, J.H.; Ro, J.Y. The Recent Advances in Molecular Diagnosis of Soft Tissue Tumors. Int. J. Mol. Sci. 2023, 24, 5934. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bi, W.L.; Hosny, A.; Schabath, M.B.; Giger, M.L.; Birkbak, N.J.; Mehrtash, A.; Allison, T.; Arnaout, O.; Abbosh, C.; Dunn, I.F.; et al. Artificial intelligence in cancer imaging: Clinical challenges and applications. CA Cancer J. Clin. 2019, 69, 127–157. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Schajowicz, F.; McGuire, M.H. Diagnostic difficulties in skeletal pathology. Clin. Orthop. Relat. Res. 1989, 240, 281–310. [Google Scholar] [CrossRef] [PubMed]
- van Griethuysen, J.J.M.; Fedorov, A.; Parmar, C.; Hosny, A.; Aucoin, N.; Narayan, V.; Beets-Tan, R.G.H.; Fillion-Robin, J.C.; Pieper, S.; Aerts, H.J.W.L. Computational Radiomics System to Decode the Radiographic Phenotype. Cancer Res. 2017, 77, e104–e107. [Google Scholar] [CrossRef] [PubMed]
- Lambin, P.; Rios-Velazquez, E.; Leijenaar, R.; Carvalho, S.; van Stiphout, R.G.P.M.; Granton, P.; Zegers, C.M.L.; Gillies, R.; Boellard, R.; Dekker, A.; et al. Radiomics: Extracting More Information from Medical Images Using Advanced Feature Analysis. Eur. J. Cancer 2012, 48, 441–446. [Google Scholar] [CrossRef] [PubMed]
- Mayerhoefer, M.E.; Materka, A.; Langs, G.; Häggström, I.; Szczypiński, P.; Gibbs, P.; Cook, G. Introduction to Radiomics. J. Nucl. Med. 2020, 61, 488–495. [Google Scholar] [CrossRef] [PubMed]
- Gillies, R.J.; Kinahan, P.E.; Hricak, H. Radiomics: Images Are More than Pictures, They Are Data. Radiology 2016, 278, 563–577. [Google Scholar] [CrossRef] [PubMed]
- Shur, J.D.; Doran, S.J.; Kumar, S.; Ap Dafydd, D.; Downey, K.; O’Connor, J.P.B.; Poptani, H.; Koh, D.M.; Orton, M.R.; Messiou, C. Radiomics in Oncology: A Practical Guide. Radiographics 2021, 41, 1717–1732. [Google Scholar] [CrossRef] [PubMed]
- Kocak, B.; Durmaz, E.S.; Ates, E.; Kilickesmez, O. Radiomics with Artificial Intelligence: A Review for the Nonradiologist. Diagn. Interv. Radiol. 2021, 27, 635–643. [Google Scholar] [CrossRef] [PubMed]
- Cè, M.; Caloro, E.; Pellegrino, M.E.; Basile, M.; Sorce, A.; Fazzini, D.; Oliva, G.; Cellina, M. Artificial Intelligence in Breast Cancer Imaging: Risk Stratification, Lesion Detection and Classification, Treatment Planning and Prognosis—A Narrative Review. Explor. Target. Antitumor Ther. 2022, 3, 795–816. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Wang, S.; Dong, D.; Wei, J.; Fang, C.; Zhou, X.; Sun, K.; Li, L.; Li, B.; Wang, M.; et al. The Applications of Radiomics in Precision Diagnosis and Treatment of Oncology: Opportunities and Challenges. Theranostics 2019, 9, 1303–1322. [Google Scholar] [CrossRef] [PubMed]
- Park, J.E.; Kim, D.; Kim, H.S.; Park, S.Y.; Kim, J.Y.; Cho, S.J.; Shin, J.H.; Kim, J.H. Quality of Science and Reporting of Radiomics in Oncologic Studies: Room for Improvement According to Radiomics Quality Score and TRIPOD Statement. Eur. Radiol. 2020, 30, 523–536. [Google Scholar] [CrossRef] [PubMed]
- Jiang, R.; Jiang, J.; Zhao, L.; Zhang, J.; Zhang, S.; Yao, Y.; Yang, S.; Shi, J.; Shen, N.; Su, C.; et al. Diffusion kurtosis imaging can efficiently assess the glioma grade and cellular proliferation. Oncotarget 2015, 6, 42380–42393. [Google Scholar] [CrossRef] [PubMed] [PubMed Central][Green Version]
- Radiomic Features—Pyradiomics 2.2.0.post35+g8da1db7 Documentation. Readthedocs.io. Published 2016. Available online: https://pyradiomics.readthedocs.io/en/latest/features.html (accessed on 5 May 2025).
- Rios Velazquez, E.; Parmar, C.; Liu, Y.; Coroller, T.P.; Cruz, G.; Stringfield, O.; Ye, Z.; Makrigiorgos, M.; Fennessy, F.; Mak, R.H.; et al. Somatic Mutations Drive Distinct Imaging Phenotypes in Lung Cancer. Cancer Res. 2017, 77, 3922–3930. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Fusco, R.; Sansone, M.; Granata, V.; Di Bonito, M.; Avino, F.; Catalano, O.; Botti, G.; Petrillo, A. Use of Quantitative Morphological and Functional Features for Assessment of Axillary Lymph Node in Breast Dynamic Contrast-Enhanced Magnetic Resonance Imaging. Biomed. Res. Int. 2018, 2018, 2610801. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zubair, A.R.; Alo, O.A. Grey Level Co-occurrence Matrix (GLCM) Based Second Order Statistics for Image Texture Analysis. arXiv. 2024. Available online: https://arxiv.org/abs/2403.04038 (accessed on 15 May 2025).
- Aerts, H.J.W.L.; Velazquez, E.R.; Leijenaar, R.T.H.; Parmar, C.; Grossmann, P.; Carvalho, S.; Bussink, J.; Monshouwer, R.; Haibe-Kains, B.; Rietveld, D.; et al. Decoding Tumour Phenotype by Noninvasive Imaging Using a Quantitative Radiomics Approach. Nat. Commun. 2014, 5, 4006. [Google Scholar] [CrossRef] [PubMed]
- Yip, S.S.F.; Aerts, H.J.W.L. Applications and Limitations of Radiomics. Phys. Med. Biol. 2016, 61, R150–R166. [Google Scholar] [CrossRef] [PubMed]
- Limkin, C.J.; Sun, R.; Dercle, L.; Zacharaki, E.I.; Robert, C.; Reuzé, S.; Schernberg, A.; Paragios, N.; Deutsch, E.; Ferté, C. Promises and Challenges for the Implementation of Computational Medical Imaging (Radiomics) in Oncology. Ann. Oncol. 2017, 28, 1191–1206. [Google Scholar] [CrossRef] [PubMed]
- Rizzo, S.; Botta, F.; Raimondi, S.; Origgi, D.; Fanciulli, M.; Morganti, A.G.; Bellomi, M. Radiomics: The Facts and the Challenges of Image Analysis. Eur. Radiol. Exp. 2018, 2, 36. [Google Scholar] [CrossRef] [PubMed]
- Leithner, D.; Horvat, J.V.; Marino, M.A.; Bernard-Davila, B.; Jochelson, M.S.; Ochoa-Albiztegui, R.E.; Martinez, D.F.; Morris, E.A.; Thakur, S.; Pinker, K. Radiomic Signatures with Contrast-Enhanced Magnetic Resonance Imaging for the Assessment of Breast Cancer Receptor Status and Molecular Subtypes: Initial Results. Breast Cancer Res. 2019, 21, 106. [Google Scholar] [CrossRef] [PubMed]
- WBodalal, Z.; Trebeschi, S.; Nguyen-Kim, T.D.L.; Schats, W.; Beets-Tan, R. Radiogenomics: Bridging Imaging and Genomics. Abdom. Radiol. 2019, 44, 1960–1984. [Google Scholar] [CrossRef] [PubMed]
- Winfield, J.M.; Miah, A.B.; Strauss, D.; Thway, K.; Collins, D.J.; deSouza, N.M.; Leach, M.O.; Morgan, V.A.; Giles, S.L.; Moskovic, E.; et al. Utility of Quantitative Magnetic Resonance Imaging Parameters in the Evaluation of Soft Tissue Sarcomas. J. Magn. Reson. Imaging 2019, 49, 472–481. [Google Scholar] [CrossRef] [PubMed]
- Crombé, A.; Périer, C.; Kind, M.; De Senneville, B.D.; Le Loarer, F.; Italiano, A.; Buy, X.; Saut, O. T2-Based MRI Delta-Radiomics Improve Response Prediction in Soft-Tissue Sarcomas Treated by Neoadjuvant Chemotherapy. J. Magn. Reson. Imaging 2019, 50, 497–510. [Google Scholar] [CrossRef] [PubMed]
- Vallières, M.; Freeman, C.R.; Skamene, S.R.; El Naqa, I. A Radiomics Model from Joint FDG-PET and MRI Texture Features for the Prediction of Lung Metastases in Soft-Tissue Sarcomas of the Extremities. Phys. Med. Biol. 2015, 60, 5471–5496. [Google Scholar] [CrossRef] [PubMed]
- Nakajo, M.; Kobayashi, H.; Natsume, T.; Shinohara, T.; Fukukura, Y.; Fujisaki, Y.; Hirahara, Y.; Nakajo, M.; Yoshiura, T. Dual-Energy CT-Derived Iodine Content and Spectral Attenuation Analysis of Metastatic Versus Nonmetastatic Lymph Nodes in Squamous Cell Carcinoma of the Oropharynx. Tomography. 2018, 4, 66–71. [Google Scholar] [CrossRef] [PubMed]
- Gaillard, F. Hounsfield Unit Formula. Case Study, Radiopaedia.org. Available online: https://doi.org/10.53347/rID-147911 (accessed on 20 May 2025).
- Peeken, J.C.; Bernhofer, M.; Wiestler, B.; Goldberg, T.; Cremers, D.; Rost, B.; Weber, M.A.; Diehl, C.D.; Zimmer, C.; Combs, S.E. Radiomics in Radiooncology—Challenging the Medical Physicist. Phys. Med. 2018, 48, 27–36. [Google Scholar] [CrossRef] [PubMed]
- Long, H.; Zhang, P.; Bi, Y.; Yang, C.; Wu, M.; He, D.; Huang, S.; Yang, K.; Qi, S.; Wang, J. MRI Radiomic Features of Peritumoral Edema May Predict the Recurrence Sites of Glioblastoma Multiforme. Front. Oncol. 2023, 12, 1042498. [Google Scholar] [CrossRef] [PubMed]
- Gao, M.; Huang, S.; Pan, X.; Liao, X.; Yang, R.; Zhou, G.; Liu, Y.; Yang, Z.; Liu, J.; Qin, G. Machine Learning-Based Radiomics for Prediction of Epidermal Growth Factor Receptor Mutations in Lung Adenocarcinoma. J. Comput. Assist. Tomogr. 2023, 47, 947–953. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Sporn, K.; Ong, J.; Waisberg, E.; Paladugu, P.; Vaja, S.; Hage, T.; Sekhar, T.C.; Vadhera, A.S.; Ngo, A.; et al. Integrating Artificial Intelligence in Orthopedic Care: Advancements in Bone Care and Future Directions. Bioengineering 2025, 12, 513. [Google Scholar] [CrossRef]
- De Angelis, R.; Casale, R.; Coquelet, N.; Ikhlef, S.; Mokhtari, A.; Simoni, P.; Bali, M.A. The Impact of Radiomics in the Management of Soft Tissue Sarcoma. Discov. Oncol. 2024, 15, 62. [Google Scholar] [CrossRef] [PubMed]
- Bailly, C.; Bodet-Milin, C.; Couespel, S.; Necib, H.; Kraeber-Bodéré, F.; Ansquer, C.; Carlier, T. Revisiting the Robustness of PET-Based Textural Features in the Context of Multi-Centric Trials. PLoS ONE 2016, 11, e0159984. [Google Scholar] [CrossRef] [PubMed]
- Lovinfosse, P.; Polus, M.; Van Daele, D.; Martinive, P.; Daenen, F.; Hatt, M.; Visvikis, D.; Hustinx, R.; Withofs, N. FDG PET/CT Radiomics for Predicting the Outcome of Locally Advanced Rectal Cancer. Eur. J. Nucl. Med. Mol. Imaging 2018, 45, 365–375. [Google Scholar] [CrossRef] [PubMed]
- Larson, S.M.; Erdi, Y.; Akhurst, T.; Mazumdar, M.; Macapinlac, H.A.; Finn, R.D.; Casilla, C.; Fazzari, M.; Srivastava, N.; Yeung, H.W.D.; et al. Tumor treatment response based on visual and quantitative changes in global tumor glycolysis using PET-FDG imaging. The visual response score and the change in total lesion glycolysis. Clin. Positron Imaging 1999, 2, 159–171. [Google Scholar] [CrossRef] [PubMed]
- Im, H.J.; Bradshaw, T.; Solaiyappan, M.; Cho, S.Y. Current Methods to Define Metabolic Tumor Volume in Positron Emission Tomography: Which One is Better? Nucl. Med. Mol. Imaging 2018, 52, 5–15. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ha, S.; Choi, H.; Paeng, J.C.; Cheon, G.J. Radiomics in Oncological PET/CT: A Methodological Overview. Nucl. Med. Mol. Imaging 2019, 53, 14–29. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, H.; Nishio, M.; Nakanishi, H.; Hanai, N.; Hirakawa, H.; Kodaira, T.; Tamaki, T.; Hasegawa, Y. Impact of total lesion glycolysis measured by 18F-FDG-PET/CT on overall survival and distant metastasis in hypopharyngeal cancer. Oncol Lett. 2016, 12, 1493–1500. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Orlhac, F.; Boughdad, S.; Philippe, C.; Stalla-Bourdillon, H.; Nioche, C.; Champion, L.; Soussan, M.; Frouin, F.; Frouin, V.; Buvat, I. A Postreconstruction Harmonization Method for Multicenter Radiomic Studies in PET. J. Nucl. Med. 2018, 59, 1321–1328. [Google Scholar] [CrossRef] [PubMed]
- Sun, R.; Orlhac, F.; Robert, C.; Reuzé, S.; Schernberg, A.; Buvat, I.; Deutsch, E.; Ferté, C. In Regard to Mattonen et al. Int. J. Radiat. Oncol. Biol. Phys. 2016, 95, 1542–1543. [Google Scholar] [CrossRef] [PubMed]
- Traverso, A.; Wee, L.; Dekker, A.; Gillies, R. Repeatability and Reproducibility of Radiomic Features: A Systematic Review. Int. J. Radiat. Oncol. Biol. Phys. 2018, 102, 1143–1158. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.; Gu, Y.; Basu, S.; Berglund, A.; Eschrich, S.A.; Schabath, M.B.; Forster, K.; Aerts, H.J.W.L.; Dekker, A.; Fenstermacher, D.; et al. Radiomics: The Process and the Challenges. Magn. Reson. Imaging 2012, 30, 1234–1248. [Google Scholar] [CrossRef] [PubMed]
- File:TORNAI-SpectrumOfMedicalImaging.jpg. Wikimedia Commons. Published 5 November 2024. Available online: https://commons.wikimedia.org/w/index.php?title=File:TORNAI-SpectrumOfMedicalImaging.jpg&oldid=953058884 (accessed on 20 May 2025).
- Parmar, C.; Grossmann, P.; Bussink, J.; Lambin, P.; Aerts, H.J.W.L. Machine Learning Methods for Quantitative Radiomic Biomarkers. Sci. Rep. 2015, 5, 13087. [Google Scholar] [CrossRef] [PubMed]
- Leger, S.; Zwanenburg, A.; Pilz, K.; Lohaus, F.; Linge, A.; Zöphel, K.; Kotzerke, J.; Schreiber, A.; Tissot, V.; Dreveau, C.; et al. A Comparative Study of Machine Learning Methods for Time-to-Event Survival Data for Radiomics Risk Modelling. Sci. Rep. 2017, 7, 13206. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.Y.; Lee, K.S.; Seo, B.K.; Cho, K.R.; Woo, O.H.; Song, S.E.; Kim, E.K.; Lee, H.Y.; Kim, J.S.; Cha, J. Radiomic Machine Learning for Predicting Prognostic Biomarkers and Molecular Subtypes of Breast Cancer Using Tumor Heterogeneity and Angiogenesis Properties on MRI. Eur. Radiol. 2022, 32, 650–660. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.J.; Lu, M.Y.; Chen, T.Y.; Williamson, D.F.K.; Mahmood, F. Synthetic Data in Machine Learning for Medicine and Healthcare. Nat. Biomed. Eng. 2021, 5, 493–497. [Google Scholar] [CrossRef] [PubMed]
- HajiEsmailPoor, Z.; Tabnak, P.; Baradaran, B.; Pashazadeh, F.; Aghebati-Maleki, L. Diagnostic Performance of CT Scan-Based Radiomics for Prediction of Lymph Node Metastasis in Gastric Cancer: A Systematic Review and Meta-Analysis. Front. Oncol. 2023, 13, 1185663. [Google Scholar] [CrossRef] [PubMed]
- Stanzione, A.; Cuocolo, R.; Ugga, L.; Verde, F.; Romeo, V.; Mainenti, P.P.; Brunetti, A.; Maurea, S. Radiomics in Cross-Sectional Adrenal Imaging: A Systematic Review and Quality Assessment Study. Diagnostics 2022, 12, 578. [Google Scholar] [CrossRef] [PubMed]
- Zwanenburg, A.; Vallières, M.; Abdalah, M.A.; Aerts, H.J.W.L.; Andrearczyk, V.; Apte, A.; Ashrafinia, S.; Bakas, S.; Beukinga, R.J.; Boellaard, R.; et al. The Image Biomarker Standardization Initiative: Standardized Quantitative Radiomics for High-Throughput Image-Based Phenotyping. Radiology 2020, 295, 328–338. [Google Scholar] [CrossRef] [PubMed]
- Orlhac, F.; Frouin, F.; Nioche, C.; Ayache, N.; Buvat, I. Validation of A Method to Compensate Multicenter Effects Affecting CT Radiomics. Radiology 2019, 291, 53–59. [Google Scholar] [CrossRef] [PubMed]
- Da-Ano, R.; Visvikis, D.; Hatt, M. Harmonization Strategies for Multicenter Radiomics Investigations. Phys. Med. Biol. 2020, 65, 24TR02. [Google Scholar] [CrossRef] [PubMed]
- Scalco, E.; Rizzo, G. Texture Analysis of Medical Images in Radiomics: Review. Phys. Med. 2020, 79, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Berenguer, R.; Pastor-Juan, M.D.R.; Canales-Vázquez, J.; Castro-García, M.; Villas, M.V.; Mansilla Legorburo, F.; Sabaté-Llobera, A. Radiomics of CT Features May Be Nonreproducible and Redundant: Influence of CT Acquisition Parameters. Radiology 2018, 288, 407–415. [Google Scholar] [CrossRef] [PubMed]
- Schwier, M.; van Griethuysen, J.; Vangel, M.G.; Pieper, S.; Peled, S.; Tempany, C.; Aerts, H.J.W.L.; Kikinis, R.; Fennessy, F.M.; Fedorov, A. Repeatability of Multiparametric Prostate MRI Radiomics Features. Sci. Rep. 2019, 9, 9441. [Google Scholar] [CrossRef] [PubMed]
- Park, B.W.; Kim, J.K.; Lim, H.J.; Park, J.J.; Lee, J.; Byeon, J.S.; Park, H.; Kim, Y.; Lee, E.J.; Oh, C.; et al. Temporal Changes of Quantitative CT Findings from 102 Patients with COVID-19 in Wuhan, China: A Longitudinal Study. J. Korean Med. Sci. 2021, 36, e50. [Google Scholar] [CrossRef] [PubMed]
- “File:K-fold Cross Validation EN.svg.” Wikimedia Commons. 3 October 2024. Available online: https://commons.wikimedia.org/w/index.php?title=File:K-fold_cross_validation_EN.svg&oldid=932198002 (accessed on 20 May 2025).
- van Timmeren, J.E.; Cester, D.; Tanadini-Lang, S.; Alkadhi, H.; Baessler, B. Radiomics in Medical Imaging—“How-To” Guide and Critical Reflection. Insights Imaging 2020, 11, 91. [Google Scholar] [CrossRef] [PubMed]
- Jha, A.K.; Mithun, S.; Sherkhane, U.B.; Dwivedi, P.; Puts, S.; Osong, B.; Traverso, A.; Purandare, N.; Wee, L.; Rangarajan, V.; et al. Emerging Role of Quantitative Imaging (Radiomics) and Artificial Intelligence in Precision Oncology. Explor. Target. Antitumor Ther. 2023, 4, 569–582. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, A.; Primakov, S.; Beuque, M.; Woodruff, H.C.; Halilaj, I.; Wu, G.; Refaee, T.; Granzier, R.; Widaatalla, Y.; Hustinx, R.; et al. Radiomics for Precision Medicine: Current Challenges, Future Prospects, and the Proposed 6Rs Framework. Methods 2021, 188, 20–29. [Google Scholar] [CrossRef] [PubMed]
- Zhao, B.; Tan, Y.; Tsai, W.Y.; Qi, J.; Xie, C.; Lu, L.; Schwartz, L.H. Reproducibility of Radiomics for Deciphering Tumor Phenotype with Imaging. Sci. Rep. 2016, 6, 23428. [Google Scholar] [CrossRef] [PubMed]
- Altazi, B.A.; Zhang, G.G.; Fernandez, D.C.; Montejo, M.E.; Hunt, D.; Werner, J.; Biagioli, M.C.; Moros, E.G. Reproducibility and Generalizability in Radiomics Modeling: Possible Strategies in Radiologic and Statistical Modeling. BJR Open 2019, 1, 20190026. [Google Scholar] [CrossRef] [PubMed]
- Tixier, F.; Um, H.; Bermudez, D.; Iyer, A.; Apte, A.; Graham, M.S.; Neff, C.M.; Sutton, E.J.; Deasy, J.O.; Hatzoglou, V.; et al. Preoperative MRI-Radiomics Features Improve Prediction of Survival in Glioblastoma Patients over MGMT Methylation Status Alone. Oncotarget 2019, 10, 660–672. [Google Scholar] [CrossRef] [PubMed]
- Fornacon-Wood, I.; Faivre-Finn, C.; O’Connor, J.P.B.; Price, G.J. Radiomics as a Personalized Medicine Tool in Lung Cancer: Predicting Treatment Response from Baseline Imaging. Clin. Lung Cancer 2022, 23, 173–179. [Google Scholar]
- “File:Real-to-Voxel-Intensity-Range.png.” Wikimedia Commons. 12 May 2024. Available online: https://commons.wikimedia.org/w/index.php?title=File:Real-to-Voxel-intensity-range.png&oldid=876059423 (accessed on 20 May 2025).
- Zwanenburg, A.; Leger, S.; Vallières, M.; Löck, S. For the Image Biomarker Standardization Initiative. Image Biomarker Standardisation Initiative. arXiv 2016, arXiv:1612.07003. [Google Scholar] [CrossRef]
- Pati, S.; Singh, A.; Rathore, S.; Gastounioti, A.; Bergman, M.; Ngo, P.; Ha, S.M.; Bounias, D.; Minock, J.; Murphy, G.; et al. The Cancer Imaging Phenomics Toolkit (CaPTk): Technical Overview. Brainlesion 2020, 11993, 380–394. [Google Scholar] [CrossRef] [PubMed]
- Chalkidou, A.; O’Doherty, M.J.; Marsden, P.K. False Discovery Rates in PET and CT Studies with Texture Features: A Systematic Review. PLoS ONE 2015, 10, e0124165. [Google Scholar] [CrossRef] [PubMed]
- Sauerbrei, W.; Perperoglou, A.; Schmid, M.; Abrahamowicz, M.; Becher, H.; Binder, H.; Dunkler, D.; Harrell, F.E.; Royston, P.; Heinze, G.; et al. State of the Art in Selection of Variables and Functional Forms in Multivariable Analysis—Outstanding Issues. Diagn. Progn. Res. 2020, 4, 3. [Google Scholar] [CrossRef] [PubMed]
- Welch, M.L.; McIntosh, C.; Haibe-Kains, B.; Milosevic, M.F.; Wee, L.; Dekker, A.; Huang, S.H.; Purdie, T.G.; O’Sullivan, B.; Aerts, H.J.W.L.; et al. Vulnerabilities of Radiomic Signature Development: The need for safeguards. Radiother Oncol. 2019, 130, 2–9. [Google Scholar] [CrossRef]
- Abeshouse, A.; Adebamowo, C.; Adebamowo, S.N.; Akbani, R.; Akeredolu, T.; Ally, A.; Anderson, M.L.; Anur, P.; Appelbaum, E.L.; Armenia, J.; et al. Comprehensive and Integrated Genomic Characterization of Adult Soft Tissue Sarcomas. Cell 2017, 171, 950–965.e28. [Google Scholar] [CrossRef] [PubMed]
- D’Angelo, S.P.; Antonescu, C.R. Targeted Therapies for Sarcomas: New Frontiers in Precision Medicine. J. Mol. Diagn. 2021, 23, 385–394. [Google Scholar] [CrossRef] [PubMed]
- Sayles, L.C.; Breese, M.R.; Koehne, A.L.; Leung, S.G.; Lee, A.G.; Liu, H.Y.; Spillinger, A.; Shah, A.T.; Tanasa, B.; Straessler, K.M.; et al. Genome-Informed Targeted Therapy for Osteosarcoma. Cancer Discov. 2019, 9, 46–63. [Google Scholar] [CrossRef] [PubMed]
- Sheffield, N.C.; Pierron, G.; Klughammer, J.; Datlinger, P.; Schönegger, A.; Schuster, M.; Hadler, J.; Surdez, D.; Guillemot, D.; Lapouble, E.; et al. DNA Methylation Heterogeneity Defines a Disease Spectrum in Ewing Sarcoma. Nat. Med. 2017, 23, 386–395. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Bahrami, A.; Pappo, A.; Easton, J.; Dalton, J.; Hedlund, E.; Ellison, D.; Shurtleff, S.; Wu, G.; Wei, L.; et al. Recurrent Somatic Structural Variations Contribute to Tumorigenesis in Pediatric Osteosarcoma. Cell Rep. 2014, 7, 104–112. [Google Scholar] [CrossRef] [PubMed]
- Sadikovic, B.; Yoshimoto, M.; Chilton-MacNeill, S.; Thorner, P.; Squire, J.A.; Zielenska, M. Identification of Interactive Networks of Gene Expression Associated with Osteosarcoma Oncogenesis by Integrated Molecular Profiling. Hum. Mol. Genet. 2009, 18, 1962–1975. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.C.; Beird, H.C.; Andrew Livingston, J.; Advani, S.; Akdemir, K.C.; Mitra, A.; Kim, S.; Ingram, D.R.; Wang, W.L.; Lazar, A.J.; et al. Immuno-Genomic Landscape of Osteosarcoma. Front. Immunol. 2020, 11, 1922. [Google Scholar] [CrossRef] [PubMed]
- Riggi, N.; Knoechel, B.; Gillespie, S.M.; Rheinbay, E.; Boulay, G.; Suvà, M.L.; Rossetti, N.E.; Boonseng, W.E.; Oksuz, O.; Cook, E.B.; et al. EWS-FLI1 Utilizes Divergent Chromatin Remodeling Mechanisms to Directly Activate or Repress Enhancer Elements in Ewing Sarcoma. Cancer Cell 2014, 26, 668–681. [Google Scholar] [CrossRef] [PubMed]
- Gorkin, D.U.; Barozzi, I.; Zhao, Y.; Zhang, Y.; Huang, H.; Lee, A.Y.; Li, B.; Chiou, J.; Wildberg, A.; Ding, B.; et al. An Atlas of Dynamic Chromatin Landscapes in Mouse Fetal Development. Nature 2020, 583, 744–751. [Google Scholar] [CrossRef] [PubMed]
- Orrapin, S.; Moonmuang, S.; Udomruk, S.; Yongpitakwattana, P.; Pruksakorn, D.; Chaiyawat, P. Unlocking the Tumor-Immune Microenvironment in Osteosarcoma: Insights into the Immune Landscape and Mechanisms. Front. Immunol. 2024, 15, 1394284. [Google Scholar] [CrossRef] [PubMed]
- Tatsuno, R.; Komohara, Y.; Pan, C.; Kawasaki, T.; Enomoto, A.; Jubashi, T.; Kono, H.; Wako, M.; Ashizawa, T.; Haro, H.; et al. Surface Markers and Chemokines/Cytokines of Tumor-Associated Macrophages in Osteosarcoma and Other Carcinoma Microenvironments—Contradictions and Comparisons. Cancers 2024, 16, 2801. [Google Scholar] [CrossRef] [PubMed]
- Gong, L.; Sun, X.; Jia, M. New Gene Signature from the Dominant Infiltration Immune Cell Type in Osteosarcoma Predicts Overall Survival. Sci. Rep. 2023, 13, 18271. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Zhao, L.; Xiao, Y.; Xie, S.; Long, Y.; Wei, Y.; Meng, Q.; Li, X.; Luo, H.; Zhu, H. The Expression of Cytokine Profiles and Related Receptors in Idiopathic Inflammatory Myopathies. Front. Pharmacol. 2022, 13, 852055. [Google Scholar] [CrossRef] [PubMed]
- Kany, S.; Vollrath, J.T.; Relja, B. Cytokines in Inflammatory Disease. Int. J. Mol. Sci. 2019, 20, 6008. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kartikasari, A.E.R.; Huertas, C.S.; Mitchell, A.; Plebanski, M. Tumor-Induced Inflammatory Cytokines and the Emerging Diagnostic Devices for Cancer Detection and Prognosis. Front. Oncol. 2021, 11, 692142. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Italiano, A.; Mathoulin-Pelissier, S.; Le Cesne, A.; Terrier, P.; Bonvalot, S.; Collin, F.; Michels, J.J.; Blay, J.Y.; Coindre, J.M.; Bui, B.N. Trends in Survival for Patients with Metastatic Soft-Tissue Sarcoma. Cancer 2011, 117, 1049–1054. [Google Scholar] [CrossRef] [PubMed]
- Davis, L.E.; Jeng, S.; Svalina, M.N.; Huang, E.; Pittsenbarger, J.; Cantor, E.L.; Berlow, N.; Seguin, B.; Mansoor, A.; McWeeney, S.K.; et al. Integration of genomic, transcriptomic and functional profiles of aggressive osteosarcomas across multiple species. Oncotarget 2017, 8, 76241–76256. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Frazzette, N.; Jour, G. Novel Molecular Methods in Soft Tissue Sarcomas: From Diagnostics to Theragnostics. Cancers 2025, 17, 1215. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zou, Z.; Sun, W.; Xu, Y.; Liu, W.; Zhong, J.; Lin, X.; Chen, Y. Application of Multi-Omics Approach in Sarcomas: A Tool for Studying Mechanism, Biomarkers, and Therapeutic Targets. Front. Oncol. 2022, 12, 946022. [Google Scholar] [CrossRef]
- Gooding, S.; Wang, C.-Y.; Baker, W.; Watson, E.; Davis, S.; Fischer, R.; McCallion, O.; Hester, J.; Issa, F.; Rao, S.; et al. Development of a Spatial Multiomics Platform to Integrate Genomic, Transcriptomic and Proteomic Features for Translational Research in Multiple Myeloma. Blood 2024, 144 (Suppl. S1), 6848. [Google Scholar] [CrossRef]
- He, W.; Huang, W.; Zhang, L.; Wu, X.; Zhang, S.; Zhang, B. Radiogenomics: Bridging the gap between imaging and genomics for precision oncology. MedComm. (2020) 2024, 5, e722. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yoo, C.; Jeong, H.; Jeong, J.H.; Kim, K.P.; Lee, S.; Ryoo, B.Y.; Hwang, D.W.; Lee, J.H.; Moon, D.B.; Kim, K.H.; et al. Circulating tumor DNA status and dynamics predict recurrence in patients with resected extrahepatic cholangiocarcinoma. J. Hepatol. 2025, 82, 861–870. [Google Scholar] [CrossRef] [PubMed]
- Sharpe, M.; Mount, N. Genetically Modified T Cells in Cancer Therapy: Opportunities and Challenges. Dis. Model. Mech. 2015, 8, 337–350. [Google Scholar] [CrossRef] [PubMed]
- Su, G.H.; Xiao, Y.; You, C.; Zheng, R.C.; Zhao, S.; Sun, S.Y.; Zhou, J.Y.; Lin, L.Y.; Wang, H.; Shao, Z.M.; et al. Radiogenomic-Based Multiomic Analysis Reveals Imaging Intratumor Heterogeneity Phenotypes and Therapeutic Targets. Sci. Adv. 2023, 9, eadf0837. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Leung, A.; Echegaray, S.; Gentles, A.; Shrager, J.B.; Jensen, K.C.; Berry, G.J.; Plevritis, S.K.; Rubin, D.L.; Napel, S.; et al. Non-Small Cell Lung Cancer Radiogenomics Map Identifies Relationships between Molecular and Imaging Phenotypes with Prognostic Implications. Radiology 2018, 286, 307–315. [Google Scholar] [CrossRef] [PubMed]
- O’Sullivan, N.J.; Temperley, H.C.; Horan, M.T.; Curtain, B.M.M.; O’Neill, M.; Donohoe, C.; Ravi, N.; Corr, A.; Meaney, J.F.M.; Reynolds, J.V.; et al. Computed Tomography (CT) Derived Radiomics to Predict Post-Operative Disease Recurrence in Gastric Cancer: A Systematic Review and Meta-Analysis. Curr. Probl. Diagn. Radiol. 2024, 53, 717–722. [Google Scholar] [CrossRef] [PubMed]
- Choi, W.; Oh, J.H.; Riyahi, S.; Liu, C.J.; Feng, F.; Chen, W.; Huang, C.; Lu, W.; Saha, P.K.; Torigian, D.A.; et al. Radiologic and Genomic Evolution of Individual Metastases during HER2 Blockade in Colorectal Cancer. Cancer Cell 2018, 34, 148–162.e7. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Deng, Y.; Jiang, W.; Li, H.; Luo, Y.; Zhang, H.; Wu, H. Single Cell Transcriptomic Analysis Reveals Tumor Immune Infiltration by Macrophage Cells Gene Signature in Lung Adenocarcinoma. Discov. Oncol. 2025, 16, 261. [Google Scholar] [CrossRef] [PubMed]
- Spratt, D.E.; Alshalalfa, M.; Fishbane, N.; Weiner, A.B.; Mehra, R.; Mahal, B.A.; Lehrer, J.; Liu, Y.; Zhao, S.G.; Speers, C.; et al. Transcriptomic Heterogeneity of Gleason Grade Group 5 Prostate Cancer. Eur. Urol. 2020, 78, 327–332. [Google Scholar] [CrossRef] [PubMed]
- Gibault, L.; Pérot, G.; Chibon, F.; Filhine-Trésarrieu, P.; Laplanche, A.; Michon, J.; Coindre, J.M.; Lagarde, P.; Terrier, P.; Mauduit, O.; et al. New Insights into the Prognostic Value of Genomic Classification in Synovial Sarcoma. Eur. J. Cancer 2024, 203, 114048. [Google Scholar] [CrossRef] [PubMed]
- Weigelt, B.; Vargas, H.A.; Selenica, P.; Geyer, F.C.; Mazaheri, Y.; Blecua, P.; Conlon, N.; Hoang, L.N.; Jungbluth, A.A.; Snyder, A.; et al. Radiogenomics Analysis of Intratumor Heterogeneity in a Patient with High-Grade Serous Ovarian Cancer. JCO Precis. Oncol. 2019, 3, PO1800410. [Google Scholar] [CrossRef] [PubMed]
- Gitto, S.; Cuocolo, R.; Huisman, M.; Messina, C.; Albano, D.; Omoumi, P.; Kotter, E.; Maas, M.; Van Ooijen, P.; Sconfienza, L.M. CT and MRI Radiomics of Bone and Soft-Tissue Sarcomas: An Updated Systematic Review of Reproducibility and Validation Strategies. Insights Imaging 2024, 15, 54. [Google Scholar] [CrossRef] [PubMed]
- Peeken, J.C.; Spraker, M.B.; Knebel, C.; Dapper, H.; Pfeiffer, D.; Devecka, M.; Thamer, A.; Shouman, M.A.; Ott, A.; von Eisenhart-Rothe, R.; et al. Tumor Grading of Soft Tissue Sarcomas Using MRI-Based Radiomics. EBioMedicine 2019, 48, 332–340. [Google Scholar] [CrossRef] [PubMed]
- Xie, W.; Reder, N.P.; Koyuncu, C.; Leo, P.; Hawley, S.; Huang, H.; Mao, C.; Postupna, N.; Kang, S.; Serafin, R.; et al. Prostate Cancer Risk Stratification via Nondestructive 3D Pathology with Deep Learning-Assisted Gland Analysis. Cancer Res. 2022, 82, 334–345. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lafata, K.J.; Corradetti, M.N.; Gao, J.; Jacobs, C.D.; Weng, J.; Chang, Y.; Wang, C.; Hatch, A.; Xanthopoulos, E.; Jones, G.; et al. Radiogenomic Analysis of Locally Advanced Lung Cancer Based on CT Imaging and Intratreatment Changes in Cell-Free DNA. Radiol. Imaging Cancer 2021, 3, e200157. [Google Scholar] [CrossRef] [PubMed]
- Mendes Serrão, E.; Klug, M.; Moloney, B.M.; Jhaveri, A.; Lo Gullo, R.; Pinker, K.; Luker, G.; Haider, M.A.; Shinagare, A.B.; Liu, X. Current Status of Cancer Genomics and Imaging Phenotypes: What Radiologists Need to Know. Radiol. Imaging Cancer 2023, 5, e220153. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yamamoto, S.; Maki, D.D.; Korn, R.L.; Kuo, M. Radiogenomic Analysis of Breast Cancer Using MRI: A Preliminary Study to Define the Landscape. Am. J. Roentgenol. 2012, 199, 654–663. [Google Scholar] [CrossRef]
- Fan, M.; Xia, P.; Clarke, R.; Wang, Y.; Li, L. Radiogenomic signatures reveal multiscale intratumour heterogeneity associated with biological functions and survival in breast cancer. Nat. Commun. 2020, 11, 4861. [Google Scholar] [CrossRef]
- Tian, H.; Cao, J.; Li, B.; Nice, E.C.; Mao, H.; Zhang, Y.; Huang, C. Managing the Immune Microenvironment of Osteosarcoma: The Outlook for Osteosarcoma Treatment. Bone Res. 2023, 11, 11. [Google Scholar] [CrossRef] [PubMed]
- Zeng, J.; Wang, D.; Tong, Z.; Li, Z.; Wang, G.; Du, Y.; Li, J.; Miao, J.; Chen, S. Development of a Prognostic Model for Osteosarcoma Based on Macrophage Polarization-Related Genes Using Machine Learning: Implications for Personalized Therapy. Clin. Exp. Med. 2025, 25, 146. [Google Scholar] [CrossRef] [PubMed]
- Jeys, L.M.; Thorne, C.J.; Parry, M.; Gaston, C.L.; Sumathi, V.P.; Grimer, J.R. A Novel System for the Surgical Staging of Primary High-Grade Osteosarcoma: The Birmingham Classification. Clin. Orthop. Relat. Res. 2017, 475, 842–850. [Google Scholar] [CrossRef] [PubMed]
- Bacci, G.; Longhi, A.; Versari, M.; Mercuri, M.; Briccoli, A.; Picci, P. Prognostic Factors for Osteosarcoma of the Extremity Treated with Neoadjuvant Chemotherapy: 15-Year Experience in 789 Patients Treated at a Single Institution. Cancer 2006, 106, 1154–1161. [Google Scholar] [CrossRef] [PubMed]
- Bielack, S.S.; Kempf-Bielack, B.; Delling, G.; Exner, G.U.; Flege, S.; Helmke, K.; Kotz, R.; Salzer-Kuntschik, M.; Werner, M.; Winkelmann, W.; et al. Prognostic Factors in High-Grade Osteosarcoma of the Extremities or Trunk: An Analysis of 1,702 Patients Treated on Neoadjuvant Cooperative Osteosarcoma Study Group Protocols. J. Clin. Oncol. 2002, 20, 776–790. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, S.; Bertoni, F.; Mercuri, M.; Picci, P.; Giacomini, S.; Longhi, A.; Bacci, G. Predictive Factors of Disease-Free Survival for Non-Metastatic Osteosarcoma of the Extremity: An. Analysis of 300 Patients Treated at the Rizzoli Institute. Ann. Oncol. 2001, 12, 1145–1150. [Google Scholar] [CrossRef] [PubMed]
- Palmerini, E.; Torricelli, E.; Cascinu, S.; Pierini, M.; De Paolis, M.; Donati, D.; Cesari, M.; Longhi, A.; Abate, M.; Paioli, A.; et al. Is There a Role for Chemotherapy after Local Relapse in High-Grade Osteosarcoma? Pediatr. Blood Cancer 2019, 66, e27792. [Google Scholar] [CrossRef] [PubMed]
- Smeland, S.; Bielack, S.S.; Whelan, J.; Bernstein, M.; Hogendoorn, P.; Krailo, M.D.; Gorlick, R.; Janeway, K.A.; Ingleby, F.C.; Anninga, J.; et al. Survival and Prognosis with Osteosarcoma: Outcomes in More than 2000 Patients in the EURAMOS-1 (European and American Osteosarcoma Study) Cohort. Eur. J. Cancer 2019, 109, 36–50. [Google Scholar] [CrossRef] [PubMed]
- Whelan, J.S.; Jinks, R.C.; McTiernan, A.; Sydes, M.R.; Hook, J.M.; Trani, L.; Uscinska, B.; Bramwell, V.; Lewis, I.J.; Nooij, M.A.; et al. Survival from High-Grade Localised Extremity Osteosarcoma: Combined Results and Prognostic Factors from Three European Osteosarcoma Intergroup Randomised Controlled Trials. Ann. Oncol. 2012, 23, 1607–1616. [Google Scholar] [CrossRef] [PubMed]
- Strauss, S.J.; Frezza, A.M.; Abecassis, N.; Bajpai, J.; Bauer, S.; Biagini, R.; Bielack, S.; Blay, J.Y.; Bolle, S.; Bonvalot, S.; et al. ESMO Guidelines Committee, EURACAN, GENTURIS, and ERN PaedCan. Bone Sarcomas: ESMO–EURACAN–GENTURIS–ERN PaedCan Clinical Practice Guideline for Diagnosis, Treatment and Follow-Up. Ann. Oncol. 2021, 32, 1520–1536. [Google Scholar] [CrossRef] [PubMed]
- Isakoff, M.S.; Bielack, S.S.; Meltzer, P.; Gorlick, R. Osteosarcoma: Current Treatment and a Collaborative Pathway to Success. J. Clin. Oncol. 2015, 33, 3029–3035. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Gokavarapu, S.; Shen, L.; Liu, F.; Cao, W.; Ling, Y.; Ji, T. Chemotherapy in Head and Neck Osteosarcoma: A Retrospective Analysis of 27 Patients from a Single Institution. Oral Oncol. 2017, 74, 155–160. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Xu, Y.; Liu, W.; Zhang, J.; Wang, F.; Jian, Q.; Huang, G.; Zou, C.; Xie, X.; Kim, A.H.; et al. Tumor-Informed Deep Sequencing of ctDNA Detects Minimal Residual Disease and Predicts Relapse in Osteosarcoma. EClinicalMedicine 2024, 73, 102697. [Google Scholar] [CrossRef] [PubMed]
- Meltzer, P.S.; Helman, L.J. New Horizons in the Treatment of Osteosarcoma. N. Engl. J. Med. 2021, 385, 2066–2076. [Google Scholar] [CrossRef] [PubMed]
- Lilienthal, I.; Herold, N. Targeting Molecular Mechanisms Underlying Treatment Efficacy and Resistance in Osteosarcoma: A Review of Current and Future Strategies. Int. J. Mol. Sci. 2020, 21, 6885. [Google Scholar] [CrossRef] [PubMed]
- Eaton, B.R.; Schwarz, R.; Vatner, R.; Yeh, B.; Claude, L.; Indelicato, D.J.; Laack, N. Osteosarcoma. Pediatr. Blood Cancer 2021, 68, e28352. [Google Scholar] [CrossRef] [PubMed]
- Ritter, J.; Bielack, S.S. Osteosarcoma. Ann. Oncol. 2010, 21, vii320–vii325. [Google Scholar] [CrossRef] [PubMed]
- Kansara, M.; Teng, M.W.; Smyth, M.J.; Thomas, D.M. Translational Biology of Osteosarcoma. Nat. Rev. Cancer 2014, 14, 722–735. [Google Scholar] [CrossRef] [PubMed]
- File:SVM explain.png. Wikimedia Commons. Published 22 February 2022. Available online: https://commons.wikimedia.org/w/index.php?title=File:SVM_explain.png&oldid=631507021 (accessed on 20 May 2025).
- File:Random forest explain.png. Wikimedia Commons. Published 20 November 2021. Available online: https://commons.wikimedia.org/w/index.php?title=File:Random_forest_explain.png&oldid=608403035 (accessed on 20 May 2025).
- Anderson, M.E. Update on Survival in Osteosarcoma. Orthop. Clin. N. Am. 2016, 47, 283–292. [Google Scholar] [CrossRef] [PubMed]
- Meazza, C.; Scanagatta, P. Metastatic Osteosarcoma: A Challenging Multidisciplinary Treatment. Expert. Rev. Anticancer Ther. 2016, 16, 543–556. [Google Scholar] [CrossRef] [PubMed]
- Harrison, D.J.; Geller, D.S.; Gill, J.D.; Lewis, V.O.; Gorlick, R. Current and Future Therapeutic Approaches for Osteosarcoma. Expert. Rev. Anticancer Ther. 2018, 18, 39–50. [Google Scholar] [CrossRef] [PubMed]
- Kager, L.; Tamamyan, G.; Bielack, S. Novel Insights and Therapeutic Interventions for Pediatric Osteosarcoma. Future Oncol. 2017, 13, 357–368. [Google Scholar] [CrossRef] [PubMed]
- van Maldegem, A.M.; Bhosale, A.; Gelderblom, H.J.; Hogendoorn, P.C.; Hassan, A.B. Comprehensive Analysis of Published Phase I/II Clinical Trials for Osteosarcoma and Ewing Sarcoma. Eur. J. Cancer 2016, 65, 89–99. [Google Scholar] [CrossRef] [PubMed]
- Lagmay, J.P.; Krailo, M.D.; Dang, H.; Kim, A.; Hawkins, D.S.; Beaty, O.; Widemann, B.C.; Zwerdling, T.; Bomgaars, L.; Langevin, A.M.; et al. Outcome of Patients with Recurrent Osteosarcoma Enrolled in Seven Phase II Trials through Children’s Cancer Group, Pediatric Oncology Group, and Children’s Oncology Group. Pediatr. Blood Cancer 2016, 63, 1748–1754. [Google Scholar] [CrossRef] [PubMed]
- “File:Feature Learning Diagram.png.” Wikimedia Commons. 9 October 2022. Available online: https://commons.wikimedia.org/w/index.php?title=File:Feature_Learning_Diagram.png&oldid=695252798 (accessed on 20 May 2025).
- Omer, N.; Le Deley, M.C.; Piperno-Neumann, S.; Marec-Berard, P.; Italiano, A.; Corradini, N.; Bellera, C.; Brugières, L.; Gaspar, N. Phase-II Trials in Osteosarcoma Recurrences: A Systematic Review of Past Experience. Eur. J. Cancer 2017, 75, 98–108. [Google Scholar] [CrossRef] [PubMed]
- Benjamin, R.S. Osteosarcoma: Better Treatment through Better Trial Design. Lancet Oncol. 2015, 16, 12–13. [Google Scholar] [CrossRef] [PubMed]
- Meyers, P.A.; Schwartz, C.L.; Krailo, M.D.; Healey, J.H.; Bernstein, M.L.; Betcher, D.; Ferguson, W.S.; Gebhardt, M.C.; Goorin, A.M.; Harris, M.; et al. Children’s Oncology Group Osteosarcoma: The Addition of Muramyl Tripeptide to Chemotherapy Improves Overall Survival—AReport from the Children’s Oncology Group. J. Clin. Oncol. 2008, 26, 633–638. [Google Scholar] [CrossRef] [PubMed]
- Gaspar, N.; Hawkins, D.S.; Dirksen, U.; Lewis, I.J.; Ferrari, S.; Le Deley, M.C.; Kovar, H.; Grimer, R.; Whelan, J.; Claude, L.; et al. Ewing Sarcoma: Current Management and Future Approaches through Collaboration. J. Clin. Oncol. 2015, 33, 3036–3046. [Google Scholar] [CrossRef] [PubMed]
- Womer, R.B.; West, D.C.; Krailo, M.D.; Dickman, P.S.; Pawel, B.R.; Grier, H.E.; Marcus, K.; Sailer, S.; Healey, J.H.; Dormans, J.P.; et al. Children’s Oncology Group Randomized Controlled Trial of Interval-Compressed Chemotherapy for the Treatment of Localized Ewing Sarcoma: AReport from the Children’s Oncology Group. J. Clin. Oncol. 2012, 30, 4148–4154. [Google Scholar] [CrossRef] [PubMed]
- Granowetter, L.; Womer, R.; Devidas, M.; Krailo, M.; Wang, C.; Bernstein, M.; Marina, N.; Leavey, P.; Gebhardt, M.; Healey, J.; et al. Dose-Intensified Compared with Standard Chemotherapy for Nonmetastatic Ewing Sarcoma Family of Tumors: A Children’s Oncology Group Study. J. Clin. Oncol. 2009, 27, 2536–2541. [Google Scholar] [CrossRef] [PubMed]
- Grier, H.E.; Krailo, M.D.; Tarbell, N.J.; Link, M.P.; Fryer, C.J.; Pritchard, D.J.; Gebhardt, M.C.; Dickman, P.S.; Perlman, E.J.; Meyers, P.A.; et al. Addition of Ifosfamide and Etoposide to Standard Chemotherapy for Ewing’s Sarcoma and Primitive Neuroectodermal Tumor of Bone. N. Engl. J. Med. 2003, 348, 694–701. [Google Scholar] [CrossRef] [PubMed]
- Casali, P.G.; Bielack, S.; Abecassis, N.; Aro, H.T.; Bauer, S.; Biagini, R.; Bonvalot, S.; Boukovinas, I.; Bovee, J.V.M.G.; Brennan, B.; et al. Bone Sarcomas: ESMO-PaedCan-EURACAN Clinical Practice Guidelines for Diagnosis, Treatment and Follow-Up. Ann. Oncol. 2018, 29, iv79–iv95. [Google Scholar] [CrossRef] [PubMed]
- Paladugu, P.; Kumar, R.; Ong, J.; Waisberg, E.; Sporn, K. Virtual Reality-Enhanced Rehabilitation for Improving Musculoskeletal Function and Recovery after Trauma. J. Orthop. Surg. Res. 2025, 20, 404. [Google Scholar] [CrossRef] [PubMed]
- Cotterill, S.J.; Ahrens, S.; Paulussen, M.; Jürgens, H.F.; Voûte, P.A.; Gadner, H.; Craft, A.W. Prognostic Factors in Ewing’s Sarcoma of Bone: Analysis of 975 Patients from the European Intergroup Cooperative Ewing’s Sarcoma Study Group. J. Clin. Oncol. 2000, 18, 3108–3114. [Google Scholar] [CrossRef] [PubMed]
- Oberlin, O.; Deley, M.C.L.; Bui, B.N.; Gentet, J.C.; Philip, T.; Terrier, P.; Carrie, C.; Mechinaud, F.; Schmitt, C.; Babin-Boillettot, A.; et al. French Society of Paediatric Oncology Prognostic Factors in Localized Ewing’s Tumours Peripheral Neuroectodermal Tumours: The Third Study of the French Society of Paediatric Oncology (EW88 Study). Br. J. Cancer 2001, 85, 1646–1654. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Galindo, C.; Liu, T.; Krasin, M.J.; Wu, J.; Billups, C.A.; Daw, N.C.; Spunt, S.L.; Arndt, C.; Santana, V.M.; Navid, F. Analysis of Prognostic Factors in Ewing Sarcoma Family of Tumors: Review of St. Jude Children’s Research Hospital Studies. Cancer 2007, 110, 375–384. [Google Scholar] [CrossRef] [PubMed]
- Averaged Chest Radiographs for all Participants and Heatmap of Regions’ Association with Aging (Saliency Maps from the External Test Dataset) [Internet]. Wikimedia Commons. 21 September 2024. Available online: https://commons.wikimedia.org/w/index.php?title=File:Averaged_chest_radiographs_for_all_participants_and_heatmap_of_regions%27_association_with_aging_(saliency_maps_from_the_external_test_dataset).jpg&oldid=926962397 (accessed on 20 May 2025).
- Ladenstein, R.; Pötschger, U.; Le Deley, M.C.; Whelan, J.; Paulussen, M.; Oberlin, O.; van den Berg, H.; Dirksen, U.; Hjorth, L.; Michon, J.; et al. Primary Disseminated Multifocal Ewing Sarcoma: Results of the Euro-EWING 99 Trial. J. Clin. Oncol. 2010, 28, 3284–3291. [Google Scholar] [CrossRef] [PubMed]
- Fritz, B.; Yi, P.H.; Kijowski, R.; Fritz, J. Radiomics and Deep Learning for Disease Detection in Musculoskeletal Radiology: An Overview of Novel MRI- and CT-Based Approaches. Investig. Radiol. 2023, 58, 3–13. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Sun, M.; Sun, J.; Wang, Q.; Wang, G.; Wang, X.; Meng, X.; Wang, Z.; Yu, H. Advancing musculoskeletal tumor diagnosis: Automated segmentation and predictive classification using deep learning and radiomics. Comput. Biol. Med. 2024, 175, 108502. [Google Scholar] [CrossRef] [PubMed]
- Lemore, A.; Vogt, N.; Oster, J.; Germain, E.; Fauvel, M.; Gillet, R.; Sirveaux, F.; Marie, B.; Sans, N.; Faruch, M.; et al. Enhanced CT and MRI Focal Bone Tumor Classification with Machine Learning–based Stratification: A Multicenter Retrospective Study. Radiology 2025, 315, e232834. [Google Scholar] [CrossRef]
- Wang, H.; He, Y.; Wan, L.; Li, C.; Li, Z.; Li, Z.; Xu, H.; Tu, C. Deep learning models in classifying primary bone tumors and bone infections based on radiographs. npj Precis. Oncol. 2025, 9, 72. [Google Scholar] [CrossRef]
- von Schacky, C.E.; Wilhelm, N.J.; Schäfer, V.S.; Leonhardt, Y.; Gassert, F.G.; Foreman, S.C.; Gassert, F.T.; Jung, M.; Jungmann, P.M.; Russe, M.F.; et al. Multitask Deep Learning for Segmentation and Classification of Primary Bone Tumors on Radiographs. Radiology 2021, 301, 398–406. [Google Scholar] [CrossRef]
- Leavey, P.J.; Mascarenhas, L.; Marina, N.; Chen, Z.; Krailo, M.; Miser, J.; Brown, K.; Tarbell, N.; Bernstein, M.L.; Granowetter, L.; et al. Prognostic Factors for Patients with Ewing Sarcoma (EWS) at First Recurrence Following Multi-Modality Therapy: A Report from the Children’s Oncology Group. Pediatr. Blood Cancer 2008, 51, 334–338. [Google Scholar] [CrossRef] [PubMed]
- Barker, L.M.; Pendergrass, T.W.; Sanders, J.E.; Hawkins, D.S. Survival after Recurrence of Ewing’s Sarcoma Family of Tumors. J. Clin. Oncol. 2005, 23, 4354–4362. [Google Scholar] [CrossRef] [PubMed]
- Stahl, M.; Ranft, A.; Paulussen, M.; Bölling, T.; Vieth, V.; Bielack, S.; Görtitz, I.; Braun-Munzinger, G.; Hardes, J.; Jürgens, H.; et al. Risk of Recurrence and Survival after Relapse in Patients with Ewing Sarcoma. Pediatr. Blood Cancer 2011, 57, 549–553. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, S.; Luksch, R.; Sundby Hall, K.; Fagioli, F.; Prete, A.; Tamburini, A.; Tienghi, A.; Erba, P.; Melchionda, F.; Di Giulio, G.; et al. Post-Relapse Survival in Patients with Ewing Sarcoma. Pediatr. Blood Cancer 2015, 62, 994–999. [Google Scholar] [CrossRef] [PubMed]
- Bacci, G.; Ferrari, S.; Longhi, A.; Donati, D.; De Paolis, M.; Forni, C.; Versari, M.; Setola, E.; Briccoli, A.; Barbieri, E. Therapy and Survival after Recurrence of Ewing’s Tumors: The Rizzoli Experience in 195 Patients Treated with Adjuvant and Neoadjuvant Chemotherapy from 1979 to 1997. Ann. Oncol. 2003, 14, 1654–1659. [Google Scholar] [CrossRef] [PubMed]
- McTiernan, A.; Driver, D.; Michelagnoli, M.P.; Kilby, A.M.; Whelan, J.S. High Dose Chemotherapy with Bone Marrow or Peripheral Stem Cell Rescue Is an Effective Treatment Option for Patients with Relapsed or Progressive Ewing’s Sarcoma Family of Tumours. Ann. Oncol. 2006, 17, 1301–1305. [Google Scholar] [CrossRef] [PubMed]
- Gardner, S.L.; Carreras, J.; Bjerre, J.; Cheung, N.K.; Dunkel, I.J.; Finlay, J.; Gardner, S.; Goldman, S.; Grant, G.; Kalapurakal, J.; et al. Phase II Study of Oral Capecitabine in Children with Relapsed/Refractory Ewing Sarcoma or Osteosarcoma: A Report from the Children’s Oncology Group (COG). J. Clin. Oncol. 2010, 28, 9546. [Google Scholar] [CrossRef]
- Fox, E.; Patel, S.; Wathen, J.K.; Schuetze, S.; Chawla, S.; Harmon, D.; Reinke, D.; Chugh, R.; Benjamin, R.S.; Helman, L.J. Phase II Study of Sequential Gemcitabine Followed by Docetaxel for Recurrent Ewing Sarcoma, Osteosarcoma, or Unresectable or Locally Recurrent Chondrosarcoma: Results of Sarcoma Alliance for Research Through Collaboration Study 003. Oncologist 2012, 17, 321. [Google Scholar] [CrossRef] [PubMed]
- Casey, D.A.; Wexler, L.H.; Merchant, M.S.; Chou, A.J.; Merola, P.R.; Price, A.P.; Meyers, P.A. Irinotecan and Temozolomide for Ewing Sarcoma: The Memorial Sloan-Kettering Experience. Pediatr. Blood Cancer 2009, 53, 1029–1034. [Google Scholar] [CrossRef] [PubMed]
- Schafer, E.S.; Rau, R.E.; Berg, S.; Liu, X.; Minard, C.G.; D’Adamo, D.; Scott, R.; Reyderman, L.; Martinez, G.; Devarajan, S.; et al. A Phase 1 Study of Eribulin Mesylate (E7389), a Novel Microtubule-Targeting Chemotherapeutic Agent, in Children with Refractory or Recurrent Solid Tumors: A Children’s Oncology Group Phase 1 Consortium Study (ADVL1314). Pediatr. Blood Cancer 2018, 65, e27066. [Google Scholar] [CrossRef] [PubMed]
- Grignani, G.; Palmerini, E.; Dileo, P.; Asaftei, S.D.; D’Ambrosio, L.; Pignochino, Y.; Mercuri, M.; Picci, P.; Fagioli, F.; Casali, P.G.; et al. A Phase II Trial of Sorafenib in Relapsed and Unresectable High-Grade Osteosarcoma after Failure of Standard Multimodal Therapy: An Italian Sarcoma Group Study. Ann. Oncol. 2012, 23, 508–516. [Google Scholar] [CrossRef] [PubMed]
- Duffaud, F.; Mir, O.; Boudou-Rouquette, P.; Piperno-Neumann, S.; Penel, N.; Bompas, E.; Delcambre, C.; Kalbacher, E.; Italiano, A.; Collard, O.; et al. Efficacy and Safety of Regorafenib in Adult Patients with Metastatic Osteosarcoma: A Non-Comparative, Randomised, Double-Blind, Placebo-Controlled, Phase 2 Study. Lancet Oncol. 2019, 20, 120–133. [Google Scholar] [CrossRef] [PubMed]
- Davis, L.E.; Bolejack, V.; Ryan, C.W.; Ganjoo, K.N.; Loggers, E.T.; Chawla, S.; Agulnik, M.; Livingston, M.B.; Park, S.; Reed, D.R.; et al. Randomized Double-Blind Phase II Study of Regorafenib in Patients with Metastatic Osteosarcoma. J. Clin. Oncol. 2019, 37, 1424–1431. [Google Scholar] [CrossRef] [PubMed]
- Wedekind, M.F.; Wagner, L.M.; Cripe, T.P. Immunotherapy for Osteosarcoma: Where Do We Go from Here? Pediatr. Blood Cancer 2018, 65, e27227. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, N.; Brawley, V.S.; Hegde, M.; Robertson, C.; Ghazi, A.; Gerken, C.; Liu, E.; Dakhova, O.; Ashoori, A.; Corder, A.; et al. Human Epidermal Growth Factor Receptor 2 (HER2)–Specific Chimeric Antigen Receptor–Modified T Cells for the Immunotherapy of HER2-Positive Sarcoma. J. Clin. Oncol. 2015, 33, 1688–1696. [Google Scholar] [CrossRef] [PubMed]
- Merchant, M.S.; Wright, M.; Baird, K.; Wexler, L.H.; Rodriguez-Galindo, C.; Bernstein, D.; Delbrook, C.; Lodish, M.; Bishop, R.; Wolchok, J.D.; et al. Phase I Clinical Trial of Ipilimumab in Pediatric Patients with Advanced Solid Tumors. Clin. Cancer Res. 2016, 22, 1364–1370. [Google Scholar] [CrossRef] [PubMed]
- Tawbi, H.A.; Burgess, M.; Bolejack, V.; Van Tine, B.A.; Schuetze, S.M.; Hu, J.; D’Angelo, S.; Attia, S.; Riedel, R.F.; Priebat, D.A.; et al. Pembrolizumab in Advanced Soft-Tissue Sarcoma and Bone Sarcoma (SARC028): A Multicentre, Two-Cohort, Single-Arm, Open-Label, Phase 2 Trial. Lancet Oncol. 2017, 18, 1493–1501. [Google Scholar] [CrossRef] [PubMed]
- D’Angelo, S.P.; Mahoney, M.R.; Van Tine, B.A.; Atkins, J.; Milhem, M.M.; Jahagirdar, B.N.; Antonescu, C.R.; Horvath, L.E.; Tap, W.D.; Schwartz, G.K.; et al. Nivolumab with or without Ipilimumab Treatment for Metastatic Sarcoma (Alliance A091401): Two Open-Label, Non-Comparative, Randomised, Phase 2 Trials. Lancet Oncol. 2018, 19, 416–426. [Google Scholar] [CrossRef] [PubMed]
- Le Cesne, A.; Marec-Berard, P.; Blay, J.Y.; Gaspar, N.; Bertucci, F.; Penel, N.; Bompas, E.; Cousin, S.; Toulmonde, M.; Bessede, A.; et al. Programmed Cell Death 1 (PD-1) Targeting in Patients with Advanced Osteosarcomas: Results from the PEMBROSARC Study. J. Clin. Oncol. 2018, 36, 11504. [Google Scholar] [CrossRef]
- Boye, K.; Longhi, A.; Guren, T.; Lorenz, S.; Næss, S.; Pierini, M.; Taksdal, I.; Lobmaier, I.; Cesari, M.; Paioli, A.; et al. Pembrolizumab in Advanced Osteosarcoma: Results of a Single-Arm, Open-Label, Phase 2 Trial. Cancer Immunol. Immunother. 2021, 70, 2617–2624. [Google Scholar] [CrossRef] [PubMed]
- Thanindratarn, P.; Dean, D.C.; Nelson, S.D.; Hornicek, F.J.; Duan, Z. Advances in Immune Checkpoint Inhibitors for Bone Sarcoma Therapy. J. Bone Oncol. 2019, 15, 100221. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Bui, N.; Bolourchi, S.; Lohman, M.; Elicer, O.; Dupuy, A.G.; Lizardo, M.M.; Allen, J.; Caldwell, A.; Withers, S.; et al. Targeting the Tumor Microenvironment of Ewing Sarcoma with the Combination of Dinutuximab and Trabectedin: A New Immunotherapeutic Strategy. J. Clin. Oncol. 2023, 41, 11508. [Google Scholar] [CrossRef]
- Rainusso, N.; Brawley, V.S.; Ghazi, A.; Hicks, J.; Gottschalk, S.; Rosen, J.M.; Ahmed, N.; Hegde, M.; Wu, M.F.; Liu, H.; et al. Immunotherapy Targeting HER2 with Genetically Modified T Cells Eliminates Tumor-Initiating Cells in Osteosarcoma. Cancer Gene Ther. 2012, 19, 212–217. [Google Scholar] [CrossRef] [PubMed]
- Long, A.H.; Highfill, S.L.; Cui, Y.; Smith, J.P.; Walker, A.J.; Ramakrishna, S.; El-Etriby, R.; Orentas, R.J.; Tran, T.; Mackall, C.L. Reduction of MDSCs with All-Trans Retinoic Acid Improves CAR Therapy Efficacy Against Sarcomas. J. Clin. Oncol. 2016, 34, 10506. [Google Scholar] [CrossRef]
- Majzner, R.G.; Theruvath, J.L.; Nellan, A.; Heitzeneder, S.; Cui, Y.; Mount, C.W.; Rietberg, S.P.; Linde, M.H.; Xu, P.; Rota, C.; et al. GD2-CAR T Cell Therapy for H3K27M-Mutated Diffuse Midline Gliomas. Nature 2022, 603, 934–941. [Google Scholar] [CrossRef] [PubMed]
- Hegde, M.; Joseph, S.K.; Pashankar, F.; DeRenzo, C.; Sanber, K.; Navai, S.; Byrd, T.T.; Hicks, J.; Xu, M.L.; Gerken, C.; et al. Tumor Response and Endogenous Immune Reactivity Following Administration of HER2 CAR T Cells in a Child with Metastatic Rhabdomyosarcoma. Nat. Commun. 2020, 11, 3549. [Google Scholar] [CrossRef] [PubMed]
- Navai, S.A.; Derenzo, C.; Joseph, S.; Sanber, K.; Byrd, T.; Zhang, H.; Mata, M.; Gerken, C.; Shree, A.; Mathew, P.R.; et al. Administration of HER2-Chimeric Antigen Receptor (CAR) Expressing T Cells for Sarcoma Is Safe, with Preliminary Evidence of Efficacy in HER2 Low-Expressing Tumors. J. Clin. Oncol. 2018, 36, 10507. [Google Scholar] [CrossRef]
- Chulanetra, M.; Morchang, A.; Sayour, E.; Eldjerou, L.; Milner, R.; Lagmay, J.; Slayton, W.; Ligon, J.; Simon, E.; Schafer, B.; et al. GD2 Chimeric Antigen Receptor T-Cell Therapy for Tyrosine Hydroxylase-Positive Stage 4 Neuroblastomas and Sarcomas. J. Clin. Oncol. 2020, 38, 10529. [Google Scholar] [CrossRef]
- Hingorani, P.; Janeway, K.; Crompton, B.D.; Kadoch, C.; Mackall, C.L.; Khan, J.; Shern, J.F.; Schiffman, J.; Mirabello, L.; Savage, S.A.; et al. Current State of Pediatric Sarcoma Biology and Opportunities for Future Discovery: A Report from the Sarcoma Translational Research Workshop. Cancer Genet. 2016, 209, 182–194. [Google Scholar] [CrossRef] [PubMed]
- Sporn, K.; Kumar, R.; Paladugu, P.; Ong, J.; Sekhar, T.; Vaja, S.; Hage, T.; Waisberg, E.; Gowda, C.; Jagadeesan, R.; et al. Artificial Intelligence in Orthopedic Medical Education: A Comprehensive Review of Emerging Technologies and Their Applications. Int. Med. Educ. 2025, 4, 14. [Google Scholar] [CrossRef]
- Roberts, R.D.; Lizardo, M.M.; Reed, D.R.; Hingorani, P.; Glover, J.; Allen-Rhoades, W.; Fan, T.; Khanna, C.; Sweet-Cordero, E.A.; Cash, T.; et al. Provocative Questions in Osteosarcoma Research: An Update from the Children’s Oncology Group. Cancer 2019, 125, 3514–3525. [Google Scholar] [CrossRef] [PubMed]
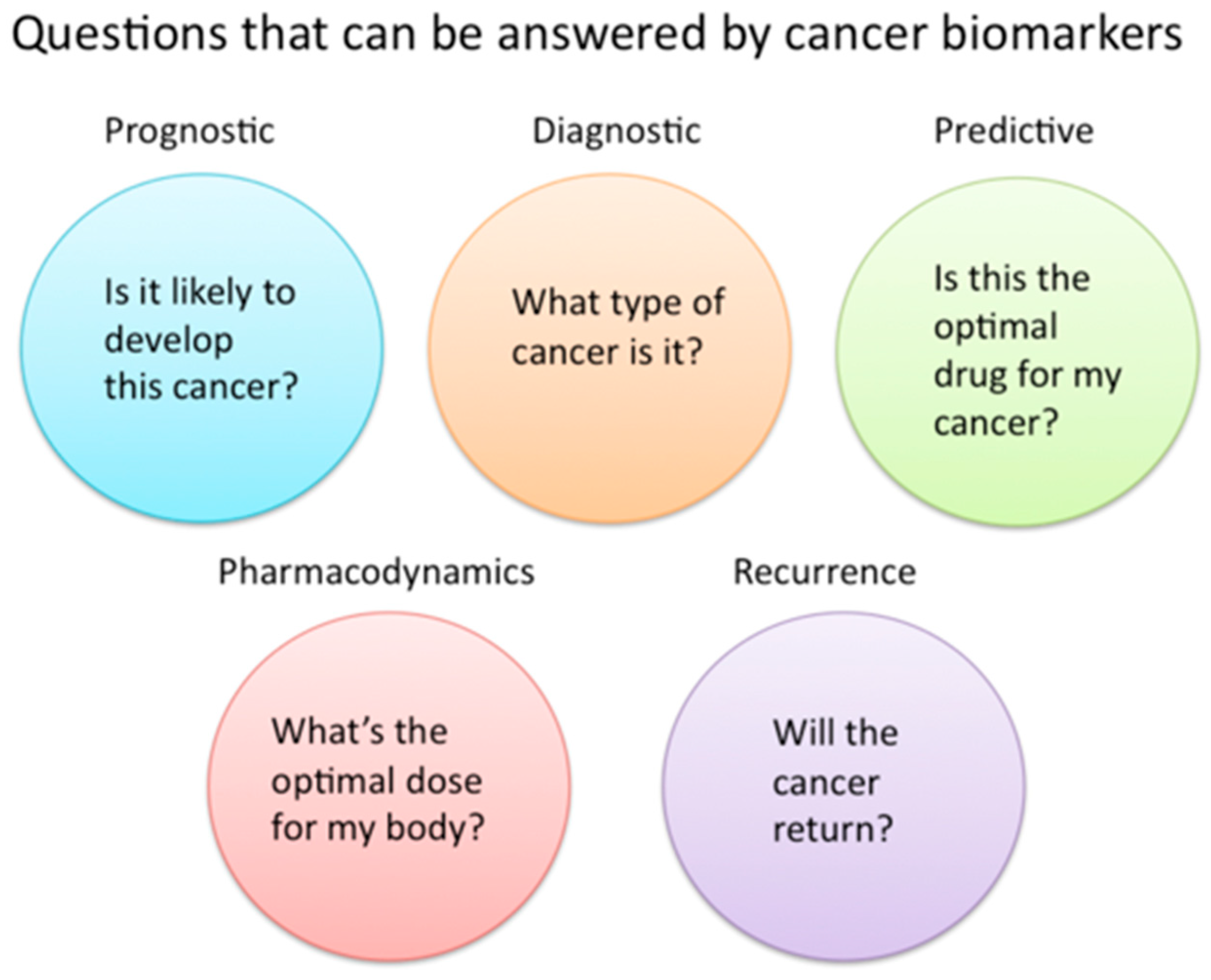
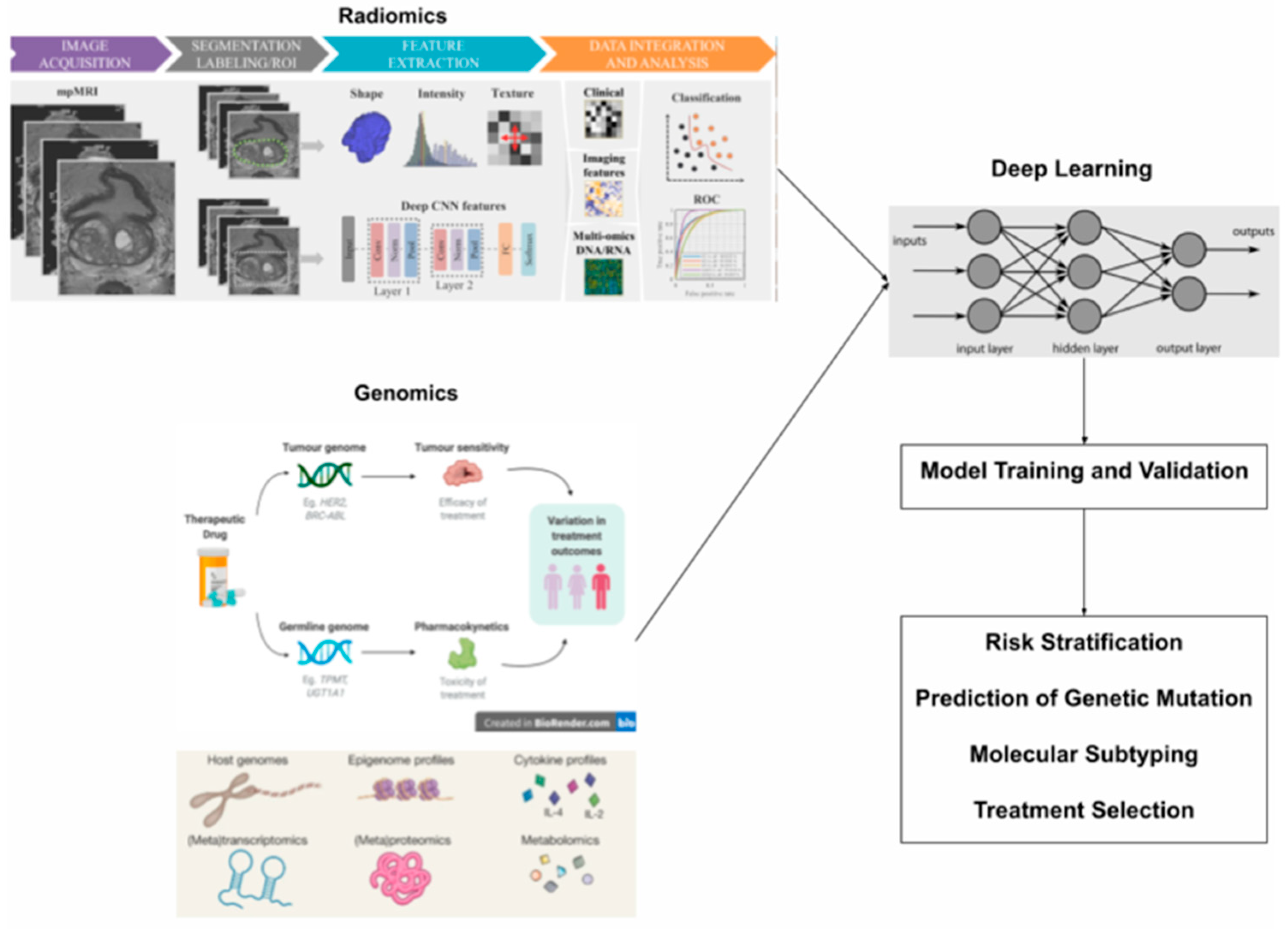
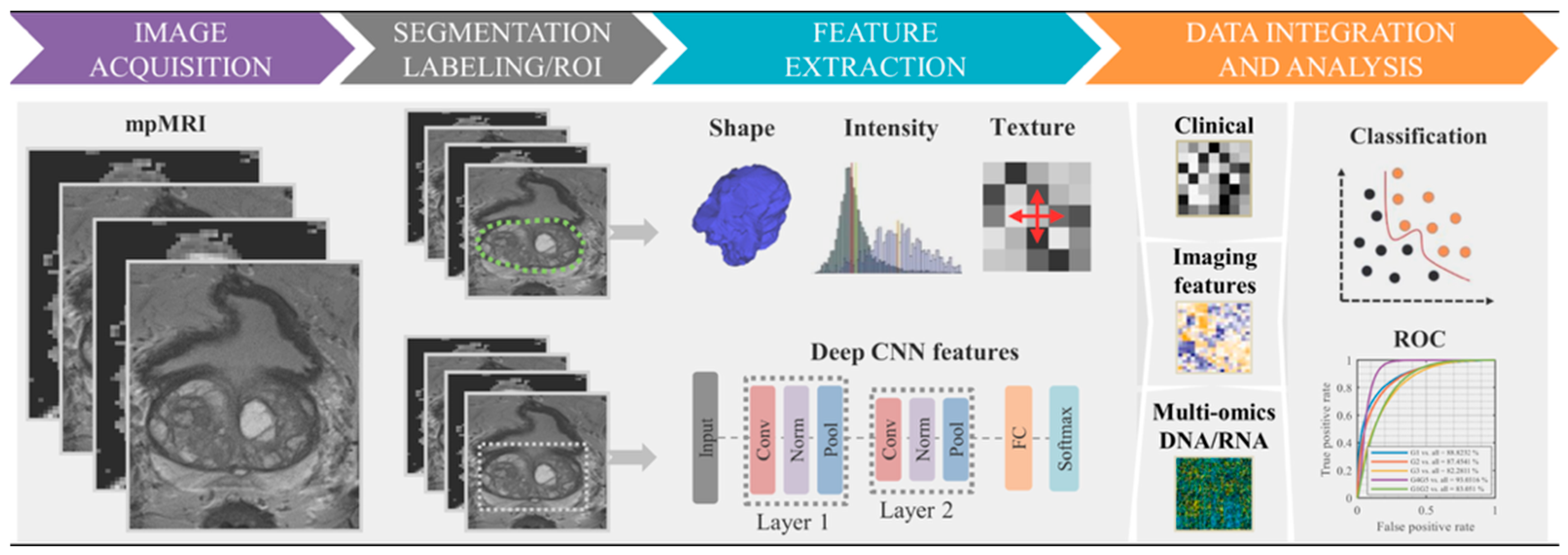
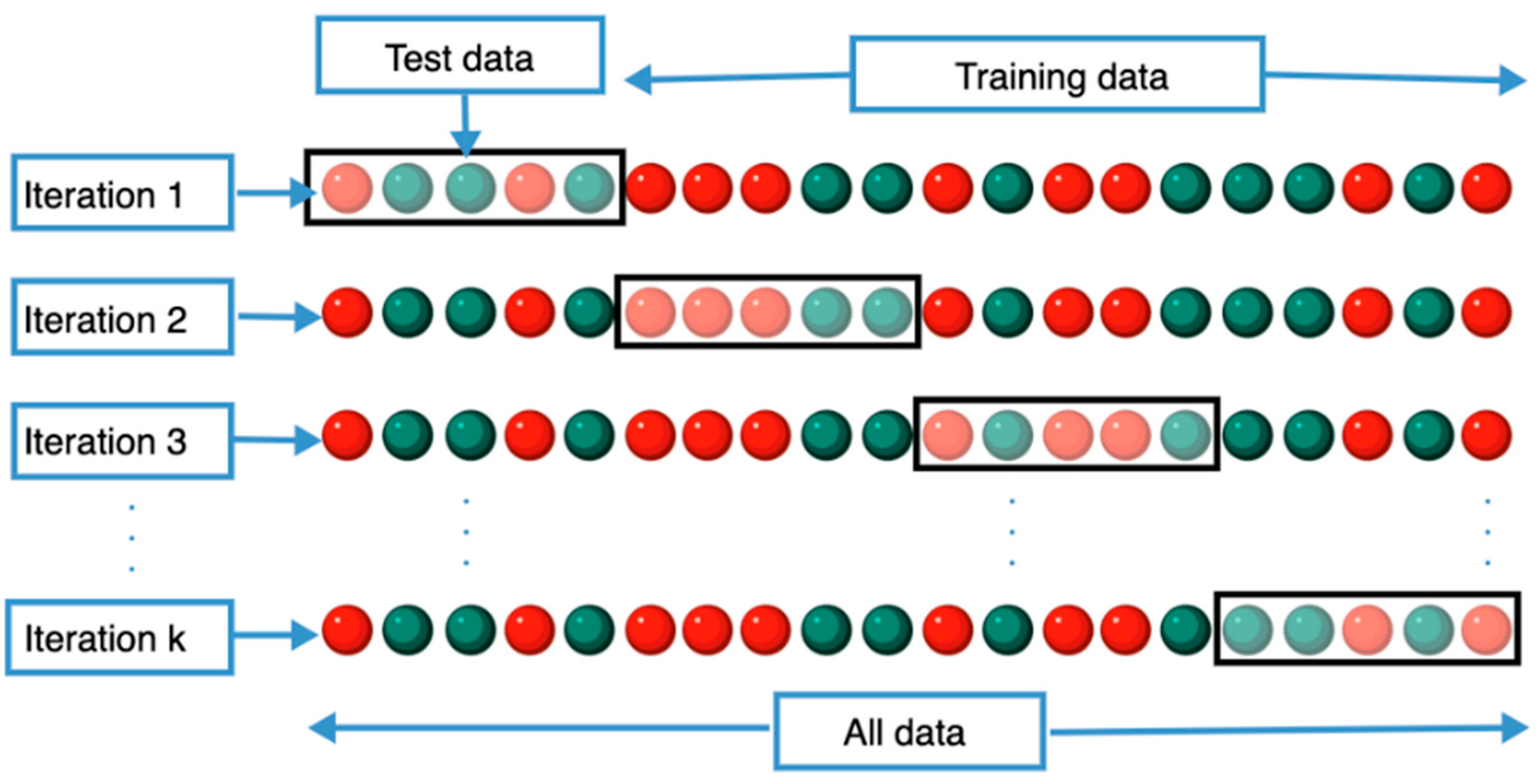

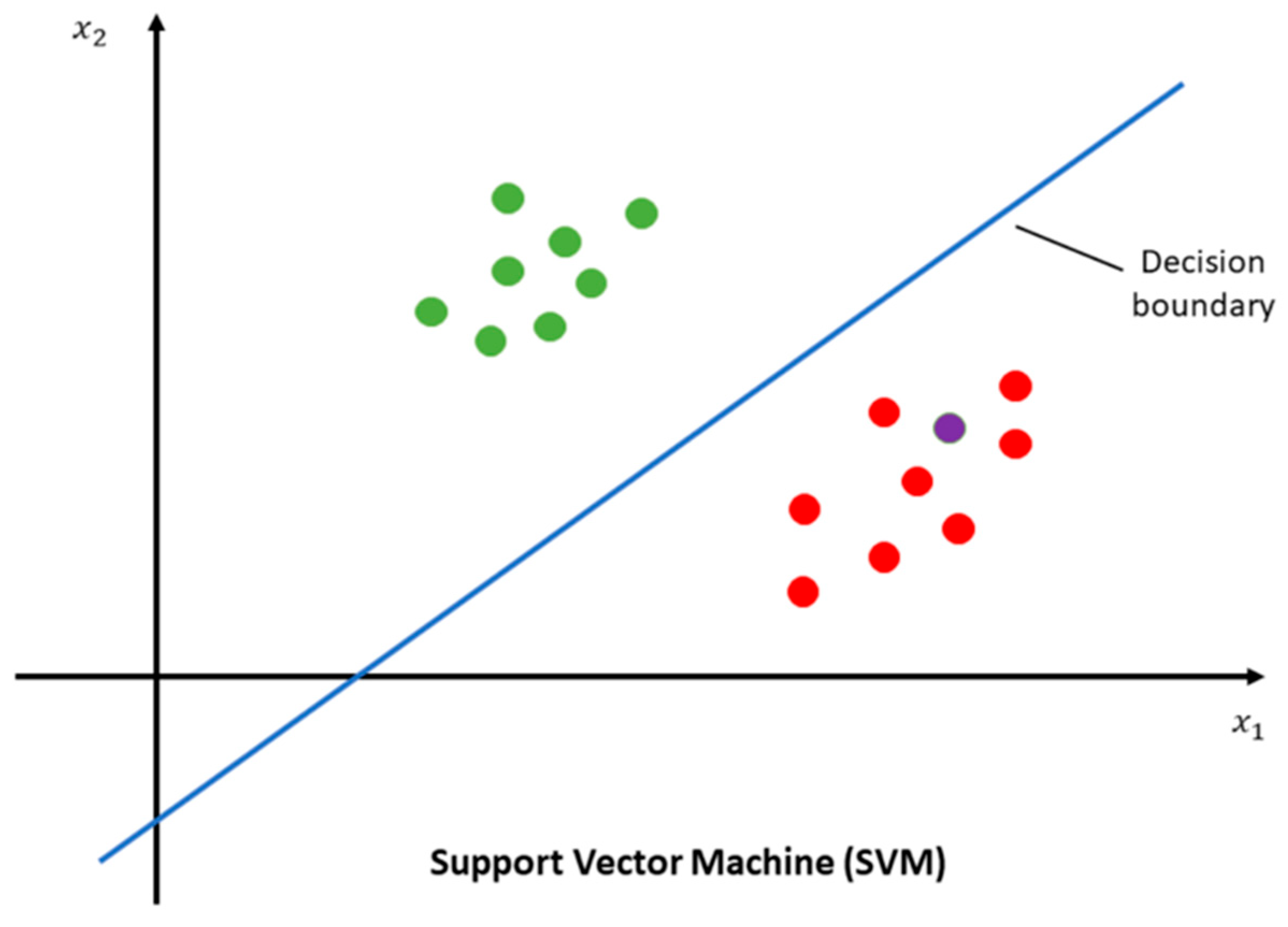
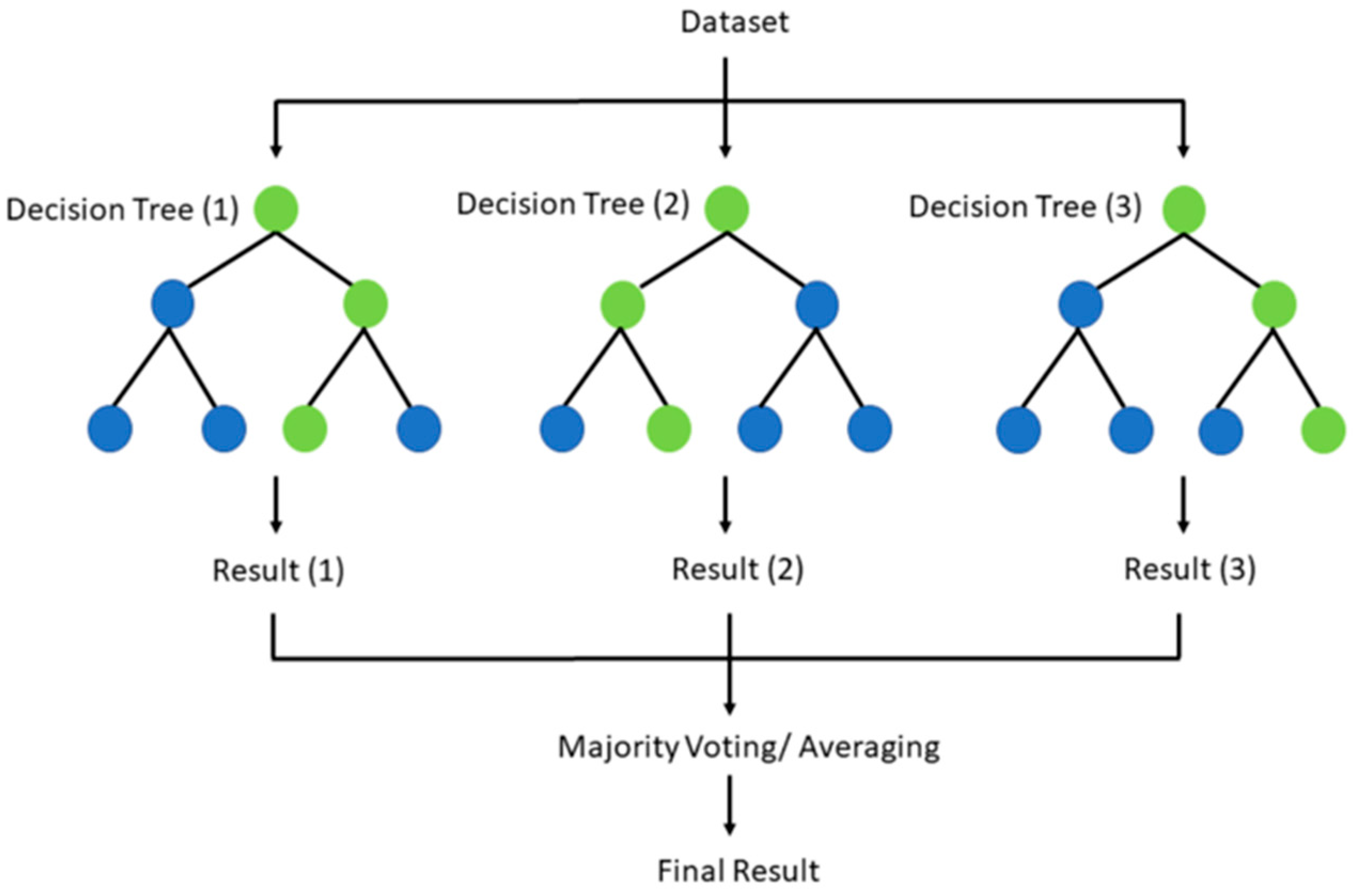
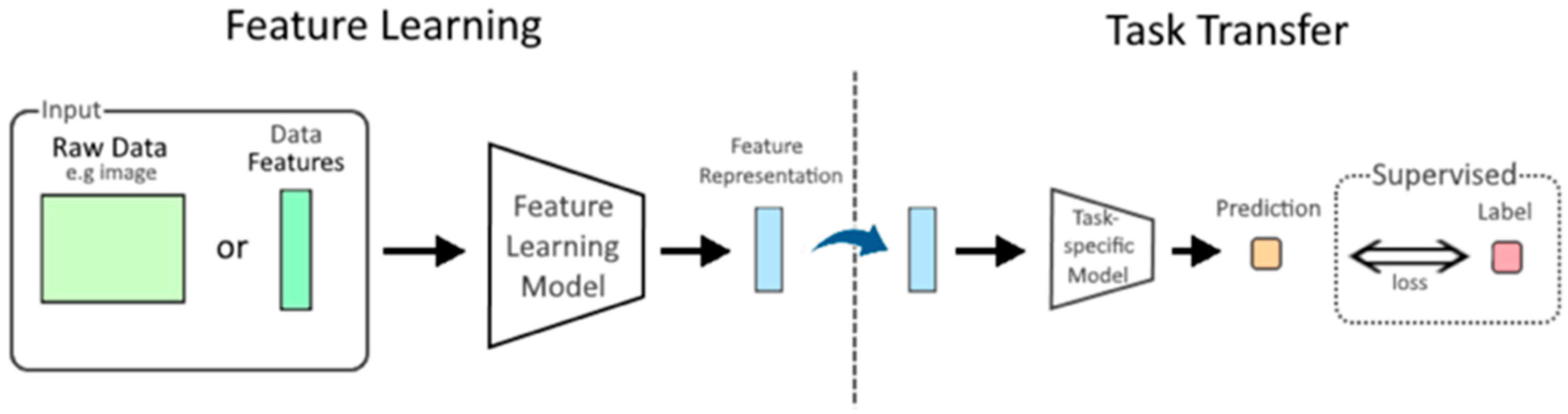

| Diagnostic Challenge | Clinical Impact |
|---|---|
| Low clinical exposure due to rarity (e.g., Ewing sarcoma, chordoma) | Delayed or missed diagnosis due to limited familiarity among general radiologists and clinicians |
| High histological heterogeneity (e.g., differentiation between liposarcoma subtypes) | Requires expert pathology and imaging interpretation; misclassification can affect treatment selection |
| Difficult tissue access in underserved or resource-limited settings | Suboptimal biopsy access and diagnostic delays, leading to delayed interventions |
| Variable anatomical origin (bone, cartilage, soft tissue, e.g., osteosarcoma, synovial sarcoma) | Complicates imaging interpretation, surgical planning, and multidisciplinary coordination |
| Poor delineation of tumor margins with conventional imaging (e.g., T1-weighted imaging) | Inadequate surgical margin assessment increases the risk of local recurrence or incomplete resection |
| Unreliable differentiation of tumor grade/type via imaging alone | May lead to incorrect prognostication or therapy selection without histological confirmation |
| Inaccuracy of Response Evaluation Criteria in Solid Tumors (RECIST) in measuring therapy response, especially in necrotic or cystic tumors | Can lead to misjudgment of therapeutic effectiveness, potentially affecting patient inclusion in clinical trials or treatment continuation |
| Invasiveness, data requirements, and resource demands of current AI model training (eg., deep learning with 3D MRI) | Limits the scalability and adoption of AI tools in low-resource or community settings |
| Diagnostic Challenge | Tumor Type | AI Solution | References |
|---|---|---|---|
| Morphological overlap with benign lesions | Soft tissue tumors | Deep learning models analyze histopathology slides to distinguish benign vs. malignant subtypes with 84–95% accuracy | [78] |
| Ambiguous radiographic features | Bone tumors | Convolutional neural networks (CNNs) detect subtle patterns in X-rays/MRI (e.g., “moth-eaten” appearance seen in Ewing sarcoma vs. “sunburst” appearance in Osteosarcoma) with 86–91% specificity | [79,80] |
| Molecular heterogeneity | Both | Machine learning integrates transcriptomic, immunohistochemical, and imaging data to predict tumor-specific mutations (e.g., EWSR1 translocations in Ewing sarcoma) | [78,81,82] |
| Inter-observer variability in biopsy interpretation | Both | AI algorithms standardize biopsy analysis by quantifying cellular features (e.g., mitotic count, necrosis) to reduce diagnostic discordance | [79,81] |
| Time-intensive manual tumor grading | Soft tissue tumors | Automated segmentation tools measure tumor volume and heterogeneity on MRI/CT, reducing grading time by 30–50% | [80,82] |
| Differentiating round-cell tumors | Bone tumors | AI-driven FISH/RT-PCR prioritization identifies high-risk molecular markers (e.g., CD99 for Ewing sarcoma) with 95% concordance | [79,83] |
| Tumor Type | Genomic/Transcriptomic Feature | Imaging Feature | Integration Method | Reference |
|---|---|---|---|---|
| Osteosarcoma | PLK1 pathway activation; glucose metabolism genes | Heterogeneous Contrast Enhancement on MRI/CT | Multi-omic pathway analysis (WES, RNA-seq, drug screens) | [168] |
| Soft Tissue Sarcoma | Gene fusions (e.g., EWSR1); methylation profiles | Necrosis/hemorrhage patterns on T2-weighted MRI | Spatial transcriptomics + MRI radiomics | [169,170] |
| Multiple Myeloma | Copy number alterations; HLA-G/LILRB1 interactions | Focal bone lesions on PET/CT | Spatial multi-omics (IF, IMC, LC/MS proteomics) | [171] |
| Bone Metastases | Circulating tumor DNA (ctDNA); AR-variant expression | Osteolytic/osteoblastic changes on CT | Radiogenomic correlation (CTCs, cfDNA) | [172,173,174] |
| Sarcomas (general) | Subtype-specific mutations (e.g., TP53, RB1) | Tumor texture/heterogeneity on dynamic contrast MRI | Multi-omics clustering (PET/MRI + RNA-seq) | [175] |
| Imaging Phenotype | Genomic Signature/Molecular Feature | Tumor Type/Context | Key Findings/Correlation | Reference |
|---|---|---|---|---|
| Irregular tumor margins and intratumoral necrosis | Poor response-associated gene expression profiles | Various cancers including musculoskeletal tumors | Presence correlates with poor neoadjuvant chemotherapy response; imaging features reflect aggressive genomic behavior | [187] |
| Tumor heterogeneity on MRI (contrast enhancement patterns) | Gene expression subtypes related to immune response (e.g., interferon-related genes) | Breast cancer (model for musculoskeletal tumors) | Heterogeneous enhancement correlates with specific gene expression subtypes linked to prognosis | [188] |
| Low or absent T2 signal intensity on MRI | Poor prognosis gene sets including van’t Veer 70-gene signature | Breast cancer (analogous to fibrotic/malignant features in musculoskeletal tumors) | Low T2 signal correlates with poor prognosis gene signatures, reflecting collagen-rich fibrotic tissue | [188] |
| Radiomic texture and entropy features | Tumor mutational burden (TMB) and neoantigen load | Bone malignancies and sarcomas | Radiomic features correlate with genomic markers of tumor aggressiveness and immune evasion | [172] |
| Spatial heterogeneity in imaging | Intratumoral genomic subclones identified by transcriptomics | Breast cancer (framework applicable to musculoskeletal tumors) | Radiogenomic signatures link imaging heterogeneity to genomic subclone composition, predicting survival outcomes | [189] |
| Imaging features of bone metastases (osteolytic/osteoblastic changes) | Circulating tumor DNA (ctDNA) and gene expression alterations | Bone metastases | Imaging phenotypes correlate with liquid biopsy genomic markers, enabling non-invasive monitoring | [172] |
| Peritumoral edema and necrosis on MRI | Gene expression signatures related to hypoxia and wound healing | Various solid tumors including sarcomas | Imaging features correlate with gene sets associated with tumor microenvironment and aggressive biology | [187,188] |
| Radiomic Process Step or Modality | Significance for Musculoskeletal Tumor Assessment | Key Considerations/Outputs |
|---|---|---|
| Imaging Acquisition Standardization | Minimizes inter-scan and inter-site variability for model training and cross-center reproducibility | Protocol harmonization; consistent voxel spacing, timing, and field strength |
| ROI Segmentation (Manual, Semi-/Automated) | Defines tumor boundaries for downstream analysis; crucial for accurate feature mapping, prone to variability | Affected by operator variability; accuracy impacts all subsequent model inputs |
| Quantitative Feature Extraction | Converts image data into quantifiable metrics representing tumor morphology and texture | Includes shape, intensity, texture (GLCM, GLRLM), and wavelet features |
| MRI Functional Imaging (e.g., DWI and ADC mapping) | Reflects tumor cellularity and treatment response using ADC values and other water diffusion metrics | ADC values inversely correlate with cell density; useful in response monitoring |
| CT with Dual-Energy and Spectral Imaging | Enhances tissue characterization, particularly in bone tumors and mineral content | Enables separation of materials (e.g., calcium vs. iodine); improves lesion conspicuity |
| PET/CT and PET/MRI Metabolic Profiling | Evaluates tumors aggressiveness and viability through metabolic markers such as MTV and TLG | Includes metabolic tumor volume (MTV) and total lesion glycolysis (TLG) |
| Multiparametric Imaging Integration | Provides a comprehensive view by combining functional, anatomical, and metabolic imaging for robust modeling | Fuses MRI, PET, and CT data for superior classification and response assessment |
| Feature Selection and Overfitting Prevention | Prioritizes robust, predictive features to avoid in small datasets | Uses LASSO, recursive feature elimination, or embedded CNN layers to improve generalizability |
| Standardization using ComBat and NestedComBat Techniques | Adjusts for scanner and protocol-induced variability to enable pooled analysis | Batch effect correlation enhances multi-site model compatibility |
| Transcriptomic Integration with Imaging | Links radiomic phenotypes with gene expression for personalized diagnosis based on molecular subtypes | Enables radiogenomic profiling and non-invasive surrogate biomarkers of oncogenic pathways |
| Immune-Related Radiomic Signatures | Maps imaging biomarkers associated with immune infiltration or systemic inflammation | Correlates with IL-6, TNF- expression; may predict immunotherapy response |
| Deep Learning and CNN-Driven Radiogenomics | Enhances automated classification and prognostication from raw scans | Improves classification, survival prediction, and uncovering latent imaging-genomic patterns |
| Algorithm/Model | Application | Performance | Reference |
|---|---|---|---|
| Deep Convolutional Neural Network (CNN) | Classifying benign vs. malignant bone lesions, subtyping (e.g., cartilaginous vs. osteogenic) | Top 1 error rate of 0.25; 93% accuracy in matrix classification, outperforming average radiologists (70%) | [80] |
| Pre-trained ResNet50 Classifier | Predicting malignant potential of bone tumors on MRI | 93.7% accuracy (T1-weighted); 86.7% accuracy (T2-weighted) | [80] |
| Ensemble Deep Learning Network | Integrating multicenter radiographs and clinical features | High accuracy for primary bone tumor classification | [234] |
| Multitask Deep Learning Model | Simultaneous segmentation and classification on radiographs | 80.2% accuracy, 62.9% sensitivity, 88.2% specificity; performance comparable to fellowship-trained radiologists | [235] |
| Radiomics-based Machine Learning | Differentiating atypical cartilaginous neoplasms from chondrosarcoma (MRI/CT) | 92% accuracy (MRI), 78% AUC (CT), similar to specialized radiologists (98% accuracy) | [231] |
| Automated Segmentation (MSAPN) + Radiomics | MRI-based segmentation and classification | Dice score 0.871 (test set); 0.890 accuracy for benign vs. malignant classification | [232] |
| Risk Stratification System (BTI-RADS 2.0) + ML | Standardized bone lesion grading on CT/MRI | Sensitivity of 96% for malignant lesions; F1-score 0.81 (slightly below radiologists at 0.83) | [233] |
| Translational Barrier or Challenge | Description | Implication for Clinical Translation |
|---|---|---|
| Heterogeneous Imaging Acquisition Protocols | Variations in scanner models, sequences, and reconstruction algorithms across sites | Introduces batch effects and non-biological variability |
| Inconsistent Transcriptomic Profiling Methods | Differences in RNA extraction, sequencing platforms, and bioinformatic pipelines | Compromises data quality and model reliability |
| Integrating Multimodal Data (Imaging + Genomics) | Challenges in aligning data with different resolutions, formats, and missing values | Limits comprehensive tumor profiling; demands advanced fusion models and data imputation strategies |
| Low Model Interpretability and Explainability | DL models often operate as “black boxes” without transparent decision logic | Limits clinician trust and slows regulatory acceptance; explainable AI (XAI) methods are essential (e.g., saliency maps) |
| Segmentation Variability and Lack of Standardization | Inter- and intra-observe variability in manual or semi-automated tumor delineation | Reduces feature reproducibility; highlights the need for robust auto-segmentation algorithms and consensus protocols |
| Regulatory Hurdles and Clinical Validation Challenges for Adaptive AI Models | Continuously learning systems evolve post-deployment, making validation and certification complex | Raises legal and ethical concerns; requires new regulatory frameworks and post-market surveillance mechanisms |
| Algorithmic Bias and Performance Disparities Acros Subgroups | Underrepresentation of demographic subgroups during training | Can lead to inaccurate predictions in minorities; underscores the need for diverse datasets and fairness audits |
| Lack of Federated Learning Implementation in Low-Resource Clinical Settings | Limited infrastructure in low- and middle-income countries for decentralized model training | Exacerbates global disparities in AI access; federated learning could enable secure, local model deployment |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kumar, R.; Sporn, K.; Khanna, A.; Paladugu, P.; Gowda, C.; Ngo, A.; Jagadeesan, R.; Zaman, N.; Tavakkoli, A. Integrating Radiogenomics and Machine Learning in Musculoskeletal Oncology Care. Diagnostics 2025, 15, 1377. https://doi.org/10.3390/diagnostics15111377
Kumar R, Sporn K, Khanna A, Paladugu P, Gowda C, Ngo A, Jagadeesan R, Zaman N, Tavakkoli A. Integrating Radiogenomics and Machine Learning in Musculoskeletal Oncology Care. Diagnostics. 2025; 15(11):1377. https://doi.org/10.3390/diagnostics15111377
Chicago/Turabian StyleKumar, Rahul, Kyle Sporn, Akshay Khanna, Phani Paladugu, Chirag Gowda, Alex Ngo, Ram Jagadeesan, Nasif Zaman, and Alireza Tavakkoli. 2025. "Integrating Radiogenomics and Machine Learning in Musculoskeletal Oncology Care" Diagnostics 15, no. 11: 1377. https://doi.org/10.3390/diagnostics15111377
APA StyleKumar, R., Sporn, K., Khanna, A., Paladugu, P., Gowda, C., Ngo, A., Jagadeesan, R., Zaman, N., & Tavakkoli, A. (2025). Integrating Radiogenomics and Machine Learning in Musculoskeletal Oncology Care. Diagnostics, 15(11), 1377. https://doi.org/10.3390/diagnostics15111377






