Low-keV Virtual Monoenergetic Imaging for Bronchial Artery Visualization on Photon-Counting Detector Computed Tomography
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. CT Image Acquisition
2.3. Objective Image Analysis
2.4. Subjective Image Analysis
2.5. Representative Image
2.6. Statistical Analysis
3. Results
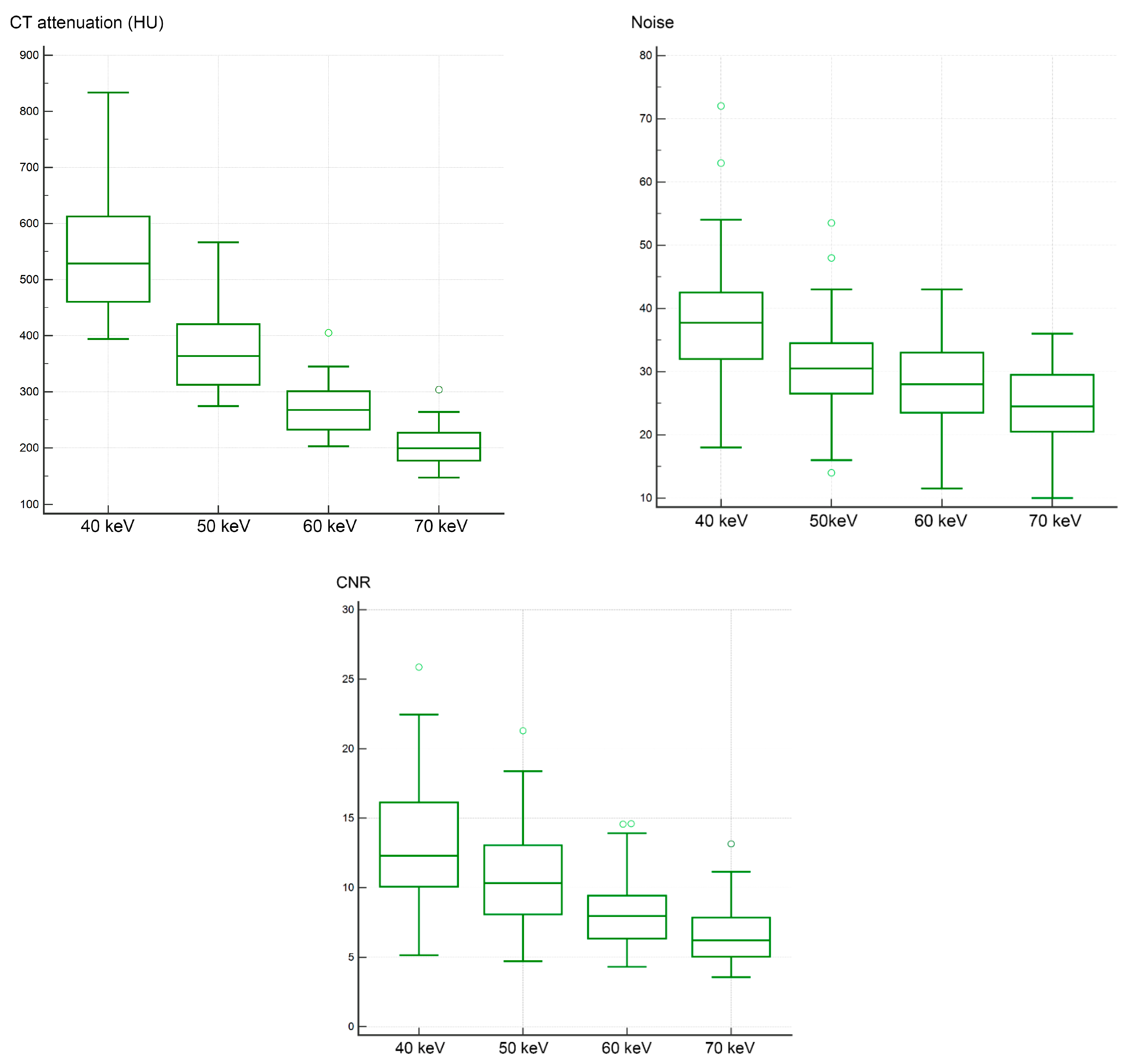
3.1. Objective Image Analysis
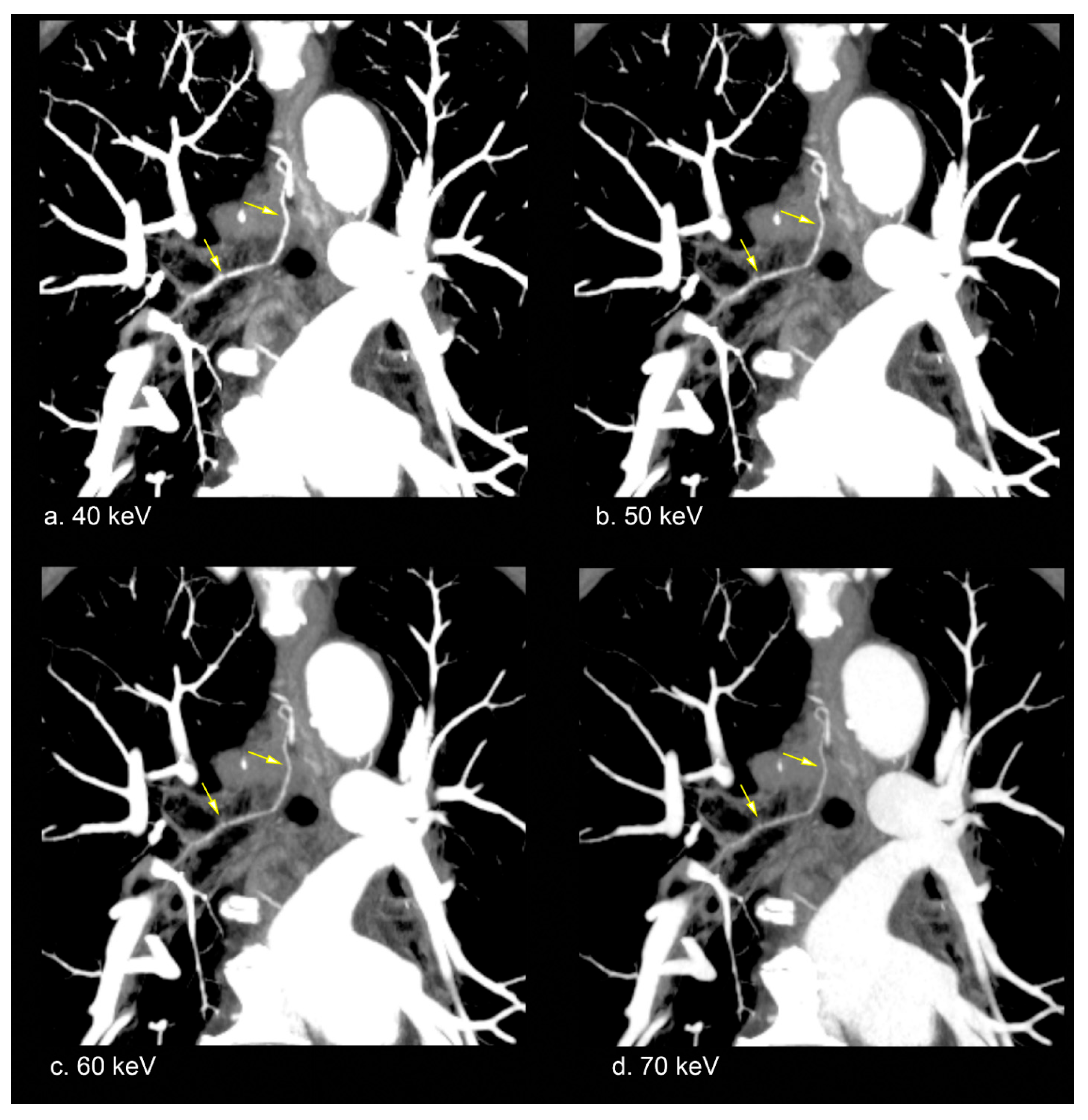
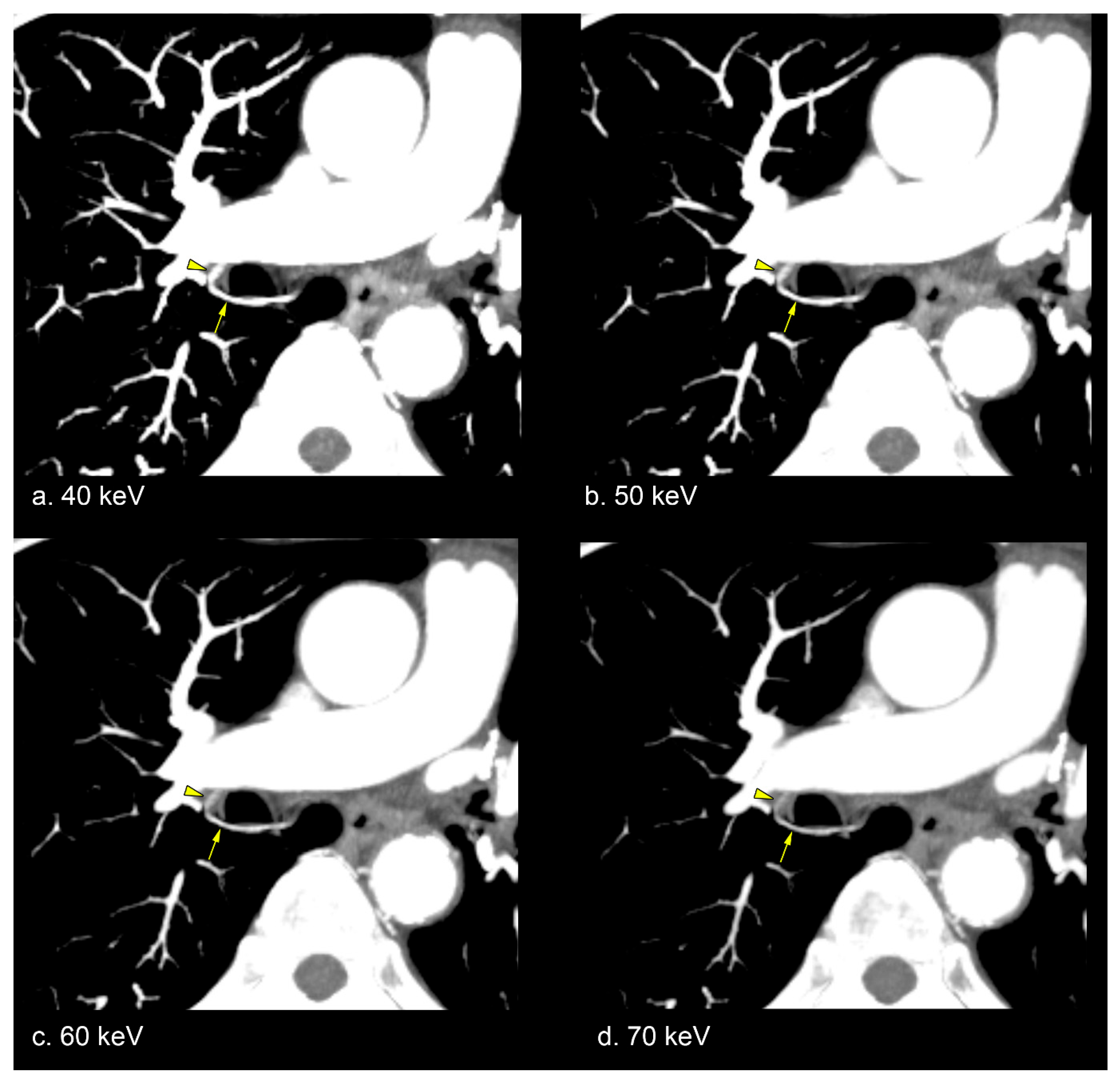
3.2. Subjective Image Analysis
4. Discussion
4.1. Objective Image Analysis
4.2. Subjective Image Analysis
4.3. Utility of Low-keV VMI for Bronchial Artery
4.4. Comparison to EID-CT
4.5. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CNR | Contrast-to-noise ratio |
| CT | Computed tomography |
| EID | Energy-integrating detector |
| MIP | Maximum intensity projection |
| PCD | Photon-counting detector |
| ROI | Regions of interest |
| SD | Standard deviation |
| VMI | Virtual monoenergetic imaging |
| QIR | Quantum iterative reconstruction |
References
- Burke, C.T.; Mauro, M.A. Bronchial artery embolization. Semin. Intervent. Radiol. 2004, 21, 43–48. [Google Scholar] [CrossRef]
- Fei, Q.L.; Zhou, Y.Y.; Yuan, Y.X.; Sun, S.Q. An applied anatomical study of bronchial artery. Surg. Radiol. Anat. 2018, 40, 55–61. [Google Scholar] [CrossRef]
- Battal, B.; Akgun, V.; Karaman, B.; Bozlar, U.; Tasar, M. Normal anatomical features and variations of bronchial arteries: An analysis with 64-detector-row computed tomographic angiography. J. Comput. Assist. Tomogr. 2011, 35, 253–259. [Google Scholar] [CrossRef] [PubMed]
- Walker, C.M.; Rosado-de-Christenson, M.L.; Martinez-Jimenez, S.; Kunin, J.R.; Wible, B.C. Bronchial arteries: Anatomy, function, hypertrophy, and anomalies. Radiographics 2015, 35, 32–49. [Google Scholar] [CrossRef] [PubMed]
- Osiro, S.; Wear, C.; Hudson, R.; Ma, X.X.; Zurada, A.; Michalak, M.; Loukas, M. A friend to the airways: A review of the emerging clinical importance of the bronchial arterial circulation. Surg. Radiol. Anat. 2012, 34, 791–798. [Google Scholar] [CrossRef] [PubMed]
- Pramesh, C.S.; Mistry, R.C.; Sharma, S.; Pantvaidya, G.H.; Raina, S. Bronchial artery preservation during transthoracic esophagectomy. J. Surg. Oncol. 2004, 85, 202–203. [Google Scholar] [CrossRef]
- Chun, J.Y.; Belli, A.M. Immediate and long-term outcomes of bronchial and non-bronchial systemic artery embolisation for the management of haemoptysis. Eur. Radiol. 2010, 20, 558–565. [Google Scholar] [CrossRef]
- Ma, G.; He, T.; Yu, Y.; Duan, H.; Yang, C. Improving image quality of bronchial arteries with virtual monochromatic spectral CT images. PLoS ONE 2016, 11, e0150985. [Google Scholar] [CrossRef]
- D’Angelo, T.; Cicero, G.; Mazziotti, S.; Ascenti, G.; Albrecht, M.H.; Martin, S.S.; Othman, A.E.; Vogl, T.J.; Wichmann, J.L. Dual energy computed tomography virtual monoenergetic imaging: Technique and clinical applications. Br. J. Radiol. 2019, 92, 20180546. [Google Scholar] [CrossRef]
- Albrecht, M.H.; Vogl, T.J.; Martin, S.S.; Nance, J.W.; Duguay, T.M.; Wichmann, J.L.; De Cecco, C.N.; Varga-Szemes, A.; van Assen, M.; Tesche, C.; et al. Review of clinical applications for virtual monoenergetic dual-energy CT. Radiology 2019, 293, 260–271. [Google Scholar] [CrossRef]
- Chalian, H.; Kalisz, K.; Rassouli, N.; Dhanantwari, A.; Rajiah, P. Utility of virtual monoenergetic images derived from a dual-layer detector-based spectral CT in the assessment of aortic anatomy and pathology: A retrospective case control study. Clin. Imaging 2018, 52, 292–301. [Google Scholar] [CrossRef] [PubMed]
- Pinho, D.F.; Kulkarni, N.M.; Krishnaraj, A.; Kalva, S.P.; Sahani, D.V. Initial experience with single-source dual-energy CT abdominal angiography and comparison with single-energy CT angiography: Image quality, enhancement, diagnosis and radiation dose. Eur. Radiol. 2013, 23, 351–359. [Google Scholar] [CrossRef] [PubMed]
- Abel, F.; Schubert, T.; Winklhofer, S. Advanced neuroimaging with photon-counting detector CT. Investig. Radiol. 2023, 58, 472–481. [Google Scholar] [CrossRef] [PubMed]
- Esquivel, A.; Ferrero, A.; Mileto, A.; Baffour, F.; Horst, K.; Rajiah, P.S.; Inoue, A.; Leng, S.; McCollough, C.; Fletcher, J.G. Photon-counting detector CT: Key points radiologists should know. Korean J. Radiol. 2022, 23, 854–865. [Google Scholar] [CrossRef] [PubMed]
- Douek, P.C.; Boccalini, S.; Oei, E.H.G.; Cormode, D.P.; Pourmorteza, A.; Boussel, L.; Si-Mohamed, S.A.; Budde, R.P.J. Clinical applications of photon-counting CT: A review of pioneer studies and a glimpse into the future. Radiology 2023, 309, e222432. [Google Scholar] [CrossRef]
- Flohr, T.; Petersilka, M.; Henning, A.; Ulzheimer, S.; Ferda, J.; Schmidt, B. Photon-counting CT review. Phys. Med. 2020, 79, 126–136. [Google Scholar] [CrossRef]
- Tortora, M.; Gemini, L.; D’Iglio, I.; Ugga, L.; Spadarella, G.; Cuocolo, R. Spectral photon-counting computed tomography: A review on technical principles and clinical applications. J. Imaging. 2022, 8, 112. [Google Scholar] [CrossRef]
- Hagen, F.; Soschynski, M.; Weis, M.; Hagar, M.T.; Krumm, P.; Ayx, I.; Taron, J.; Krauss, T.; Hein, M.; Ruile, P.; et al. Photon-counting computed tomography—Clinical application in oncological, cardiovascular, and pediatric radiology. RoFo 2024, 196, 25–35. [Google Scholar] [CrossRef]
- Leng, S.; Bruesewitz, M.; Tao, S.; Rajendran, K.; Halaweish, A.F.; Campeau, N.G.; Fletcher, J.G.; McCollough, C.H. Photon-counting detector CT: System design and clinical applications of an emerging technology. Radiographics 2019, 39, 729–743. [Google Scholar] [CrossRef]
- Dogra, S.; Shekhrajka, N.; Moonis, G. A review of applications of photon-counting computed tomography in head and neck imaging. Br. J. Radiol. 2024, tqae226. [Google Scholar] [CrossRef]
- Zsarnoczay, E.; Fink, N.; Schoepf, U.J.; Pinos, D.; O’Doherty, J.; Allmendinger, T.; Hagenauer, J.; Griffith Iii, J.P.; Vecsey-Nagy, M.; Maurovich-Horvat, P.; et al. Accuracy of ultra-high resolution and virtual non-calcium reconstruction algorithm for stenosis evaluation with photon-counting CT: Results from a dynamic phantom study. Eur. Radiol. Exp. 2024, 8, 102. [Google Scholar] [CrossRef]
- Okazaki, T.; Niwa, T.; Yoshida, R.; Sorimachi, T.; Hashimoto, J. Visibility of intracranial perforating arteries using ultra-high-resolution photon-counting detector computed tomography (CT) angiography. Tomography 2024, 10, 1867–1880. [Google Scholar] [CrossRef]
- Willemink, M.J.; Persson, M.; Pourmorteza, A.; Pelc, N.J.; Fleischmann, D. Photon-counting CT: Technical principles and clinical prospects. Radiology 2018, 289, 293–312. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Ye, Z.; Chen, J.; Deng, L.; Song, B. Photon counting CT: Technical principles, clinical applications, and future prospects. Acad. Radiol. 2023, 30, 2362–2382. [Google Scholar] [CrossRef] [PubMed]
- Tariq, B.; Sikander, O.; Francis, N.; Alkhatib, M.; Naseer, F.; Werghi, N.; Memisoglu, E.; Maalej, N.; Raja, A. Assessment of material identification and quantification in the presence of metals using spectral photon counting CT. PLoS ONE 2024, 19, e0308658. [Google Scholar] [CrossRef]
- Feldle, P.; Scheuber, M.; Grunz, J.P.; Heidenreich, J.F.; Pannenbecker, P.; Nora, C.; Huflage, H.; Bley, T.A.; Petritsch, B. Virtual non-iodine photon-counting CT-angiography for aortic valve calcification scoring. Sci. Rep. 2024, 14, 4724. [Google Scholar] [CrossRef]
- Zanon, C.; Cademartiri, F.; Toniolo, A.; Bini, C.; Clemente, A.; Colacchio, E.C.; Cabrelle, G.; Mastro, F.; Antonello, M.; Quaia, E.; et al. Advantages of photon-counting detector CT in aortic imaging. Tomography 2023, 10, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Sartoretti, T.; Wildberger, J.E.; Flohr, T.; Alkadhi, H. Photon-counting detector CT: Early clinical experience review. Br. J. Radiol. 2023, 96, 20220544. [Google Scholar] [CrossRef]
- Euler, A.; Higashigaito, K.; Mergen, V.; Sartoretti, T.; Zanini, B.; Schmidt, B.; Flohr, T.G.; Ulzheimer, S.; Eberhard, M.; Alkadhi, H. High-pitch photon-counting detector computed tomography angiography of the aorta: Intraindividual comparison to energy-integrating detector computed tomography at equal radiation dose. Investig. Radiol. 2022, 57, 115–121. [Google Scholar] [CrossRef]
- Dillinger, D.; Overhoff, D.; Booz, C.; Kaatsch, H.L.; Piechotka, J.; Hagen, A.; Froelich, M.F.; Vogl, T.J.; Waldeck, S. Impact of CT photon-counting virtual monoenergetic imaging on visualization of abdominal arterial vessels. Diagnostics 2023, 13, 938. [Google Scholar] [CrossRef]
- Ota, T.; Onishi, H.; Itoh, T.; Fukui, H.; Tsuboyama, T.; Nakamoto, A.; Enchi, Y.; Tatsumi, M.; Tomiyama, N. Investigation of abdominal artery delineation by photon-counting detector CT. Radiol. Med. 2024, 129, 1265–1274. [Google Scholar] [CrossRef] [PubMed]
- Grosse Hokamp, N.; Gilkeson, R.; Jordan, M.K.; Laukamp, K.R.; Neuhaus, V.F.; Haneder, S.; Halliburton, S.S.; Gupta, A. Virtual monoenergetic images from spectral detector CT as a surrogate for conventional CT images: Unaltered attenuation characteristics with reduced image noise. Eur. J. Radiol. 2019, 117, 49–55. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Bao, S.; Wang, M.; Xu, Z.; Yan, F.; Yang, W. Stability and accuracy of fat quantification on photon-counting detector CT with various scan settings: A phantom study. Eur. J. Radiol. 2024, 177, 111545. [Google Scholar] [CrossRef] [PubMed]
- Higashigaito, K.; Mergen, V.; Eberhard, M.; Jungblut, L.; Hebeisen, M.; Ratzer, S.; Zanini, B.; Kobe, A.; Martini, K.; Euler, A.; et al. CT angiography of the aorta using photon-counting detector CT with reduced contrast media volume. Radiol. Cardiothorac. Imaging 2023, 5, e220140. [Google Scholar] [CrossRef]
- Almutairi, A.; Al Safran, Z.; AlZaabi, S.A.; Sun, Z. Dual energy CT angiography in peripheral arterial stents: Optimal scanning protocols with regard to image quality and radiation dose. Quant. Imaging Med. Surg. 2017, 7, 520–531. [Google Scholar] [CrossRef]
- Mergen, V.; Ried, E.; Allmendinger, T.; Sartoretti, T.; Higashigaito, K.; Manka, R.; Euler, A.; Alkadhi, H.; Eberhard, M. Epicardial adipose tissue attenuation and fat attenuation index: Phantom study and in vivo measurements with photon-counting detector CT. AJR Am. J. Roentgenol. 2022, 218, 822–829. [Google Scholar] [CrossRef]
- Panda, A.; Bhalla, A.S.; Goyal, A. Bronchial artery embolization in hemoptysis: A systematic review. Diagn. Interv. Radiol. 2017, 23, 307–317. [Google Scholar] [CrossRef]
- Kaufman, C.; Kabutey, N.K.; Sgroi, M.; Kim, D. Bronchial artery pseudoaneurysm with symptomatic mediastinal hematoma. Clin. Imaging 2014, 38, 536–539. [Google Scholar] [CrossRef]
- Wildberger, J.E.; Alkadhi, H. New horizons in vascular imaging with photon-counting detector CT. Investig. Radiol. 2023, 58, 499–504. [Google Scholar] [CrossRef]
- Zsarnoczay, E.; Varga-Szemes, A.; Emrich, T.; Szilveszter, B.; van der Werf, N.R.; Mastrodicasa, D.; Maurovich-Horvat, P.; Willemink, M.J. Characterizing the heart and the myocardium with photon-counting CT. Investig. Radiol. 2023, 58, 505–514. [Google Scholar] [CrossRef]
- Schwartz, F.R.; Samei, E.; Marin, D. Exploiting the potential of photon-counting ct in abdominal imaging. Investig. Radiol. 2023, 58, 488–498. [Google Scholar] [CrossRef] [PubMed]
- Remy-Jardin, M.; Hutt, A.; Flohr, T.; Faivre, J.B.; Felloni, P.; Khung, S.; Remy, J. Ultra-high-resolution photon-counting CT imaging of the chest: A new era for morphology and function. Investig. Radiol. 2023, 58, 482–487. [Google Scholar] [CrossRef] [PubMed]
- Pourmorteza, A.; Symons, R.; Reich, D.S.; Bagheri, M.; Cork, T.E.; Kappler, S.; Ulzheimer, S.; Bluemke, D.A. Photon-counting CT of the brain: In vivo human results and image-quality assessment. AJNR Am. J. Neuroradiol. 2017, 38, 2257–2263. [Google Scholar] [CrossRef]
- Rajagopal, J.R.; Farhadi, F.; Solomon, J.; Sahbaee, P.; Saboury, B.; Pritchard, W.F.; Jones, E.C.; Samei, E. Comparison of low dose performance of photon-counting and energy integrating CT. Acad. Radiol. 2021, 28, 1754–1760. [Google Scholar] [CrossRef] [PubMed]
| Scan Parameter | Value |
|---|---|
| Tube voltage | 120 kv |
| Collimation | 144 × 0.4 mm |
| Pitch | 2.4 |
| Gantry rotation time | 0.5 s |
| CARE keV image quality level | 80 |
| Reconstruction kernel | Qr40 |
| QIR | Strength level 2 |
| Matrix size | 512 × 512 |
| VMI at 40 keV | VMI at 50 keV | VMI at 60 keV | VMI at 70 keV | p | |
|---|---|---|---|---|---|
| CT attenuation (HU) | 528.8 (394.0–833.5) | 363.8 (274.5–566.5) | 267.5 (203.0–405.0) | 199.5 (147.5–303.5) | <0.00001 * |
| Noise | 37.8 (18.0–72.0) | 30.5 (14.0–53.5) | 28.0 (11.5–43.0) | 24.5 (10.0–36.0) | <0.00001 * |
| CNR | 12.3 (5.1–25.9) | 10.3 (4.7–21.3) | 8.0 (4.3–14.6) | 6.2 (3.6–13.1) | <0.00001 * |
| Assessment | VMI at 40 keV | VMI at 50 keV | VMI at 60 keV | p | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Score | 1 | 2 | 3 | 4 | 5 | 1 | 2 | 3 | 4 | 5 | 1 | 2 | 3 | 4 | 5 | |
| Enhancement | ||||||||||||||||
| Observer 1 | 0 | 0 | 0 | 3 | 31 | 0 | 0 | 0 | 10 | 24 | 0 | 0 | 11 | 19 | 4 | <0.00001 * |
| Observer 2 | 0 | 0 | 0 | 7 | 27 | 0 | 0 | 1 | 23 | 10 | 0 | 0 | 14 | 19 | 1 | <0.00001 * |
| Border clarity | ||||||||||||||||
| Observer 1 | 0 | 0 | 0 | 2 | 32 | 0 | 0 | 0 | 10 | 24 | 0 | 0 | 8 | 23 | 3 | <0.00001 * |
| Observer 2 | 0 | 0 | 0 | 7 | 27 | 0 | 0 | 3 | 22 | 9 | 0 | 0 | 19 | 14 | 1 | <0.00001 * |
| Peripheral visibility | ||||||||||||||||
| Observer 1 | 0 | 0 | 0 | 5 | 29 | 0 | 0 | 3 | 15 | 16 | 0 | 0 | 20 | 11 | 3 | <0.00001 * |
| Observer 2 | 0 | 0 | 0 | 6 | 28 | 0 | 0 | 3 | 21 | 10 | 0 | 0 | 24 | 9 | 1 | <0.00001 * |
| Noise | ||||||||||||||||
| Observer 1 | 9 | 24 | 1 | 0 | 0 | 3 | 22 | 9 | 0 | 0 | 1 | 10 | 23 | 0 | 0 | <0.00001 * |
| Observer 2 | 3 | 16 | 15 | 0 | 0 | 0 | 4 | 30 | 0 | 0 | 0 | 3 | 31 | 0 | 0 | <0.00001 † |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, X.; Niwa, T.; Nomura, T.; Yoshida, R.; Koyanagi, K.; Hashimoto, J. Low-keV Virtual Monoenergetic Imaging for Bronchial Artery Visualization on Photon-Counting Detector Computed Tomography. Diagnostics 2025, 15, 1354. https://doi.org/10.3390/diagnostics15111354
Sun X, Niwa T, Nomura T, Yoshida R, Koyanagi K, Hashimoto J. Low-keV Virtual Monoenergetic Imaging for Bronchial Artery Visualization on Photon-Counting Detector Computed Tomography. Diagnostics. 2025; 15(11):1354. https://doi.org/10.3390/diagnostics15111354
Chicago/Turabian StyleSun, Xuyang, Tetsu Niwa, Takakiyo Nomura, Ryoichi Yoshida, Kazuo Koyanagi, and Jun Hashimoto. 2025. "Low-keV Virtual Monoenergetic Imaging for Bronchial Artery Visualization on Photon-Counting Detector Computed Tomography" Diagnostics 15, no. 11: 1354. https://doi.org/10.3390/diagnostics15111354
APA StyleSun, X., Niwa, T., Nomura, T., Yoshida, R., Koyanagi, K., & Hashimoto, J. (2025). Low-keV Virtual Monoenergetic Imaging for Bronchial Artery Visualization on Photon-Counting Detector Computed Tomography. Diagnostics, 15(11), 1354. https://doi.org/10.3390/diagnostics15111354






