The Value of PET/CT-Based Radiomics in Predicting Adrenal Metastases in Patients with Cancer
Abstract
1. Introduction
2. Materials and Methods
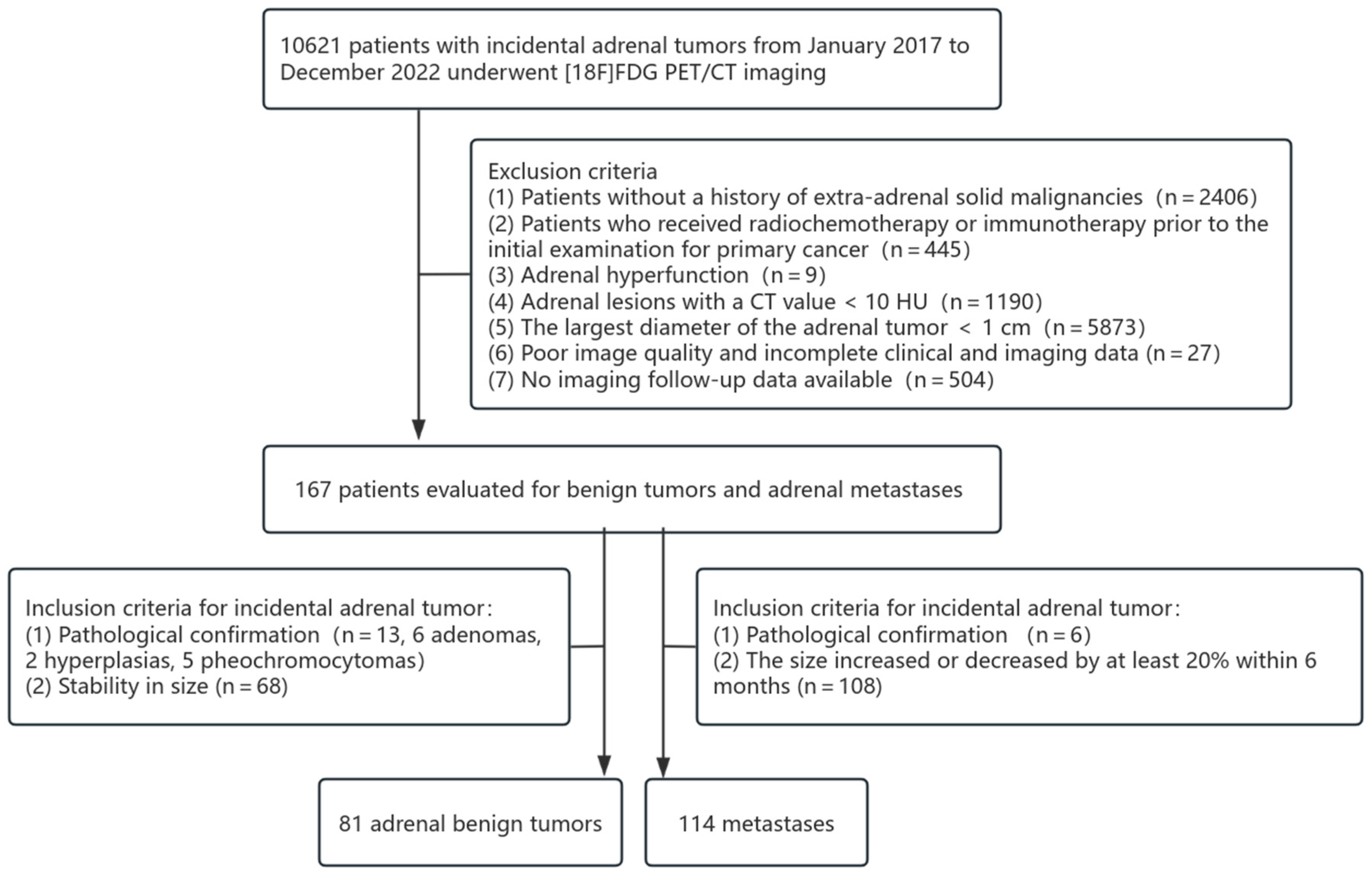
2.1. Patients
2.2. [18F]FDG PET/CT Image Acquisition and Reconstruction
2.3. Image Interpretation
2.4. Construct Models of Clinical and Conventional PET/CT Parameters
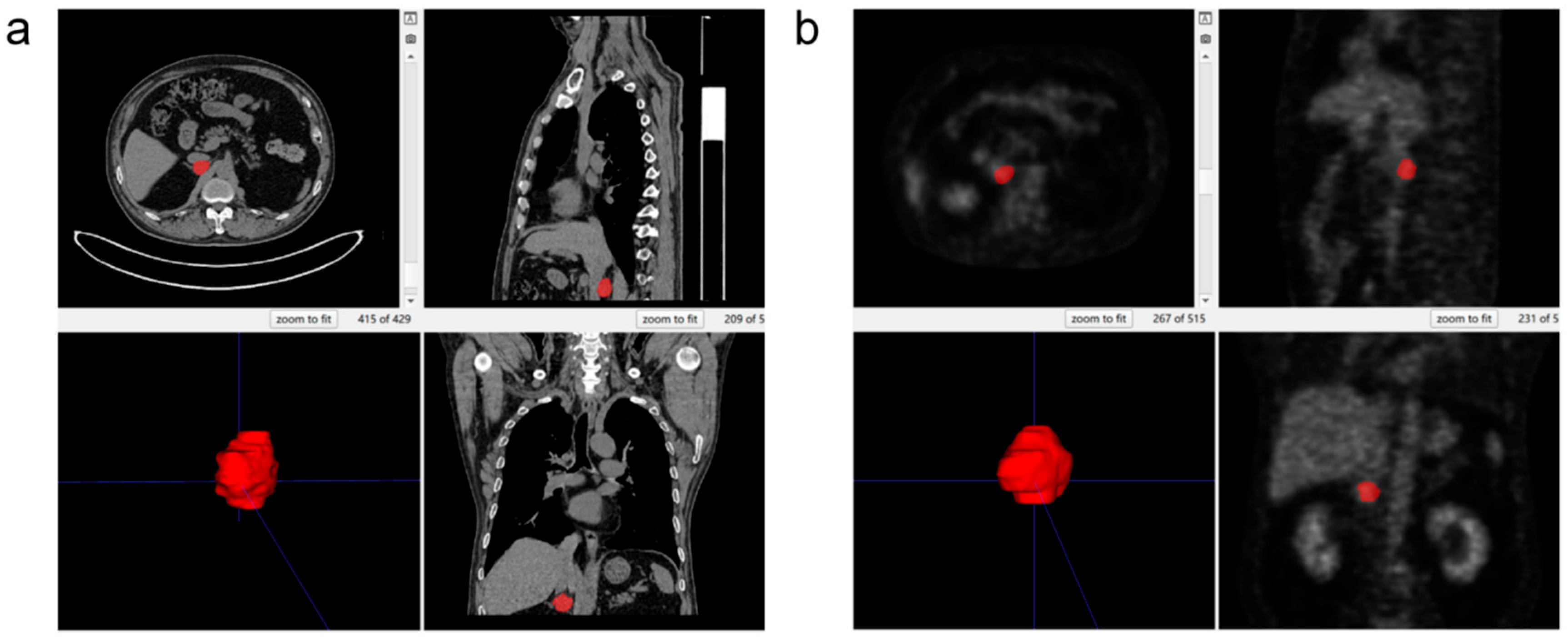
2.5. Three-Dimensional Segmentation
2.6. Feature Extraction and Screening
2.7. Constructed and Evaluated Models
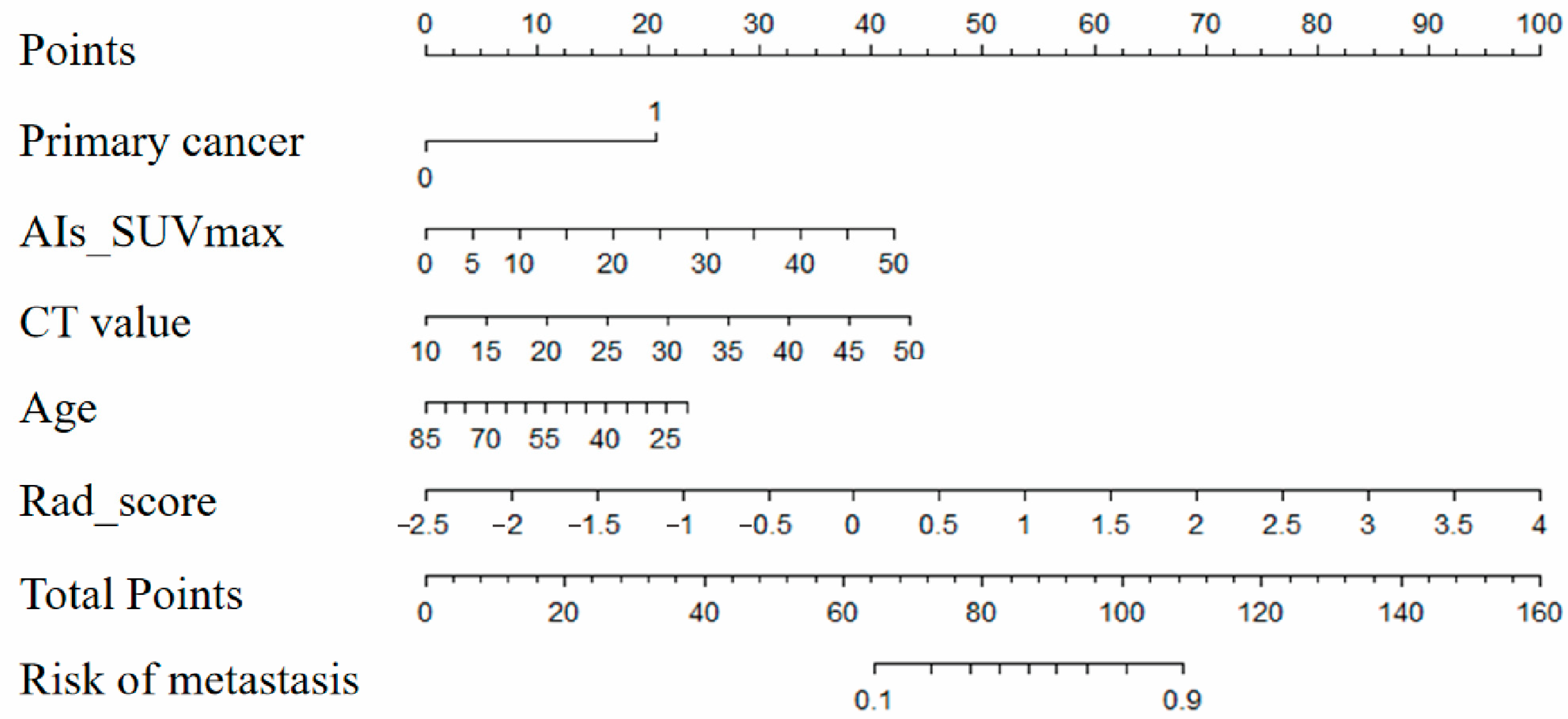
2.8. Draw and Evaluate Nomogram
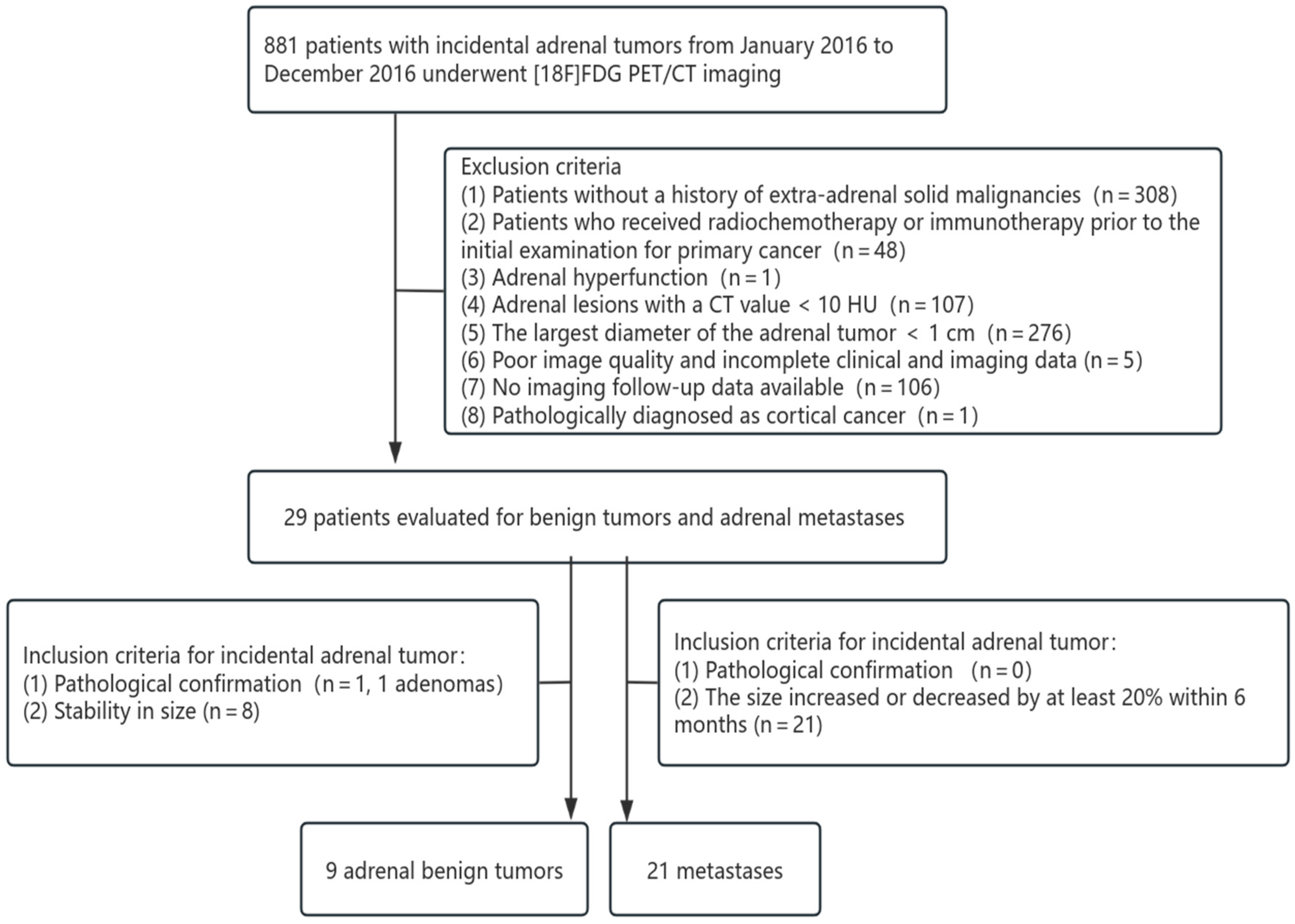
2.9. External Validation Protocol
3. Results
− 0.04 × age + 0.82 × Rad_score − 1.25
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AIs | adrenal incidentalomas |
| [18F]FDG | [18F]Fludeoxyglucose |
| PET/CT | positron emission tomography combined with computed tomography |
| SUVmax | Maximum Standardized Uptake Value |
| T/L | tumor SUVmax/liver SUVmax |
| HU | Hounsfield units |
| Dmax | maximum diameter |
| ROI | region of interest |
| LASSO | the least Absolute shrinkage and selection operator |
| MSE | mean square error |
| ROC | Receiver Operating Characteristic Curve |
| AUC | area under the curve |
| DCA | decision curve analysis |
| APW | Absolute Percentage Washout |
| RPW | Relative Percentage Washout |
| RF | Random Forest |
| AdaBoost | Adaptive Boosting |
| KNN | K-nearest Neighbor |
| GaussianNB | Gaussian Naive Bayes; |
| GDBT | Gradient Boosting Decision Tree |
| LightGBM | Light Gradient Boosting Machine |
| XGBoost | eXtreme Gradient Boosting |
| LR | logistic regression |
| SVM | Support Vector Machine |
| PPV | positive predictive value |
| NPV | negative predictive value |
| GLCM | Gray Level co-occurrence Matrix |
| GLDM | Gray Level Dependence Matrix |
| GLSZM | Gray Level Size Zone Matrix |
| NGTDM | Neighboring gray tone difference matrix |
| GLRLM | Gray-Level Run-Length Matrix |
| MIP | maximum Density projection |
References
- Fassnacht, M.; Arlt, W.; Bancos, I.; Dralle, H.; Newell-Price, J.; Sahdev, A.; Tabarin, A.; Terzolo, M.; Tsagarakis, S.; Dekkers, O.M. Management of adrenal incidentalomas: European Society of Endocrinology Clinical Practice Guideline in collaboration with the European Network for the Study of Adrenal Tumor. Eur. J. Endocrinol. 2016, 175, G1–G34. [Google Scholar] [CrossRef] [PubMed]
- Kebebew, E. Adrenal Incidentaloma. N. Engl. J. Med. 2021, 384, 1542–1551. [Google Scholar] [CrossRef] [PubMed]
- Cao, L.; Xu, W. Radiomics approach based on biphasic CT images well differentiate “early stage” of adrenal metastases from lipid-poor adenomas: A STARD Compliant Artice. Medicine 2022, 101, e30856. [Google Scholar] [CrossRef] [PubMed]
- Sherlock, M.; Scarsbrook, A.; Abbas, A.; Fraser, S.; Limumpornpetch, P.; Dineen, R.; Stewart, P.M. Adrenal incidentaloma. Endocr. Rev. 2020, 41, 775–820. [Google Scholar] [CrossRef]
- Terzolo, M.; Stigliano, A.; Chiodini, I.; Loli, P.; Furlani, L.; Arnaldi, G.; Reimondo, G.; Pia, A.; Toscano, V.; Zini, M.; et al. AME Position Statement on adrenal incidentaloma. Eur. J. Endocrinol. 2011, 164, 851–870. [Google Scholar] [CrossRef] [PubMed]
- Bovio, S.; Cataldi, A.; Reimondo, G.; Sperone, P.; Novello, S.; Berruti, A.; Borasio, P.; Fava, C.; Dogliotti, L.; Scagliotti, G.V.; et al. Prevalence of adrenal incidentaloma in a contemporary computerized tomography series. J. Endocrinol. Investig. 2006, 29, 298–302. [Google Scholar] [CrossRef]
- Zheng, Q.Y.; Zhang, G.H.; Zhang, Y.; Guo, Y.L. Adrenalectomy may increase survival of patients with adrenal metastases. Quant. Imaging Med. Surg. 2021, 11, 2013–2018. [Google Scholar] [CrossRef]
- Yuan, H.; Kang, B.; Sun, K.; Qin, S.; Ji, C.; Wang, X. CT-based radiomics nomogram for differentiation of adrenal hyperplasia from lipid-poor adenoma: An exploratory study. BMC Med. Imaging 2023, 23, 4. [Google Scholar] [CrossRef]
- Tu, W.; Abreu-Gomez, J.; Udare, A.; Alrashed, A.; Schieda, N. Utility of T2-weighted MRI to Differentiate Adrenal Metastases from Lipid-Poor Adrenal Adenomas. Radiol. Imaging Cancer 2020, 2, e200011. [Google Scholar] [CrossRef]
- Dalavia, C.C.; Goldman, S.M.; Melo, H.J.F.E.; Kater, C.E.; Szejnfeld, J.; Iared, W.; Ajzen, S.A. The value of signal intensity on T1-weighted chemical shift magnetic resonance imaging combined with proton magnetic resonance spectroscopy for the diagnosis of adrenal adenomas. Radiol. Bras. 2020, 53, 86–94. [Google Scholar] [CrossRef]
- Cingam, S.R.; Mukkamalla, S.K.R.; Karanchi, H. Adrenal Metastasis. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Taffel, M.; Haji-Momenian, S.; Nikolaidis, P.; Miller, F.H. Adrenal imaging: A comprehensive review. Radiol. Clin. N. Am. 2012, 50, 219–243. [Google Scholar] [CrossRef] [PubMed]
- Caoili, E.M.; Korobkin, M.; Francis, I.R.; Cohan, R.H.; Platt, J.F.; Dunnick, N.R.; Raghupathi, K.I. Adrenal masses: Characterization with combined unenhanced and delayed enhanced CT. Radiology 2002, 22, 629–633. [Google Scholar] [CrossRef] [PubMed]
- Schieda, N.; Siegelman, E.S. Update on CT and MRI of Adrenal Nodules. Am. J. Roentgenol. 2017, 208, 1206–1217. [Google Scholar] [CrossRef]
- Akbulut, S.; Erten, O.; Kahramangil, B.; Gokceimam, M.; Kim, Y.S.; Li, P.; Remer, E.M.; Berber, E. A critical analysis of computed tomography washout in lipid-poor adrenal incidentalomas. Ann. Surg. Oncol. 2021, 28, 2756–2762. [Google Scholar] [CrossRef]
- Ma, G.; Zhang, X.; Wang, M.; Xu, X.; Xu, B.; Guan, Z. Role of 18F-FDG PET/CT in the differential diagnosis of primary benign and malignant unilateral adrenal tumors. Quant. Imaging Med. Surg. 2021, 11, 2013–2018. [Google Scholar] [CrossRef]
- Cuocolo, R.; Caruso, M.; Perillo, T.; Ugga, L.; Petretta, M. Machine Learning in oncology: A clinical appraisal. Cancer Lett. 2020, 481, 55–62. [Google Scholar] [CrossRef]
- Stanzione, A.; Cuocolo, R.; Verde, F.; Galatola, R.; Romeo, V.; Mainenti, P.P.; Aprea, G.; Guadagno, E.; Del Basso De Caro, M.; Maurea, S. Handcrafted MRI radiomics and machine learning: Classification of indeterminate solid adrenal lesions. Magn. Reson. Imaging 2021, 79, 52–58. [Google Scholar] [CrossRef]
- Zerunian, M.; Laghi, A.; Caruso, D. Radiomics in Oncology III. Diagnostics 2023, 13, 149. [Google Scholar] [CrossRef] [PubMed]
- Ismail, M.; Craig, S.; Ahmed, R.; de Blank, P.; Tiwari, P. Opportunities and Advances in Radiomics and Radiogenomics for Pediatric Medulloblastoma Tumors. Diagnostics 2023, 13, 2727. [Google Scholar] [CrossRef]
- Zhang, H.; Meng, X.; Wang, Z.; Zhou, X.; Liu, Y.; Li, N. Predicting PD-L1 in Lung Adenocarcinoma Using 18F-FDG PET/CT Radiomic Features. Diagnostics 2025, 15, 543. [Google Scholar] [CrossRef]
- Winkelmann, M.T.; Gassenmaier, S.; Walter, S.S.; Artzner, C.; Lades, F.; Faby, S.; Nikolaou, K.; Bongers, M.N. Differentiation of adrenal adenomas from adrenal metastases in single-phased staging dual-energy CT and radiomics. Diagn. Interv. Radiol. 2022, 28, 208–216. [Google Scholar] [CrossRef] [PubMed]
- Shea, A.; Kilcoyne, A.; McDermott, E.; O’Grady, M.; McDermott, S. Can radiomic feature analysis differentiate adrenal metastases from lipid-poor adenomas on single-phase contrast-enhanced CT abdomen? Clin. Radiol. 2022, 77, e711–e718. [Google Scholar] [CrossRef] [PubMed]
- Eisenhauer, E.A.; Therasse, P.; Bogaerts, J.; Schwartz, L.H.; Sargent, D.; Ford, R.; Dancey, J.; Arbuck, S.; Gwyther, S.; Mooney, M.; et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). Eur. J. Cancer 2009, 45, 228–247. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; He, Y.; Zeng, X.; Zhu, S.; Li, F. Distinguishing between metastatic and benign adrenal masses in patients with extra-adrenal malignancies. Front. Endocrinol. 2022, 13, 978730. [Google Scholar] [CrossRef]
- Launay, N.; Silvera, S.; Tenenbaum, F.; Groussin, L.; Tissier, F.; Audureau, E.; Vignaux, O.; Dousset, B.; Bertagna, X.; Legmann, P. Value of 18-F-FDG PET/CT and CT in the Diagnosis of Indeterminate Adrenal Masses. Int. J. Endocrinol. 2015, 2015, 213875. [Google Scholar] [CrossRef]
- Kunikowska, J.; Matyskiel, R.; Toutounchi, S.; Grabowska-Derlatka, L.; Koperski, L.; Królicki, L. What parameters from 18F-FDG PET/CT are useful in evaluation of adrenal lesions? Eur. J. Nucl. Med. Mol. Imaging 2014, 41, 2273–2280. [Google Scholar] [CrossRef]
- Lee, J.H.; Kim, E.K.; Hong, A.R.; Roh, E.; Bae, J.H.; Kim, J.H.; Shin, C.S.; Kim, S.Y.; Kim, S.W. Radiographic Characteristics of Adrenal Masses in Oncologic Patients. Endocrinol. Metab. 2016, 31, 147–152. [Google Scholar] [CrossRef]
- Wachtel, H.; Roses, R.E.; Kuo, L.E.; Lindeman, B.M.; Nehs, M.A.; Tavakkoli, A.; Sareh, P.; Richard, A.H.; Douglas, L.F.; Benjamin, C.J.; et al. Adrenalectomy for Secondary Malignancy: Patients, Outcomes, and Indications. Ann. Surg. 2021, 274, 1073–1080. [Google Scholar] [CrossRef]
- McLean, K.; Lilienfeld, H.; Caracciolo, J.T.; Hoffe, S.; Tourtelot, J.B.; Carter, W.B. Management of isolated Adrenal Lesions in Cancer Patients. Cancer Control 2011, 18, 113–126. [Google Scholar] [CrossRef]
- Point du Jour, K.S.; Alwelaie, Y.; Coleman, A.; Tadros, T.; Aneja, R.; Reid, M.D. Adrenal gland fine needle aspiration: A multi-institutional analysis of 139 cases. J. Am. Soc. Cytopathol. 2021, 10, 168–174. [Google Scholar] [CrossRef]
- Boland, G.W.; Dwamena, B.A.; Jagtiani Sangwaiya, M.; Goehler, A.G.; Blake, M.A.; Hahn, P.F.; Scott, J.A.; Kalra, M.K. Characterization of adrenal masses by using FDG PET: A systematic review and meta-analysis of diagnostic test performance. Radiolog 2011, 259, 117–126. [Google Scholar] [CrossRef] [PubMed]
- Byeon, K.H.; Ha, Y.S.; Choi, S.H.; Kim, B.S.; Kim, H.T.; Yoo, E.S.; Kwon, T.G.; Lee, J.N.; Kim, T.-H. Predictive factors for adrenal metastasis in extra-adrenal malignancy patients with solitary adrenal mass. J. Surg. Oncol. 2018, 118, 1271–1276. [Google Scholar] [CrossRef] [PubMed]
- Barta, J.A.; Powell, C.A.; Wisnivesky, J.P. Global Epidemiology of Lung Cancer. Ann. Glob. Health 2019, 85, 8. [Google Scholar] [CrossRef]
- Caruso, D.; Zerunian, M.; Daffina, J.; Polici, M.; Polidori, T.; Tipaldi, M.A.; Ronconi, E.; Pucciarelli, F.; Lucertini, E.; Rossi, M.; et al. Radiomics and functional imaging in lung cancer: The importance of radiological heterogeneity beyond FDG PET/CT and lung biopsy. Eur. J. Radiol. 2021, 142, 109874. [Google Scholar] [CrossRef]
- Kang, F.; Mu, W.; Gong, J.; Wang, S.; Li, G.; Li, G.; Qin, W.; Tian, J.; Wang, J. Integrating manual diagnosis into radiomics for reducing the false positive rate of 18 F-FDG PET/CT diagnosis in patients with suspected lung cancer. Eur. J. Nucl. Med. Mol. Imaging 2019, 46, 2770–2779. [Google Scholar] [CrossRef] [PubMed]
- Iasonos, A.; Schrag, D.; Raj, G.V.; Panageas, K.S. How to build and interpret a nomogram for cancer prognosis. J. Clin. Oncolgy 2008, 26, 1364–1370. [Google Scholar] [CrossRef] [PubMed]
- Balachandran, V.P.; Gonen, M.; Smith, J.J.; DeMatteo, R.P. Nomograms in oncology: More than meets the eye. Lancet Oncol. 2015, 16, e173–e180. [Google Scholar] [CrossRef]
- Liu, C.; Zhao, W.; Xie, J.; Lin, H.; Hu, X.; Li, C.; Shang, Y.; Wang, Y.; Jiang, Y.; Ding, M.; et al. Development and validation of a radiomics-based nomogram for predicting a major pathological response to neoadjuvant immunochemotherapy for patients with potentially resectable non-small cell lung cancer. Front. Immunol. 2023, 14, 1115291. [Google Scholar] [CrossRef]
- Zhou, M.; Zhu, X. Construction and validation of a robust ferroptosis-associated gene signature predictive of prognosis in lung adenocarcinoma. Medicine 2022, 101, e29068. [Google Scholar] [CrossRef]
- Li, F.; Zhao, Y.; Wei, Y.; Xi, Y.; Bu, H. Tumor-Infiltrating Lymphocytes Improve Magee Equation-Based Prediction of Pathologic Complete Response in HR-Positive/HER2-Negative Breast Cancer. Am. J. Clin. Pathol. 2022, 158, 291–299. [Google Scholar] [CrossRef]
- Zhang, W.; Ji, L.; Wang, X.; Zhu, S.; Luo, J.; Zhang, Y.; Tong, Y.; Feng, F.; Kang, Y.; Bi, Q. Nomogram Predicts Risk and Prognostic Factors for Bone Metastasis of Pancreatic Cancer: A Population-Based Analysis. Front. Endocrinol. 2022, 12, 752176. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.; Zhang, Y. Establishment of prognostic nomogram for elderly colorectal cancer patients: A SEER database analysis. BMC Gastroenterol. 2020, 20, 347. [Google Scholar] [CrossRef]
- Lv, J.; Liu, Y.Y.; Jia, Y.T.; He, J.L.; Dai, G.Y.; Guo, P.; Zhao, Z.L.; Zhang, Y.N.; Li, Z.X. A nomogram model for predicting prognosis of obstructive colorectal cancer. World J. Surg. Oncol. 2021, 19, 337. [Google Scholar] [CrossRef] [PubMed]
- Rocco, B.; Sighinolfi, M.C.; Sandri, M.; Puliatti, S.; Bianchi, G. A novel nomogram for predicting ECE of prostate cancer. Bju Int. 2018, 122, 916–918. [Google Scholar] [CrossRef] [PubMed]
- Gitto, S.; Cuocolo, R.; Emili, I.; Tofanelli, L.; Chianca, V.; Albano, D.; Messina, C.; Imbriaco, M.; Sconfienza, L.M. Effects of Interobserver Variability on 2D and 3D CT- and MRI-Based Texture Feature Reproducibility of Cartilaginous Bone Tumors. J. Digit. Imaging 2021, 34, 820–832. [Google Scholar] [CrossRef]

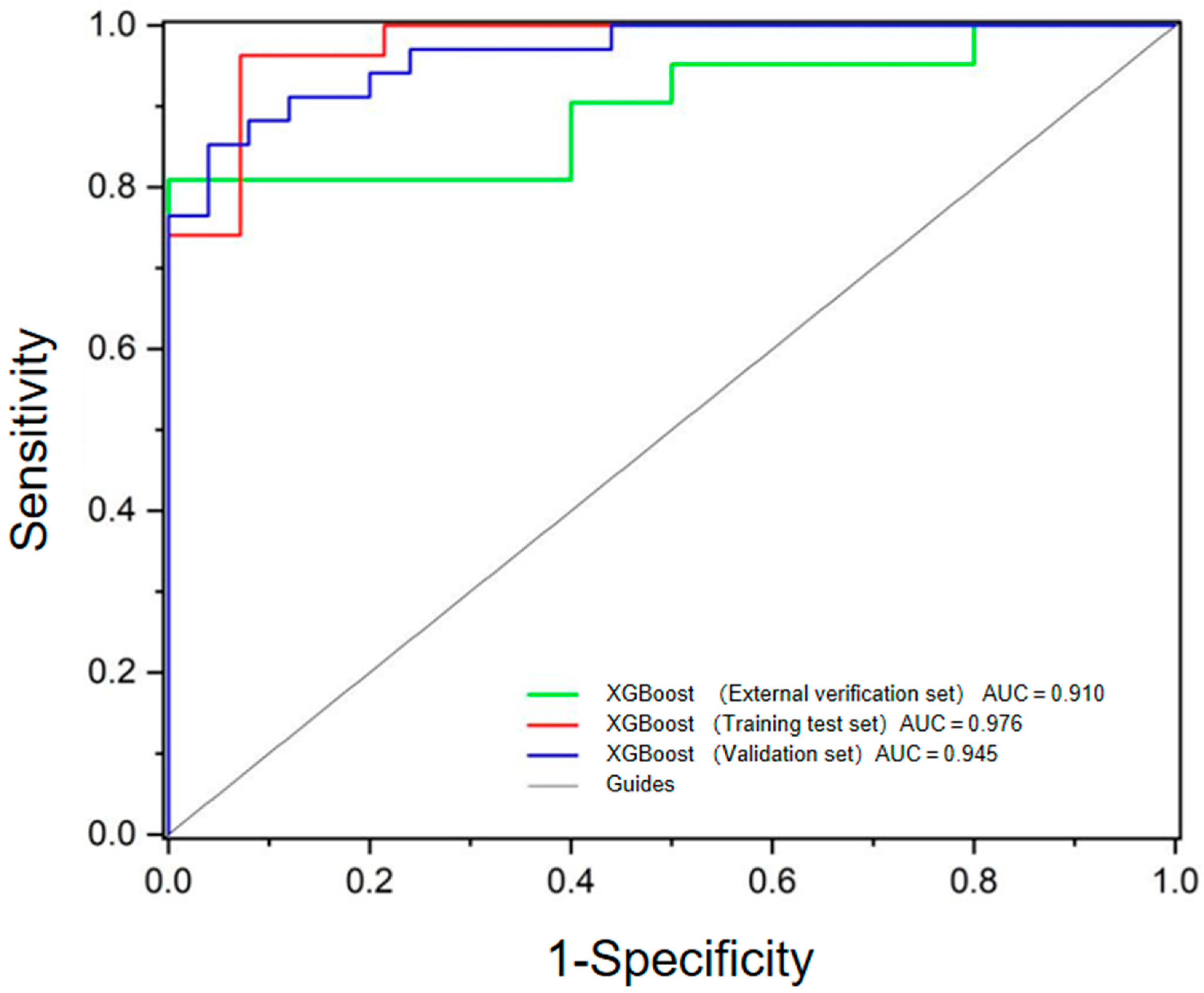
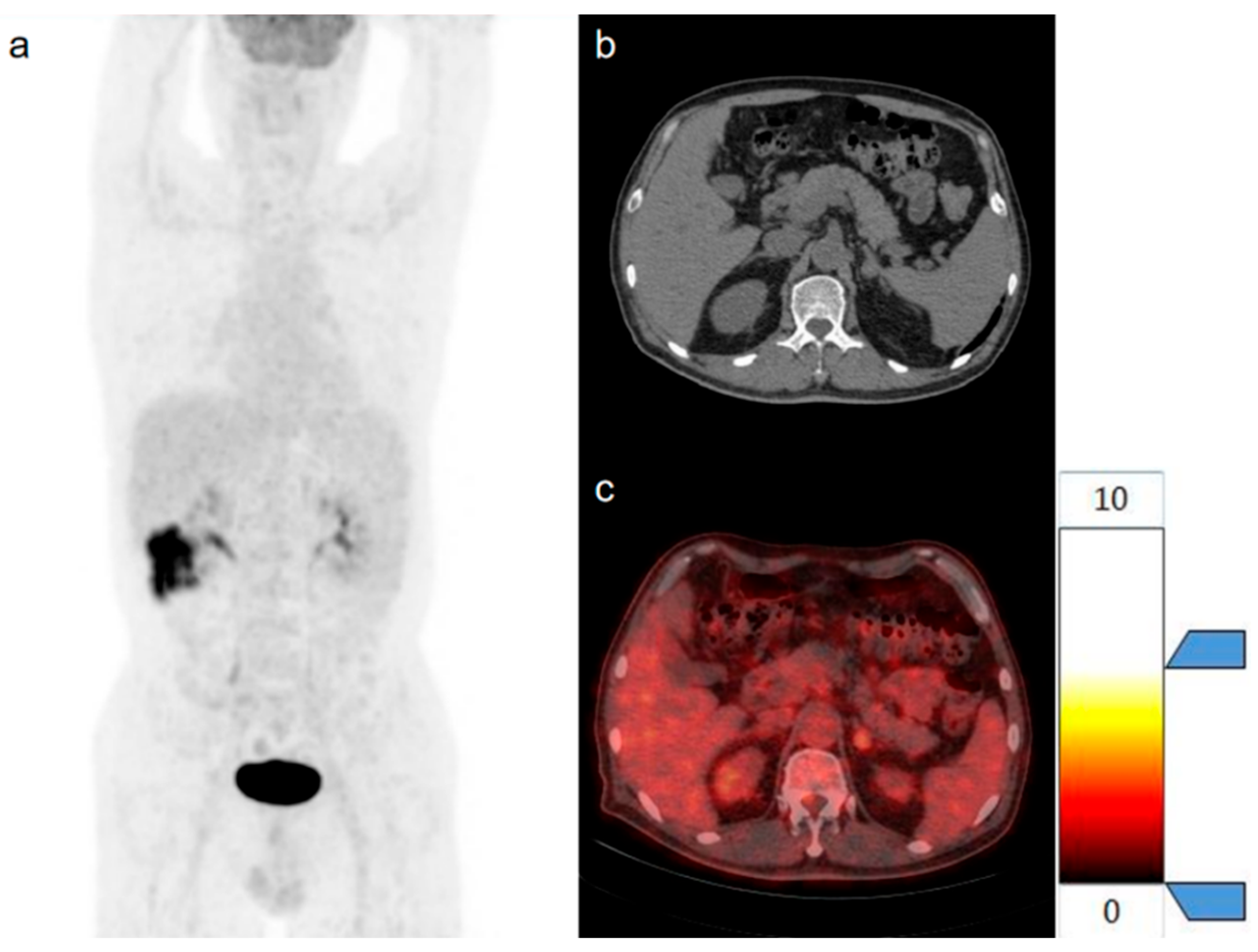
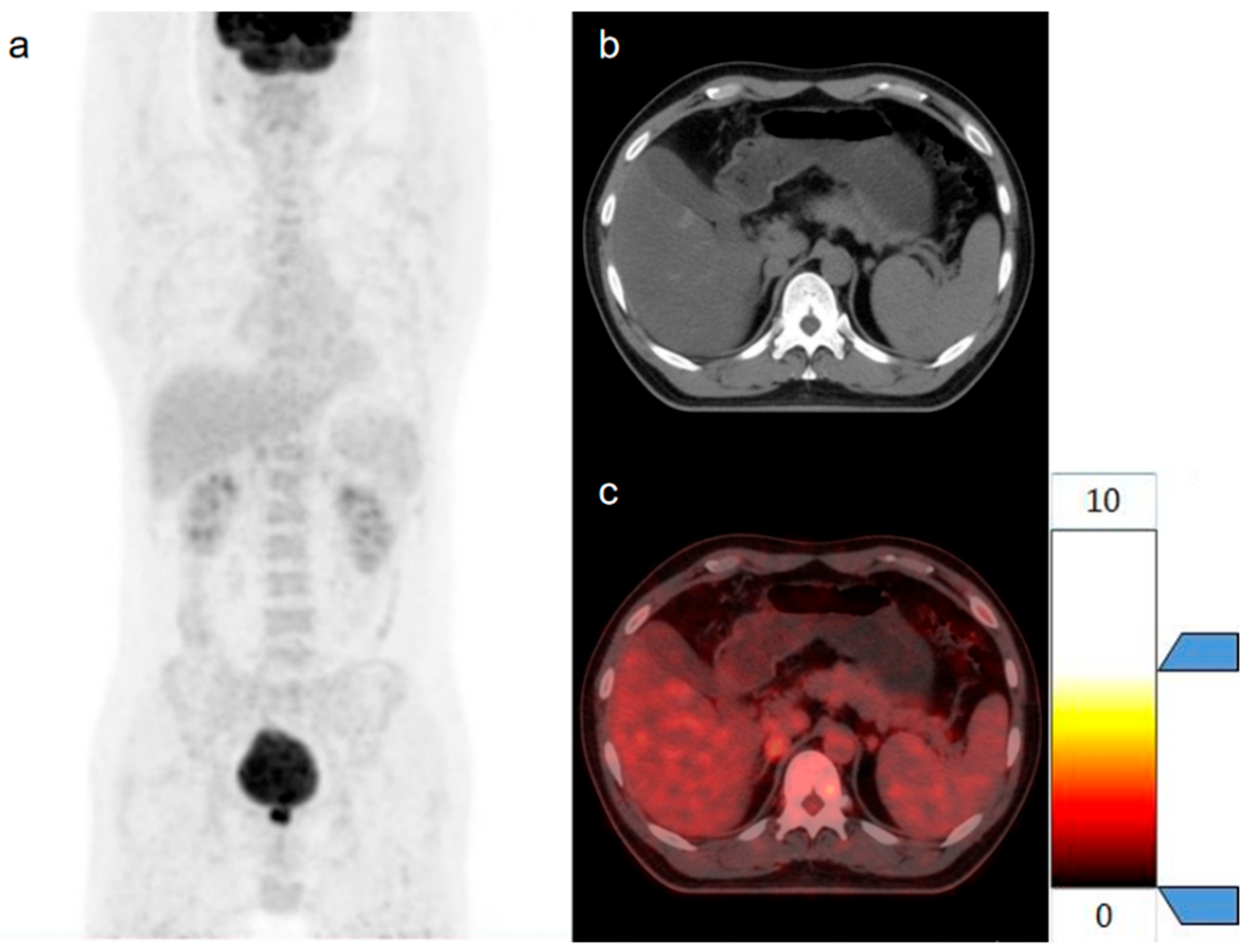
| Parameters | Benign Tumor Group | Metastases Group | p |
|---|---|---|---|
| Number of patients | 74 | 93 | |
| Number of tumors | 81 | 114 | |
| Age | 63.20 ± 7.94 | 60.55 ± 10.10 | 0.066 |
| Gender | 0.043 * | ||
| Female | 31 (41.9%) | 26 (28.0%) | |
| Male | 43 (58.1%) | 67 (72.0%) | |
| Maximum diameter (cm) | 1.90 ± 0.58 | 2.55 ± 1.28 | <0.001 * |
| Location | 0.036 * | ||
| Unilateral | 67 (90.5%) | 72 (77.4%) | |
| Bilateral | 7 (9.5%) | 21 (22.6%) | |
| CT value(HU) | 23.36 ± 8.73 | 32.36 ± 6.16 | <0.001 * |
| SUVmax | 2.8 (2.00, 3.60) | 7.5 (5.40, 10.93) | <0.001 * |
| T/L | 0.91 (0.67, 1.2) | 2.83 (1.84, 4.02) | <0.001 * |
| Primary tumor | 0.001 * | ||
| Lung cancer | 44, 59.5% | 77, 82.8% | |
| Non-lung cancer | 30, 40.5% | 16, 17.2% |
| Parameters | Univariate Analysis | Multivariate Analysis | ||
|---|---|---|---|---|
| OR (95%CI) | p | OR (95%CI) | p | |
| Lung cancer | 3.281 (1.612, 6.680) | 0.001 | 6.429 (2.190, 18.873) | 0.001 * |
| Male | 1.858 (0.973, 3.547) | 0.061 | ||
| Dmax (cm) | 2.249 (1.463, 3.457) | <0.001 | 2.099 (1.064, 4.142) | 0.033 * |
| Bilateral | 2.622 (1.043, 5.596) | 0.041 | 3.432 (0.782, 15.068) | 0.102 |
| CT value (HU) | 1.155 (1.101, 1.213) | <0.001 | 1.138 (1.069, 1.212) | <0.001 * |
| SUVmax | 1.608 (1.366, 1.894) | <0.001 | 0.694 (0.401, 1.204) | 0.194 |
| T/L | 3.876 (2.443, 6.148) | <0.001 | 5.402 (1.050, 27.802) | 0.044 * |
| Models | AUC (95%CI) | Sensitivity | Specificity | Accuracy |
|---|---|---|---|---|
| Primary tumor | 0.617 (0.530, 0.704) | 0.828 | 0.405 | 0.641 |
| Dmax | 0.664 (0.582, 0.745) | 0.742 | 0.514 | 0.623 |
| CT value | 0.783 (0.709, 0.856) | 0.860 | 0.649 | 0.766 |
| T/L | 0.920 (0.878, 0.963) | 0.925 | 0.824 | 0.844 |
| CT value + Dmax | 0.835 (0.774, 0.896) | 0.742 | 0.797 | 0.749 |
| CT value + Dmax + T/L | 0.914 (0.867, 0.960) | 0.892 | 0.824 | 0.844 |
| Primary tumor + CTvalue + Dmax | 0.870 (0.816, 0.926) | 0.849 | 0.811 | 0.814 |
| Primary tumor + CT value + Dmax + T/L | 0.919 (0.874, 0.963) | 0.849 | 0.892 | 0.844 |
| Model | Training Test Set | Validation Set | ||||
|---|---|---|---|---|---|---|
| Accuracy | Accuracy | Sensitivity | Specificity | PPV | NPV | |
| RF | 0.811 | 0.864 | 0.900 | 0.830 | 0.840 | 0.890 |
| AdaBoost | 0.824 | 0.915 | 0.910 | 0.920 | 0.940 | 0.880 |
| KNN | 0.856 | 0.831 | 0.790 | 0.880 | 0.900 | 0.760 |
| GaussianNB | 0.816 | 0.898 | 0.850 | 0.960 | 0.970 | 0.830 |
| Decision Tree | 0.787 | 0.797 | 0.820 | 0.760 | 0.820 | 0.760 |
| GDBT | 0.855 | 0.898 | 0.940 | 0.840 | 0.890 | 0.910 |
| LightGBM | 0.878 | 0.915 | 0.880 | 0.960 | 0.970 | 0.860 |
| XGBoost | 0.883 | 0.932 | 0.930 | 0.960 | 0.970 | 0.890 |
| LR | 0.826 | 0.898 | 0.940 | 0.840 | 0.890 | 0.910 |
| SVM | 0.873 | 0.864 | 0.880 | 0.840 | 0.880 | 0.840 |
| Parameters. | Benign Tumors Group | Metastases Group |
|---|---|---|
| Number of patients | 9 | 20 |
| Age | 63.89 ± 10.62 | 56.48 ± 11.30 |
| Gender | ||
| Male | 7 (77.8%) | 17 (85.0%) |
| Female | 2 (22.2%) | 3 (15.0%) |
| Location | ||
| Unilateral | 9 | 19 |
| Bilateral | 0 | 1 |
| Dmax(cm) | 1.83 ± 0.95 | 2.33 ± 1.05 |
| CT(HU) | 27.00 ± 6.61 | 29.76 ± 5.33 |
| SUVmax | 3.80 (2.85, 5.20) | 7.30 (6.05, 9.70) |
| T/L | 1.35 (0.92, 1.85) | 2.30 (1.70, 3.47) |
| Primary tumor | ||
| Lung cancer | 7, 77.8% | 15, 75.0% |
| Non-lung cancer | 2, 22.2% | 5, 25.0% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, Q.; Kong, X.; Meng, X.; Shen, X.; Li, N. The Value of PET/CT-Based Radiomics in Predicting Adrenal Metastases in Patients with Cancer. Diagnostics 2025, 15, 1356. https://doi.org/10.3390/diagnostics15111356
He Q, Kong X, Meng X, Shen X, Li N. The Value of PET/CT-Based Radiomics in Predicting Adrenal Metastases in Patients with Cancer. Diagnostics. 2025; 15(11):1356. https://doi.org/10.3390/diagnostics15111356
Chicago/Turabian StyleHe, Qiujun, Xiangxing Kong, Xiangxi Meng, Xiuling Shen, and Nan Li. 2025. "The Value of PET/CT-Based Radiomics in Predicting Adrenal Metastases in Patients with Cancer" Diagnostics 15, no. 11: 1356. https://doi.org/10.3390/diagnostics15111356
APA StyleHe, Q., Kong, X., Meng, X., Shen, X., & Li, N. (2025). The Value of PET/CT-Based Radiomics in Predicting Adrenal Metastases in Patients with Cancer. Diagnostics, 15(11), 1356. https://doi.org/10.3390/diagnostics15111356








