Primary Cutaneous Rhabdomyosarcomatous Melanomas—A Report of Two Cases and Literature Review
Abstract
1. Introduction
2. Materials and Methods
3. Results
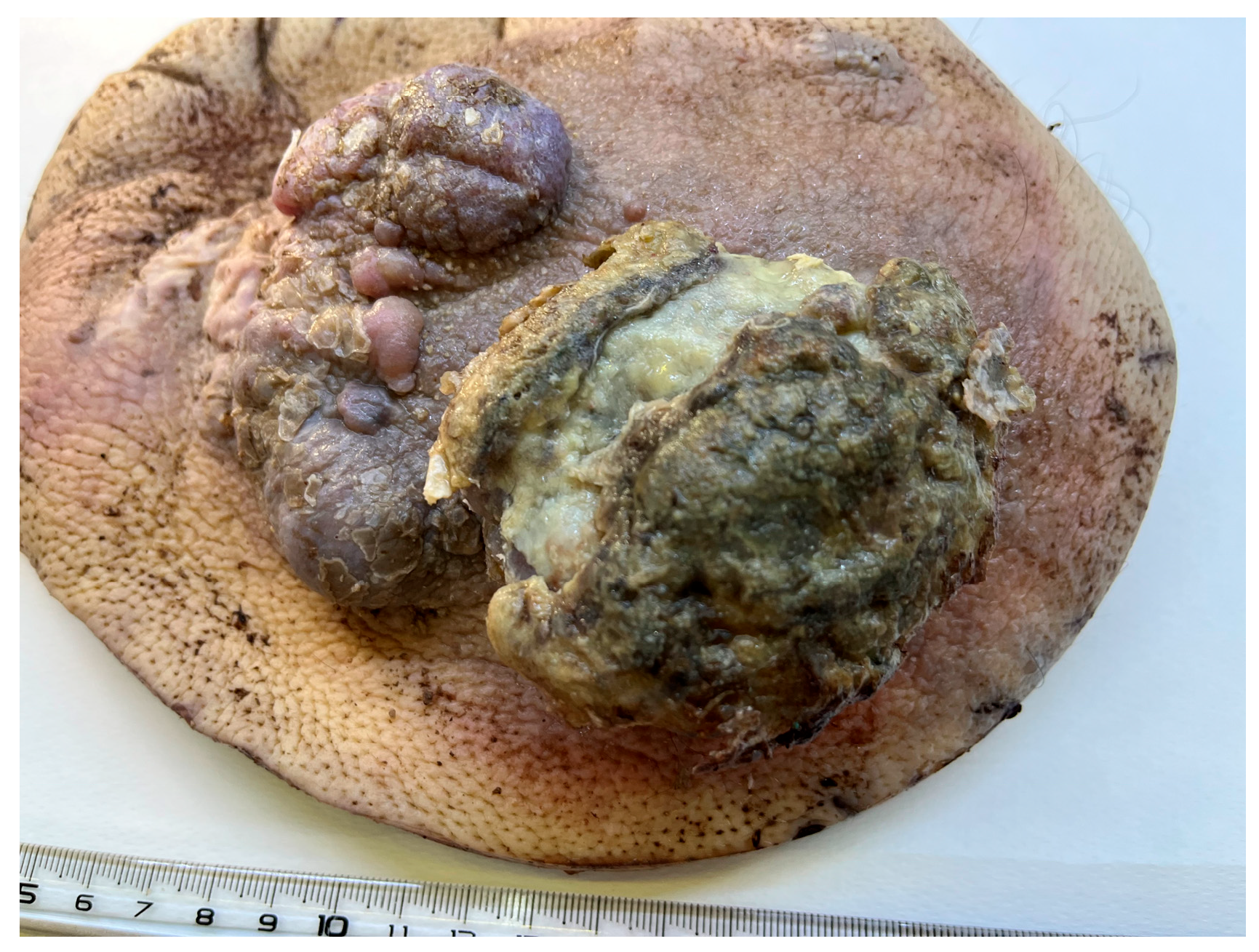
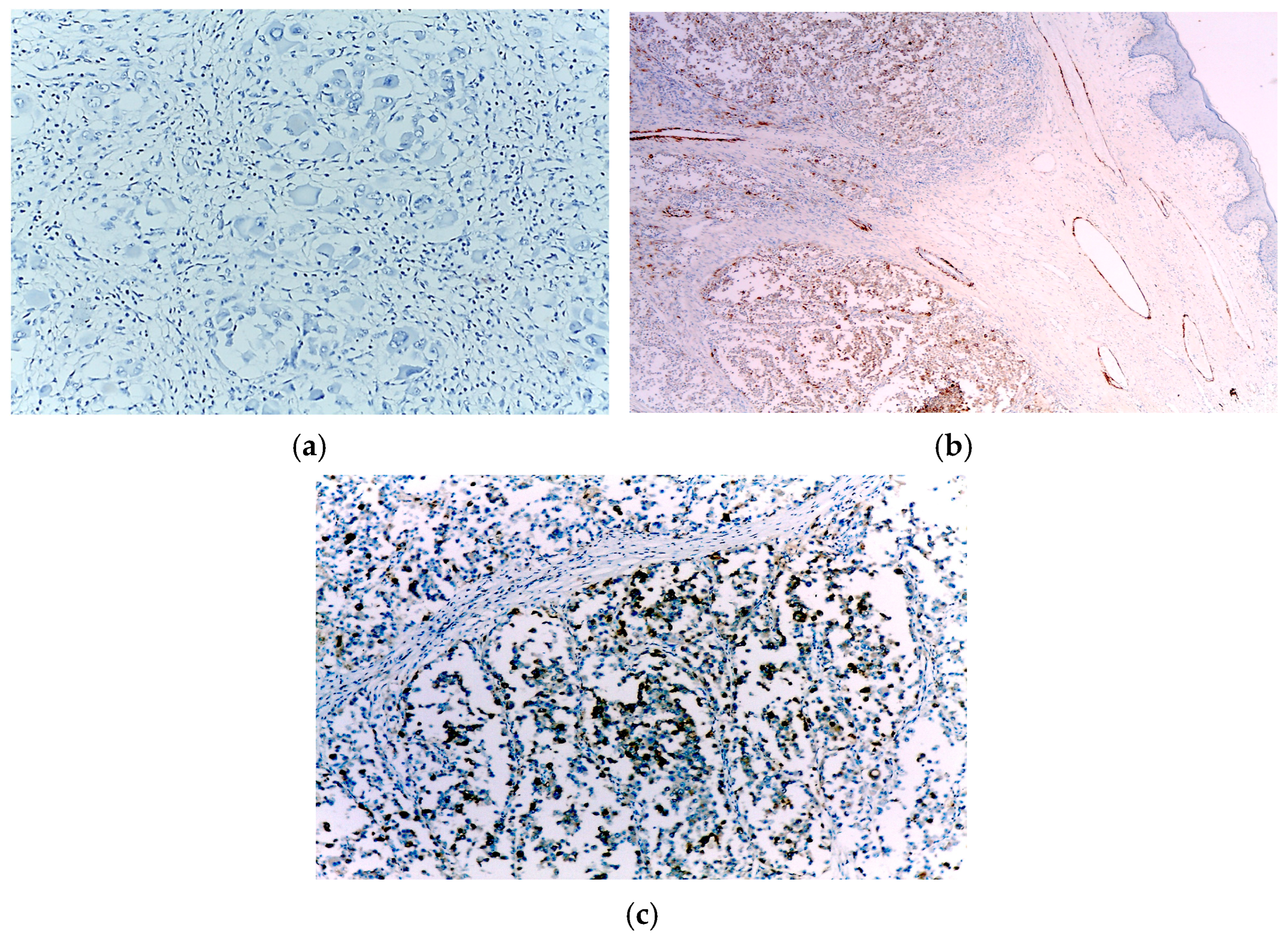
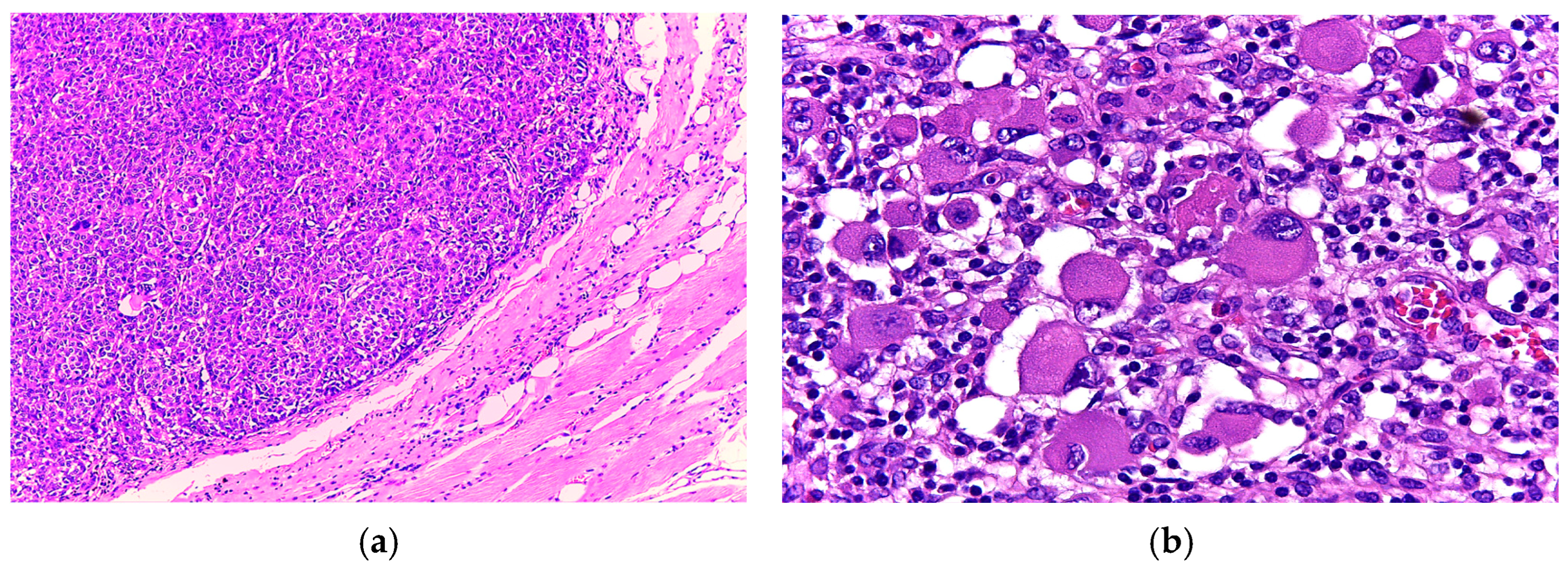
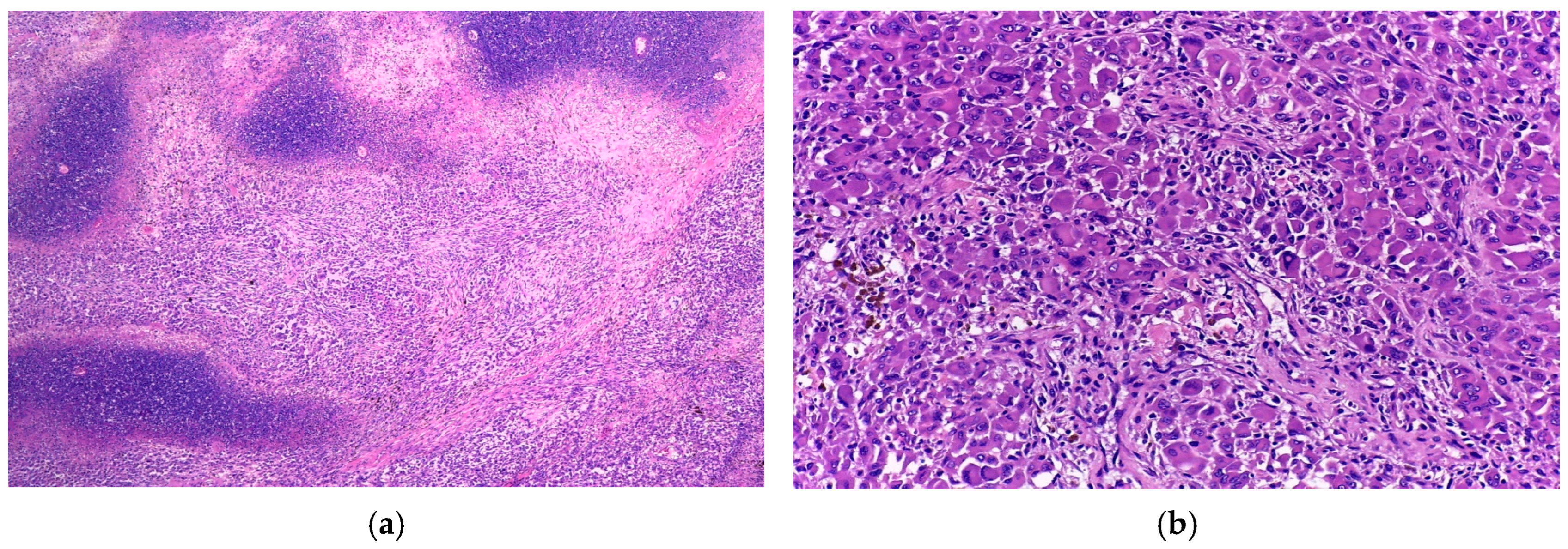
3.1. Case 1
3.2. Case 2
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Davis, L.E.; Shalin, S.C.; Tackett, A.J. Current state of melanoma diagnosis and treatment. Cancer Biol. Ther. 2019, 20, 1366–1379. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, S.S.; Eyden, B. Divergent differentiation in malignant melanomas: A review. Histopathology 2008, 52, 119–129. [Google Scholar] [CrossRef] [PubMed]
- Țăpoi, D.A.; Gheorghișan-Gălățeanu, A.-A.; Dumitru, A.V.; Ciongariu, A.M.; Furtunescu, A.R.; Marin, A.; Costache, M. Primary Undifferentiated/Dedifferentiated Cutaneous Melanomas—A Review on Histological, Immunohistochemical, and Molecular Features with Emphasis on Prognosis and Treatment. Int. J. Mol. Sci. 2023, 24, 9985. [Google Scholar] [CrossRef]
- Dumitru, A.V.; Țăpoi, D.A.; Costache, M.; Ciongariu, A.M.; Ionescu, A.I.; Liscu, H.D.; Alius, C.; Tampa, M.; Marin, A.; Furtunescu, A.R. Metastatic Nodular Melanoma with Angiosarcomatous Transdifferentiation—A Case Report and Review of the Literature. Diagnostics 2024, 14, 1323. [Google Scholar] [CrossRef]
- Bittesini, L.; Dei Tos, A.P.; Fletcher, C.D. Metastatic malignant melanoma showing a rhabdoid phenotype: Further evidence of a non-specific histological pattern. Histopathology 1992, 20, 167–170. [Google Scholar] [CrossRef]
- Laskin, W.B.; Knittel, D.R.; Frame, J.N. S100 protein and HMB-45 negative “rhabdoid” malignant melanoma: A totally dedifferentiated malignant melanoma? Am. J. Clin. Pathol. 1995, 103, 772–773. [Google Scholar] [CrossRef] [PubMed]
- Pasz-Walczak, G.; Jesionek-Kupnicka, D.; Kubiak, R.; Biernat, W.; Sygut, J.; Kordek, R. Rhabdomyosarcomatous (myoblastic?) phenotype of metastatic malignant melanoma. A case report. Pol. J. Pathol. 2002, 53, 97–100. [Google Scholar] [PubMed]
- Abbott, J.J.; Amirkhan, R.H.; Hoang, M.P. Malignant melanoma with a rhabdoid phenotype: Histologic, immunohistochemical, and ultrastructural study of a case and review of the literature. Arch. Pathol. Lab. Med. 2004, 128, 686–688. [Google Scholar] [CrossRef]
- Gharpuray-Pandit, D.; Coyne, J.; Eyden, B.; Banerjee, S.S. Rhabdomyoblastic differentiation in malignant melanoma in adults: Report of 2 cases. Int. J. Surg. Pathol. 2007, 15, 20–25. [Google Scholar] [CrossRef]
- Gavino, A.C.; Gillies, E.M. Metastatic rhabdoid melanoma: Report of a case with a comparative review of the literature. J. Cutan. Pathol. 2008, 35, 337–342. [Google Scholar] [CrossRef]
- Nakamura, T.; Matsumine, A.; Kato, H.; Kusuzaki, K.; Nishimura, K.; Murata, T.; Shiraishi, T.; Oda, Y.; Tsuneyoshi, M.; Uchida, A. Malignant melanoma with a rhabdoid phenotype exhibiting numerous solid tumor masses: A case report. Oncol. Rep. 2009, 21, 887–891. [Google Scholar] [CrossRef][Green Version]
- Guo, R.; Franco-Palacios, M.; Russell, M.; Goddard, L.; Hassell, L.; Gillies, E.; Fung, K.M. Micropthalmia transcription factor (MITF) as a diagnostic marker for metastatic melanomas negative for other melanoma markers. Int. J. Clin. Exp. Pathol. 2013, 6, 1658–1664. [Google Scholar]
- Reilly, D.J.; Volchek, M.; Ting, J.W.; Allan, P.; Findlay, M.W. Rhabdomyoblastic differentiation in metastatic melanoma: Making sense of a rare but complex form of mimicry. Int. J. Surg. Pathol. 2014, 22, 520–524. [Google Scholar] [CrossRef] [PubMed]
- Agaimy, A.; Specht, K.; Stoehr, R.; Lorey, T.; Märkl, B.; Niedobitek, G.; Straub, M.; Hager, T.; Reis, A.C.; Schilling, B.; et al. Metastatic Malignant Melanoma With Complete Loss of Differentiation Markers (Undifferentiated/Dedifferentiated Melanoma): Analysis of 14 Patients Emphasizing Phenotypic Plasticity and the Value of Molecular Testing as Surrogate Diagnostic Marker. Am. J. Surg. Pathol. 2016, 40, 181–191. [Google Scholar] [CrossRef]
- Dumitru, A.V.; Tampa, M.Ş.; Georgescu, S.R.; Păunică, S.; Matei, C.N.; Nica, A.E.; Costache, M.; Motofei, I.; Sajin, M.; Păunică, I.; et al. Immunohistochemical mismatch in a case of rhabdomyoblastic metastatic melanoma. Rom. J. Morphol. Embryol. 2018, 59, 339–344. [Google Scholar] [PubMed]
- Campbell, K.; Kumarapeli, A.R.; Gokden, N.; Cox, R.M.; Hutchins, L.; Gardner, J.M. Metastatic melanoma with dedifferentiation and extensive rhabdomyosarcomatous heterologous component. J. Cutan. Pathol. 2018, 45, 360–364. [Google Scholar] [CrossRef] [PubMed]
- Tran, T.A.N. Undifferentiated Sarcoma as Intermediate Step in Rhabdomyosarcomatous Transformation of a Metastatic Malignant Melanoma Resistant to Anti-BRAF Therapy: A Phenomenon Associated With Significant Diagnostic and Therapeutic Pitfalls. Int. J. Surg. Pathol. 2019, 27, 669–677. [Google Scholar] [CrossRef]
- Mehta, A.; Sharma, A.; Gupta, G. Rhabdoid melanoma: A diagnostic ordeal. Indian J. Cancer 2020, 57, 473–477. [Google Scholar] [CrossRef]
- Tzanavaris, K.; Pettas, E.; Thermos, G.; Georgaki, M.; Piperi, E.; Nikitakis, N.G. Base of tongue metastasis of cutaneous malignant melanoma with rhabdoid and neuroendocrine features: Report of a rare case and review of the literature. Head Neck Pathol. 2022, 16, 1230–1241. [Google Scholar] [CrossRef]
- Cilento, M.A.; Kim, C.; Chang, S.; Farshid, G.; Brown, M.P. Three cases of BRAF-mutant melanoma with divergent differentiation masquerading as sarcoma. Pathologica 2022, 114, 217–220. [Google Scholar] [CrossRef]
- Kasago, I.S.; Chatila, W.K.; Lezcano, C.M.; Febres-Aldana, C.A.; Schultz, N.; Vanderbilt, C.; Dogan, S.; Bartlett, E.K.; D’Angelo, S.P.; Tap, W.D.; et al. Undifferentiated and Dedifferentiated Metastatic Melanomas Masquerading as Soft Tissue Sarcomas: Mutational Signature Analysis and Immunotherapy Response. Mod. Pathol. 2023, 36, 100165. [Google Scholar] [CrossRef] [PubMed]
- Chang, E.S.; Wick, M.R.; Swanson, P.E.; Dehner, L.P. Metastatic malignant melanoma with “rhabdoid” features. Am. J. Clin. Pathol. 1994, 102, 426–431. [Google Scholar] [CrossRef]
- Watter, H.; Milkins, R.; Chambers, C.; O’Brien, B. Melanoma with rhabdomyosarcomatous features: A potential diagnostic pitfall. BMJ Case Rep. 2023, 16, e256427. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Wagman, R.; Kuwadekar, A.; Scoppetuolo, M.; Dardik, M.; Smith, F. Use of Immunotherapy and Radiation Treatment in the Management of Metastatic Melanoma with Rhabdomyosarcomatous Differentiation. Adv. Radiat. Oncol. 2019, 5, 134–139. [Google Scholar] [CrossRef]
- Smith, S.M.; Schmitt, A.C.; Carrau, R.L.; Iwenofu, O.H. Primary sinonasal mucosal melanoma with aberrant diffuse and strong desmin reactivity: A potential diagnostic pitfall! Head Neck Pathol. 2015, 9, 165–171. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.K.; Lin, H.; Su, C.F.; Kok, V.C. Primary Vaginal Melanoma With Rhabdoid Features: A Case Report and Literature Review. Int. J. Gynecol. Pathol. 2017, 36, 499–504. [Google Scholar] [CrossRef]
- Kim, N.R.; Lee, W.K.; Chung, D.H. Primary gastric melanoma with rhabdoid features: A case report. Korean J. Pathol. 2013, 47, 606–609. [Google Scholar] [CrossRef]
- Schindler, K.; Schicher, N.; Kunstfeld, R.; Pehamberger, H.; Toepker, M.; Haitel, A.; Hoeller, C.; Harmankaya, K. A rare case of primary rhabdoid melanoma of the urinary bladder treated with ipilimumab, an anti-CTLA 4 monoclonal antibody. Melanoma Res. 2012, 22, 320–325. [Google Scholar] [CrossRef]
- Bell, P.D.; Israel, A.K.; Dunn, A.L.; Liao, X. Primary Dedifferentiated Amelanotic Anorectal Melanoma: Report of a Rare Case. Int. J. Surg. Pathol. 2019, 27, 923–928. [Google Scholar] [CrossRef]
- Shenjere, P.; Fisher, C.; Rajab, R.; Patnaik, L.; Hazell, S.; Thway, K. Melanoma with rhabdomyosarcomatous differentiation: Two further cases of a rare pathologic pitfall. Int. J. Surg. Pathol. 2014, 22, 512–519. [Google Scholar] [CrossRef]
- Parham, D.M.; Weeks, D.A.; Beckwith, J.B. The clinicopathologic spectrum of putative extrarenal rhabdoid tumors. An analysis of 42 cases studied with immunohistochemistry or electron microscopy. Am. J. Surg. Pathol. 1994, 18, 1010–1029, Erratum in Am. J. Surg. Pathol. 1995, 19, 488–489. https://doi.org/10.1097/00000478-199504000-00018. [Google Scholar] [CrossRef]
- Borek, B.T.; McKee, P.H.; Freeman, J.A.; Maguire, B.; Brander, W.L.; Calonje, E. Primary malignant melanoma with rhabdoid features: A histologic and immunocytochemical study of three cases. Am. J. Dermatopathol. 1998, 20, 123–127. [Google Scholar] [CrossRef] [PubMed]
- Gattenlöhner, S.; Brocker, E.B.; Muller-Hermelink, H.K. Malignant melanoma with metastatic rhabdomyosarcomatoid transdifferentiation. N. Engl. J. Med. 2008, 358, 649–650. [Google Scholar] [CrossRef]
- Tallon, B.; Bhawan, J. Primary rhabdoid melanoma with clonal recurrence. Am. J. Dermatopathol. 2009, 31, 200–204. [Google Scholar] [CrossRef] [PubMed]
- Chung, B.Y.; Ahn, I.S.; Cho, S.I.; Kim, H.O.; Kim, K.H.; Park, C.W.; Lee, C.H. Primary malignant rhabdoid melanoma. Ann. Dermatol. 2011, 23 (Suppl. 2), S155–S159. [Google Scholar] [CrossRef]
- Kaneko, T.; Korekawa, A.; Akasaka, E.; Rokunohe, D.; Nakano, H.; Sawamura, D. Primary Amelanotic Rhabdoid Melanoma: A Case Report with Review of the Literature. Case Rep. Dermatol. 2015, 7, 292–297. [Google Scholar] [CrossRef]
- Fernández-Vega, I.; Santos-Juanes, J.; Fresno-Forcelledo, M.F. Primary amelanotic rhabdoid melanoma of the forehead. Br. J. Dermatol. 2016, 174, 1156–1158. [Google Scholar] [CrossRef] [PubMed]
- Antonov, N.K.; Niedt, G.W. Malignant Melanoma With Rhabdomyosarcomatous Differentiation: A Case Report. Am. J. Dermatopathol. 2016, 38, 456–460. [Google Scholar] [CrossRef]
- Prieto-Torres, L.; Alegría-Landa, V.; Llanos, C.; Córdoba, A.; Kutzner, H.; Requena, L. Cutaneous Malignant Melanoma With Rhabdoid Morphology and Smooth Muscle Differentiation: A Challenging Histopathologic Diagnosis. Am. J. Dermatopathol. 2017, 39, 397–403. [Google Scholar] [CrossRef]
- Kuwadekar, A.; Allard, J.; Dardik, M.; Smith, F. Melanoma with rhabdomyosarcomatous differentiation. BMJ Case Rep. 2018, 2018, bcr2018224263. [Google Scholar] [CrossRef]
- Murakami, T.; Ogata, D.; Arai, E.; Tsuchida, T. Case of primary hypomelanotic rhabdoid melanoma on the forehead. J. Dermatol. 2019, 46, e278–e279. [Google Scholar] [CrossRef] [PubMed]
- Tran, T.A.N.; Linos, K.; de Abreu, F.B.; Carlson, J.A. Undifferentiated Sarcoma as Intermediate Step in the Progression of Malignant Melanoma to Rhabdomyosarcoma: Histologic, Immunohistochemical, and Molecular Studies of a New Case of Malignant Melanoma With Rhabdomyosarcomatous Differentiation. Am. J. Dermatopathol. 2019, 41, 221–229. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, I.; Droop, A.; Edwards, O.; Wong, K.; Harle, V.; Habeeb, O.; Gharpuray-Pandit, D.; Houghton, J.; Wiedemeyer, K.; Mentzel, T.; et al. The clinicopathologic spectrum and genomic landscape of de-/trans-differentiated melanoma. Mod. Pathol. 2021, 34, 2009–2019. [Google Scholar] [CrossRef]
- Agaimy, A.; Stoehr, R.; Hornung, A.; Popp, J.; Erdmann, M.; Heinzerling, L.; Hartmann, A. Dedifferentiated and Undifferentiated Melanomas: Report of 35 New Cases with Literature Review and Proposal of Diagnostic Criteria. Am. J. Surg. Pathol. 2021, 45, 240–254. [Google Scholar] [CrossRef]
- Yim, S.H.; Kim, D.; Hong, D.; Jung, K.E.; Lee, Y.; Seo, Y.J.; Park, S. Primary cutaneous malignant melanoma with rhabdomyosarcomatous differentiation originating from a melanocytic nevus in a patient with myelodysplastic syndrome. J. Cutan. Pathol. 2022, 49, 875–880. [Google Scholar] [CrossRef]
- Glutsch, V.; Wobser, M.; Schilling, B.; Gesierich, A.; Goebeler, M.; Kneitz, H. PRAME Expression as Helpful Immunohistochemical Marker in Rhabdoid Melanoma. Dermatopathology 2022, 9, 148–157. [Google Scholar] [CrossRef]
- O’Neill, P.; Amanuel, B.; Mesbah Ardakani, N. Cutaneous Malignant Melanoma with Rhabdomyosarcomatous Dedifferentiation: An Immunohistological and Molecular Case Study with Literature Review. Am. J. Dermatopathol. 2023, 45, 470–474. [Google Scholar] [CrossRef]
- Choy, A.; Wang, A.; Thayaparan, G.K.; Beaumont, L.; Williams, R.; Diab, S.; Tzaikou, G.; Ajmal, A.; Nirenberg, A. The Diagnostic Utility of PRAME in Primary Cutaneous Dedifferentiated and Transdifferentiated Melanomas. J. Cutan. Pathol. 2025, 52, 212–219. [Google Scholar] [CrossRef] [PubMed]
- Weigelt, M.A.; Pattali, S.; Dermawan, J.K.; Ko, J.S.; Fritchie, K.J.; Billings, S.D. Primary Cutaneous Neoplasm With Rhabdomyosarcomatous Differentiation and a Melanoma-Like Mutational Landscape. J. Cutan. Pathol. 2025, 52, 414–417. [Google Scholar] [CrossRef]
- Huang, F.; Santinon, F.; Flores González, R.E.; Del Rincón, S.V. Melanoma Plasticity: Promoter of Metastasis and Resistance to Therapy. Front. Oncol. 2021, 11, 756001. [Google Scholar] [CrossRef]
- Diazzi, S.; Tartare-Deckert, S.; Deckert, M. The mechanical phenotypic plasticity of melanoma cell: An emerging driver of therapy cross-resistance. Oncogenesis 2023, 12, 7. [Google Scholar] [CrossRef]
- Țăpoi, D.A.; Derewicz, D.; Gheorghișan-Gălățeanu, A.-A.; Dumitru, A.V.; Ciongariu, A.M.; Costache, M. The Impact of Clinical and Histopathological Factors on Disease Progression and Survival in Thick Cutaneous Melanomas. Biomedicines 2023, 11, 2616. [Google Scholar] [CrossRef] [PubMed]
- Koyama, T.; Murakami, M.; Nishihara, O.; Masuda, T. Congenital melanoma: A case suggesting rhabdomyogenic differentiation. Pediatr. Dermatol. 1996, 13, 389–393. [Google Scholar] [CrossRef] [PubMed]
- Baltres, A.; Salhi, A.; Houlier, A.; Pissaloux, D.; Tirode, F.; Haddad, V.; Karanian, M.; Ysmail-Dahlouk, S.; Boukendakdji, F.; Dahlouk, D.; et al. Malignant melanoma with areas of rhabdomyosarcomatous differentiation arising in a giant congenital nevus with RAF1 gene fusion. Pigment. Cell Melanoma Res. 2019, 32, 708–713. [Google Scholar] [CrossRef] [PubMed]
- Gaspard, M.; Lamant, L.; Tournier, E.; Valentin, T.; Rochaix, P.; Terrier, P.; Ranchere-Vince, D.; Coindre, J.M.; Filleron, T.; Le Guellec, S. Evaluation of eight melanocytic and neural crest-associated markers in a well-characterised series of 124 malignant peripheral nerve sheath tumours (MPNST): Useful to distinguish MPNST from melanoma? Histopathology 2018, 73, 969–982. [Google Scholar] [CrossRef]
- Loras, A.; Gil-Barrachina, M.; Marqués-Torrejón, M.Á.; Perez-Pastor, G.; Martinez-Cadenas, C. UV-Induced Somatic Mutations Driving Clonal Evolution in Healthy Skin, Nevus, and Cutaneous Melanoma. Life 2022, 12, 1339. [Google Scholar] [CrossRef]
- Davis, E.J.; Johnson, D.B.; Sosman, J.A.; Chandra, S. Melanoma: What do all the mutations mean? Cancer 2018, 124, 3490–3499. [Google Scholar] [CrossRef]
- Garbe, C.; Amaral, T.; Peris, K.; Hauschild, A.; Arenberger, P.; Basset-Seguin, N.; Bastholt, L.; Bataille, V.; Del Marmol, V.; Dréno, B.; et al. European Dermatology Forum (EDF), the European Association of Dermato-Oncology (EADO), and the European Organization for Research and Treatment of Cancer (EORTC). European consensus-based interdisciplinary guideline for melanoma. Part 2: Treatment—Update 2022. Eur. J. Cancer 2022, 170, 256–284. [Google Scholar] [CrossRef]
- Johnston, L.; Starkey, S.; Mukovozov, I.; Robertson, L.; Petrella, T.; Alhusayen, R. Surveillance After a Previous Cutaneous Melanoma Diagnosis: A Scoping Review of Melanoma Follow-Up Guidelines. J. Cutan. Med. Surg. 2023, 27, 516–525. [Google Scholar] [CrossRef]
- Villani, A.; Scalvenzi, M.; Micali, G.; Lacarrubba, F.; Fornaro, L.; Martora, F.; Potestio, L. Management of Advanced Invasive Melanoma: New Strategies. Adv. Ther. 2023, 40, 3381–3394. [Google Scholar] [CrossRef]
- Kobeissi, I.; Tarhini, A.A. Systemic adjuvant therapy for high-risk cutaneous melanoma. Ther. Adv. Med. Oncol. 2022, 14, 17588359221134087. [Google Scholar] [CrossRef] [PubMed]





| Reference | Age | Gender | Primary Tumour | Site | Melanocytic Markers | Other Markers | Ultrastructural Features | Molecular Tests |
|---|---|---|---|---|---|---|---|---|
| Bittesini L et al., 1992 [5] | 74 | Male | N/A | Axillary lymph nodes | Negative: S100, HMB45 | Positive: Vimentin, desmin | Whorls of intermediate filaments; no melanosomes | NP |
| Laskin WB et al., 1995 [6] | 60 | Male | Melanoma NOS | Subcutaneous tissue | Negative: S100, HMB45 | Positive: vimentin, desmin Negative: myoglobin | Paranuclear aggregates of intermediate filaments; no tonofilaments, melanosomes, or skeletal muscle differentiation. | NP |
| Pasz-Walczak G et al., 2002 [7] | 32 | Female | Melanoma NOS | Lymph node | Positive: S100 Negative: HMB45 | Positive: desmin | NP | NP |
| 41 | Female | Melanoma NOS | Brain | Positive: HMB45 | Positive: desmin | NP | NP | |
| Abbott JJ et al., 2004 [8] | 62 | Male | N/A | Lung | Positive: S100, focally Mart-1 Negative: HMB45, MelanA, tyrosinase | Positive: Vimentin, CD56 Negative: desmin, SMA, EMA | Paranuclear whorls of intermediate filaments and no melanomas | NP |
| Gharpuray-Pandit D et al., 2007 [9] | 21 | Female | N/A | Latero-cervical lymph nodes | Focally positive: S100, HMB45, MelanA, Tyrosinase | Positive: Desmin, myoglobin, myogenin and Myo D1 in rhabdoid areas | Nonpigmented melanosomes with primitively developed lattices; no rhabdomyoblastic differentiation | NP |
| Gavino AC et al., 2008 [10] | 64 | Male | Acral melanoma | Subcutaneous tissue | Focally positive: S100, HMB45 | Positive: Vimentin Negative: desmin, SMA, MSA | Condensed, twisted sheaves of intermediate filaments | NP |
| Nakamura T et al., 2009 [11] | 44 | Female | N/A | Subcutaneous tissue | Positive: 100, MelanA Negative: HMB45 | Positive: Vimentin, EMA Negative: Desmin, CD34, cytokeratin | NP | NP |
| Guo R et al., 2013 [12] | 85 | Male | Nodular Melanoma | Parotid gland | Positive: MIFT Negative: S100, HMB45, MelanA, Tyrosinase | Desmin-, SMA- | NP | gains in 6p (RREB1), chromosome 7, and 8q11.1-q24.3 (MYC) additional copy of chromosomal 7q (BRAF), loss of chromosome 9p24.3-q13 (CDKN2A) and chromosome 4 (Melan A encoding gene) |
| Reilly DJ et al., 2014 [13] | 59 | Male | Nodular Melanoma | Axillary lymph nodes | S100, HMB45, MelanA, Tyrosinase focally positive, negative in rhabdoid areas | Positive: Desmin, myogenin Negative: SMA | NP | BRAF mutated |
| Agaimy A et al., 2016 [14] | 24 | Female | Melanoma NOS | Lung | Positive in isolated cells: S100, HMB45, PanMelanoma Negative: HMB45, MelanA | Positive: desmin, SMA, myogenin, myoglonin | NP | BRAF V6000E mutated |
| 69 | Female | N/A | Jejunum | Negative: S100, HMB45, MelanA, PanMelanoma, SOX10 | Positive: desmin, myoglobin, myogenin Negative: SMA | NP | BRAF wild-type, NRAS wild-type | |
| Dumitru AV et al., 2018 [15] | 57 | Female | N/A | Ileum | Positive: PanMelanoma, MART-1, S100 in classic areas Negative: HMB45 | Positive in rhabdoid areas: Desmin, actin, myoglobin | NP | NP |
| Campbell K et al., 2018 [16] | 52 | Female | Invasive melanoma | Axillary lymph nodes | Positive: S100, Mart-1 | Positive: desmin Negative: myogenin | NP | BRAF V6000E mutated |
| Thoracic vertebra | Focally positive: HMB45 Negative: S100, Mart-1, SOX10 | Positive: desmin, myogenin | NP | NP | ||||
| Kidney | Focally positive: HMB45, SOX10, Mart-1 Negative: S100 | Positive: desmin, myogenin | NP | NP | ||||
| Tran TAN et al., 2019 [17] | 65 | Female | Nodular Melanoma | Lung | Negative: S100, SOX10, MITF, MelanA, HMB45 | Positive: desmin, myogenin | NP | BRAF V600E mutated |
| Mehta A et al., 2020 [18] | 65 | Male | N/A | Lung | Positive: HMB45, MelanA, SOX10 Negative: S100 | Positive: Vimentin, desmin, SMA, WT1 | NP | BRAF wild-type, c-KIT mutated |
| Tzanavaris K et al., 2022 [19] | 63 | Male | Nodular Melanoma | Base of tongue | Positive: S00, HMB45, MelanA, SOX10 | Focally positive: Myogenin, Myo-D1, chromogranin and synaptophysin | NP | BRAF V600E mutated |
| Cilento MA et al., 2022 [20] | 57 | Female | Melanoma NOS | Abdominal | Focal positivity for melanocytic markers | Positive: Myogenin | NP | BRAF V600E mutated |
| Kasago IS, 2023 [21] | 69 | Female | N/A | Deep abdominal wall | Focally positive: Prame Negative: S100, HMB45, SOX10, MelanA, | Positive: Desmin, Myogenin, MyoD1 | NP | NF1 R1241 mutated |
| 21 | Female | Melanoma NOS | Positive: Prame, BRAF V600E Negative: S100, HMB45, SOX10, MelanA | Positive: Desmin, Myogenin, MyoD1 | NP | BRAF V600E mutated |
| Reference | Age | Gender | Conventional Melanoma | Site | Immunohistochemistry | Molecular Tests | Treatment and Follow-Up |
|---|---|---|---|---|---|---|---|
| Parham DM et al., 1994 [31] | 7 | Male | N/A | Back | Positive: S100, vimentin | NP | 9 months |
| Borek BT et al., 1998 [32] | 30 | Female | Nodular melanoma | Thigh | Positive: S100, vimentin Negative: HMB45, SMA, desmin | NP | 60 months |
| 36 | Female | Nodular melanoma | Scalp | Positive: S100, vimentin Negative: HMB45, SMA, desmin | NP | 12 months | |
| 59 | Male | Nodular melanoma | Back | Positive: S100, vimentin, SMA Negative: HMB45, desmin | NP | N/A | |
| Gattenlöhner S et al., 2008 [33] | 41 | Male | Primary cutaneous melanoma | Scalp | Positive: S100, MelanA, desmin, myogenin | Loss of chromosome 1q31, amplification of 1q32, gain of 12q23-qter | 6 months: DOD |
| Tallon B et al., 2009 [34] | 74 | Male | Primary cutaneous spindle cell melanoma | Back | Positive: S100, HMB45, MelanA in spindle cells, negative in rhabdoid cells, Negative: desmin | NP | 12 months: alive with pulmonary metastases |
| Chung BY et al., 2011 [35] | 54 | Male | Primary cutaneous melanoma | Forearm | Positive: S100, HMB45, Fontana-Masson silver, vimentin Negative: CD68, CD34, CD99, SMA, desmin | NP | 4 months: no progression |
| Shenjere P et al., 2014 [30] | 67 | Female | Primary cutaneous melanoma | Chest | Positive: S100, HMB45, MelanA, MITF in conventional melanoma; Desmin, myogenin, myoD1 in rhabdoid areas | BRAF wild type | 2 years: pulmonary metastases but died from unrelated causes |
| Kaneko T et al., 2015 [36] | 63 | Male | Primary cutaneous melanoma | Heel | Positive: S100, MelanA, HMB45, vimentin Negative: desmin, αSMA | BRAF wild type | Chemotherapy + immunotherapy 44 months: pulmonary metastases |
| Fernández-Vega I et al., 2016 [37] | 80 | Male | Primary cutaneous melanoma | Forehead | Weakly positive: S100, SOX10 Positive: vimentin, desmin Negative: HMB45, MelanA, SMA, myoD1 | NP | Radiotherapy 2 months: DOD |
| Antonov NK et al., 2016 [38] | 75 | Male | Primary cutaneous melanoma | Scalp | Focally positive: MelanA, S100, myogenin Positive: desmin | NP | Chemotherapy 7 months: DOD |
| Prieto-Torres L et al., 2016 [39] | 69 | Female | Primary cutaneous melanoma | Scapula | Positive: S100, SOX10, desmin, SMA Negative: MITF, MelanA, HMB45, MyoD1, myogenin, myoglobin | NP | 14 months: no progression |
| Kuwadekar A et al., 2018 [40] | 72 | Male | Superficial spreading melanoma | Scalp | Positive: desmin, myogenin Negative: S100, SOX10, HMB45, MelanA | NP | N/A |
| Murakami T et al., 2019 [41] | 78 | Male | Primary cutaneous melanoma | Forehead | Positive: S100, vimentin Negative in rhabdoid cells: MelanA, HMB45 Negative: desmin | NP | 24 months: no progression |
| Tran TAN et al., 2019 [42] | 96 | Male | Lentigo maligna melanoma | Forearm | Positive: desmin, myoD1, myogenin Negative: S100, SOX10, HMB45, MelanA | NRAS c.182A, KDR c.3434G | 5 months: local recurrences |
| Ferreira I et al., 2021 [43] | 68 | Male | Superficial spreading melanoma | Nose | Positive: desmin, myogenin, myoD1 Negative: S100, SOX10, HMB45, MelanA | NF1, TP53, CDKN2A, RAC1 | 8 months: DOD |
| 85 | Female | Desmoplastic melanoma | Chin | Positive: des-min, myogenin, myoD1 Negative: S100, SOX10, HMB45, MelanA | NF1, TP53, ATRX, RASA2 | 34 months: no progression | |
| Agaimy A et al., 2021 [44] | 55 | Female | N/A | Lower leg | Negative: S100, SOX10, HMB45, MelanA, Pan-Melanoma | BRAF V600E | Inguinal, subcutaneous lung metastasis; no follow-up |
| Yim SH et al., 2022 [45] | 64 | Male | Melanoma arising in nevus | Scalp | Positive: desmin, myoD1 Weakly positive: S100, SOX10 Negative: HMB45, MelanA, BRAF V600E | NP | Chemotherapy 2 months: DOD |
| Glutsch V et al., 2022 [46] | 72 | Female | Acral lentiginous melanoma | Ankle | Positive: S100, SOX10, MART1, HMB45, vimentin, PRAME Negative: desmin | NP | Chemotherapy 11 months: DOD |
| 74 | Male | Nodular melanoma | Chest | Positive: desmin, PRAME Unspecific/focally positive: SOX10, HMB45 Negative: S100, MART1 | NP | 2 months: DOD | |
| 75 | Male | Nodular melanoma | Scalp | Positive: S100, SOX10, MART1, HMB45, vimentin, PRAME Negative: desmin | NP | 3 months: in transit metastasis | |
| 79 | Male | Nodular melanoma | Arm | Positive: S100, SOX10, vimentin, PRAME Negative: desmin, MART1, HMB45 | NP | N/A | |
| O’Neill P et al., 2023 [47] | 74 | Male | Nodular melanoma | Chest | Positive: desmin, myoD1, myogenin Negative: SOX10, HMB45, MelanA | NRAS, TERTp, CDKN2A, NF1, FGFR2, CBL, BLM and TP53 | Immunotherapy 42 months: widespread metastasis |
| Choy A et al., 2025 [48] | 88 | Male | Desmoplastic melanoma | Scalp | Focally positive: SOX10, S100, PRAME, MelanA, desmin, MyoD1 Negative: AE1/AE3, SMA, ERG, BRAF V600E | N/A | 2 months: no progression |
| Weigelt MA et al., 2025 [49] | 83 | Female | N/A | Deltoid region | Positive: PRAME, desmin, myogenin, myoD1 Negative: cytokeratins, HMB45, S100, SOX10, BRAF V600E, NRAS Q61R | ARID2, BRCA2, CRKL, FANCB, LZTR1, TERT, APC, EGFR, ERBB2, FLCN, TP53 | Immunotherapy 10 months: no progression |
| Reference | Age | Gender | Tumour Site | Metastatic Site |
|---|---|---|---|---|
| Gattenlöhner S et al., 2008 [33] | 41 | Male | Scalp | Lung, mediastinum, abdominal organs |
| Shenjere P et al., 2014 [30] | 67 | Female | Chest | Lung |
| Antonov NK et al., 2016 [38] | 75 | Male | Scalp | Lung |
| Kuwadekar A et al., 2018 [40] | 72 | Male | Scalp | N/A |
| Tran TAN et al., 2019 [42] | 96 | Male | Forearm | Local recurrence |
| Ferreira I et al., 2021 [43] | 68 | Male | Nose | Lung, brain |
| 85 | Female | Chin | No progression | |
| Yim SH et al., 2022 [45] | 64 | Male | Scalp | Lung |
| O’Neill P et al., 2023 [47] | 74 | Male | Chest | Lung, liver, spleen, bone |
| Choy A et al., 2025 [48] | 88 | Male | Scalp | No progression |
| Weigelt MA et al., 2025 [49] | 83 | Female | Deltoid region | No progression |
| This paper | 41 | Male | Thigh | Lung, brain, abdominal organs |
| 42 | Male | Back | Lung, brain, bone | |
| Summary | Mean: 68.92 (SD:17.41) | 76.92% male (n = 10) 23.08% female (n = 3) | 53.84% head and neck (n = 7) 23.08% trunk (n = 3) 23.08% limbs (n = 2) | Metastatic cases: 66.67% (n = 8) Matastatic sites: 100% lung (n = 8), 37,5% abdominal organs (n = 3), 37,5% brain (n = 3), 20% bone (n = 2) |
| Reference | Age | Gender | Site | Immunohistochemistry | Molecular Tests | Follow-Up |
|---|---|---|---|---|---|---|
| Koyama T et al., 1996 [53] | Newborn | Female | Scalp | S100, HMB45, NKI/C3 positive in conventional area, negative in dedifferentiated area Desmin, sarcomeric actin positive in dedifferentiated area Negative: myoglobin, myosin, SMA | NP | N/A |
| Baltres, A et al., 2019 [54] | 15 months | Female | Lumbosacral | Negative: SOX10, MiTF, HMB45, MelanA Positive: myoD1, myogenin, desmin | SASS6-RAF1 fusion | 9 months: lung and liver metastasis |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iliesiu, A.; Nimigean, V.; Tapoi, D.A.; Costache, M. Primary Cutaneous Rhabdomyosarcomatous Melanomas—A Report of Two Cases and Literature Review. Diagnostics 2025, 15, 1357. https://doi.org/10.3390/diagnostics15111357
Iliesiu A, Nimigean V, Tapoi DA, Costache M. Primary Cutaneous Rhabdomyosarcomatous Melanomas—A Report of Two Cases and Literature Review. Diagnostics. 2025; 15(11):1357. https://doi.org/10.3390/diagnostics15111357
Chicago/Turabian StyleIliesiu, Andreea, Victor Nimigean, Dana Antonia Tapoi, and Mariana Costache. 2025. "Primary Cutaneous Rhabdomyosarcomatous Melanomas—A Report of Two Cases and Literature Review" Diagnostics 15, no. 11: 1357. https://doi.org/10.3390/diagnostics15111357
APA StyleIliesiu, A., Nimigean, V., Tapoi, D. A., & Costache, M. (2025). Primary Cutaneous Rhabdomyosarcomatous Melanomas—A Report of Two Cases and Literature Review. Diagnostics, 15(11), 1357. https://doi.org/10.3390/diagnostics15111357






