The Use of the Modified Brixia Score for Predicting Mortality and Acute Respiratory Distress Syndrome in Patients with COVID-19 Pneumonia: What Have We Learned?
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
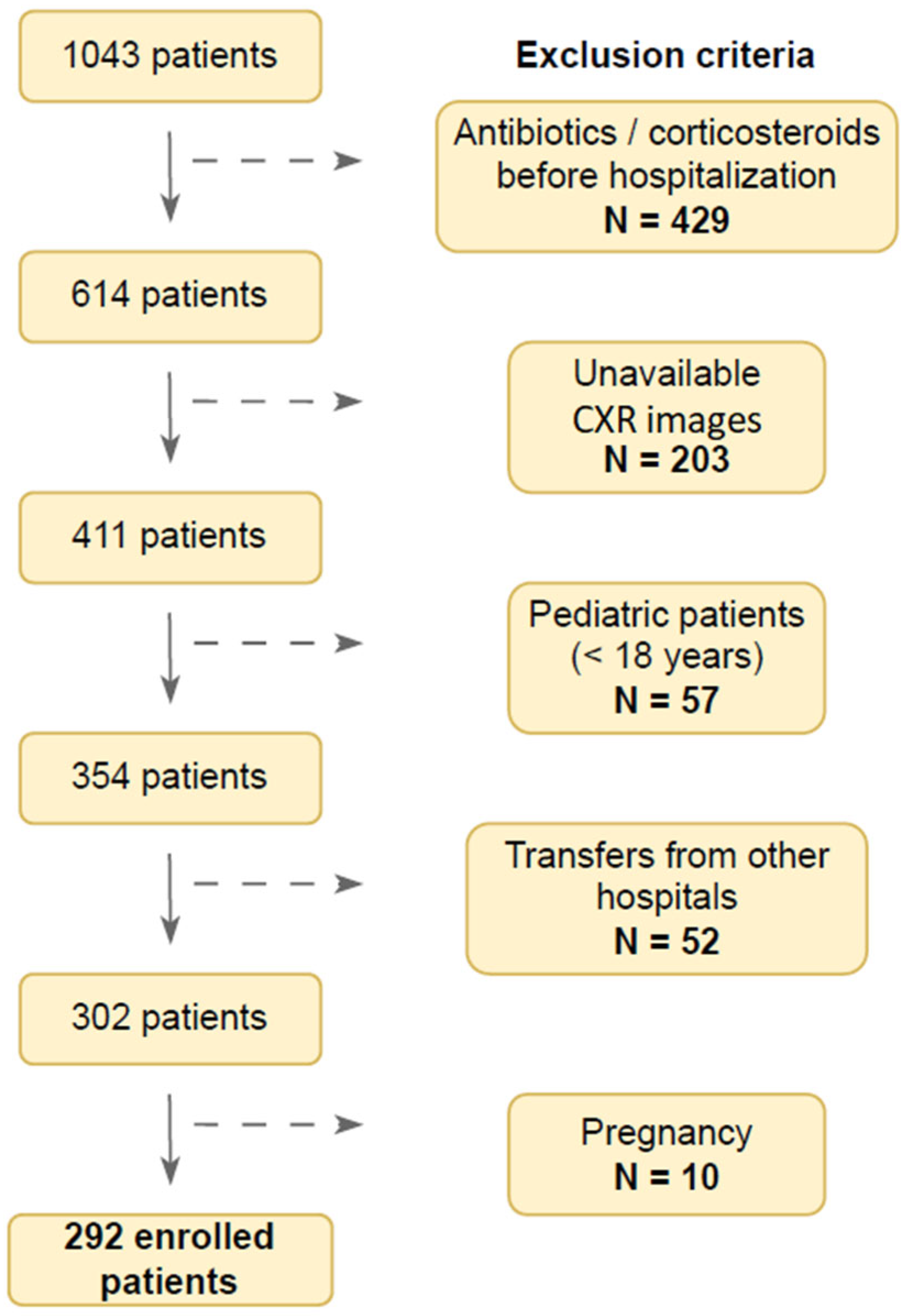
2.2. Flow Chart of the Study Population
2.3. Data Collection
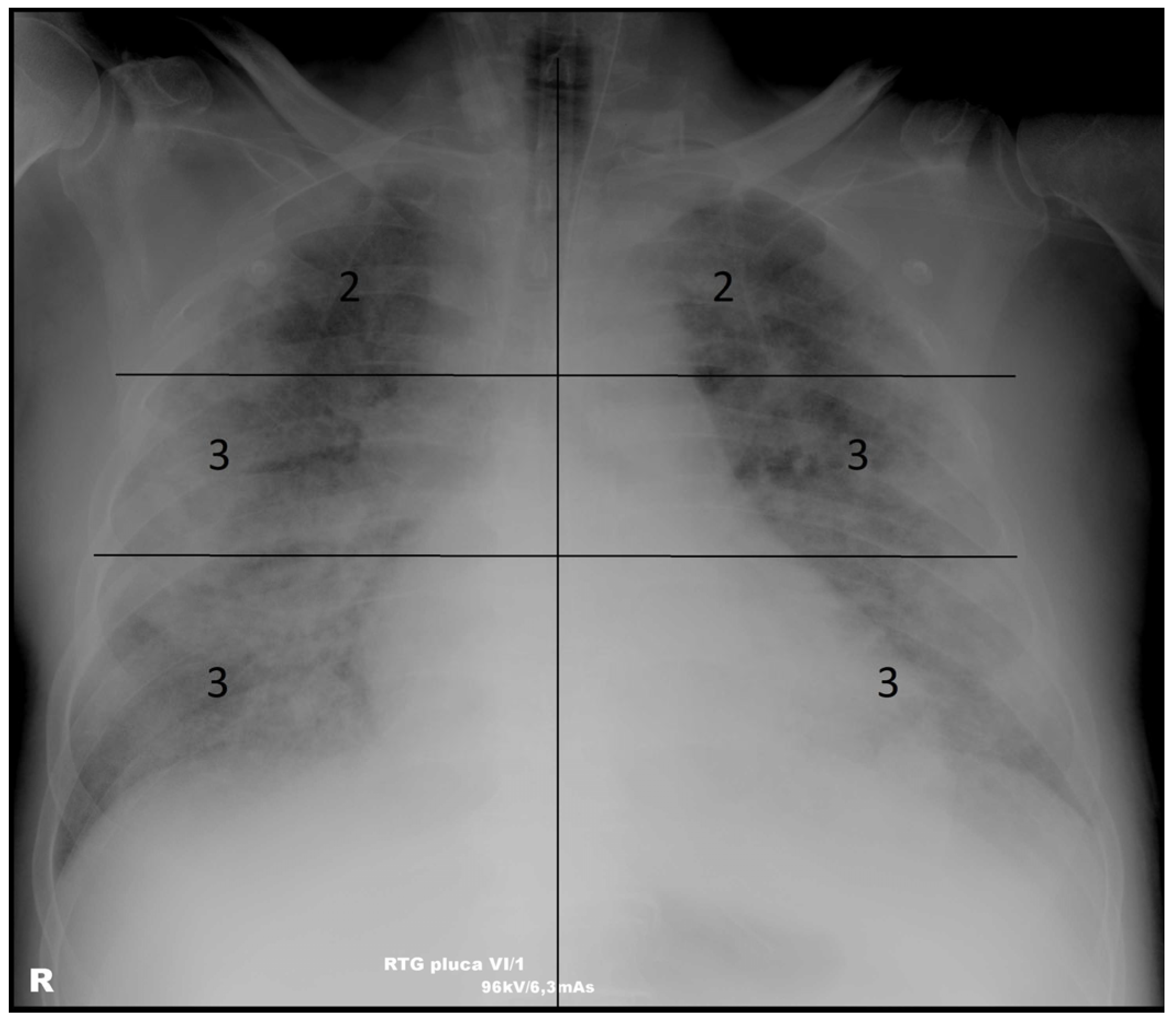
2.4. Scoring System
2.5. Statistical Analysis
3. Results
3.1. Patient Characteristics
3.2. The Association of the Modified Brixia Score with Demographic, Clinical, and Laboratory Parameters
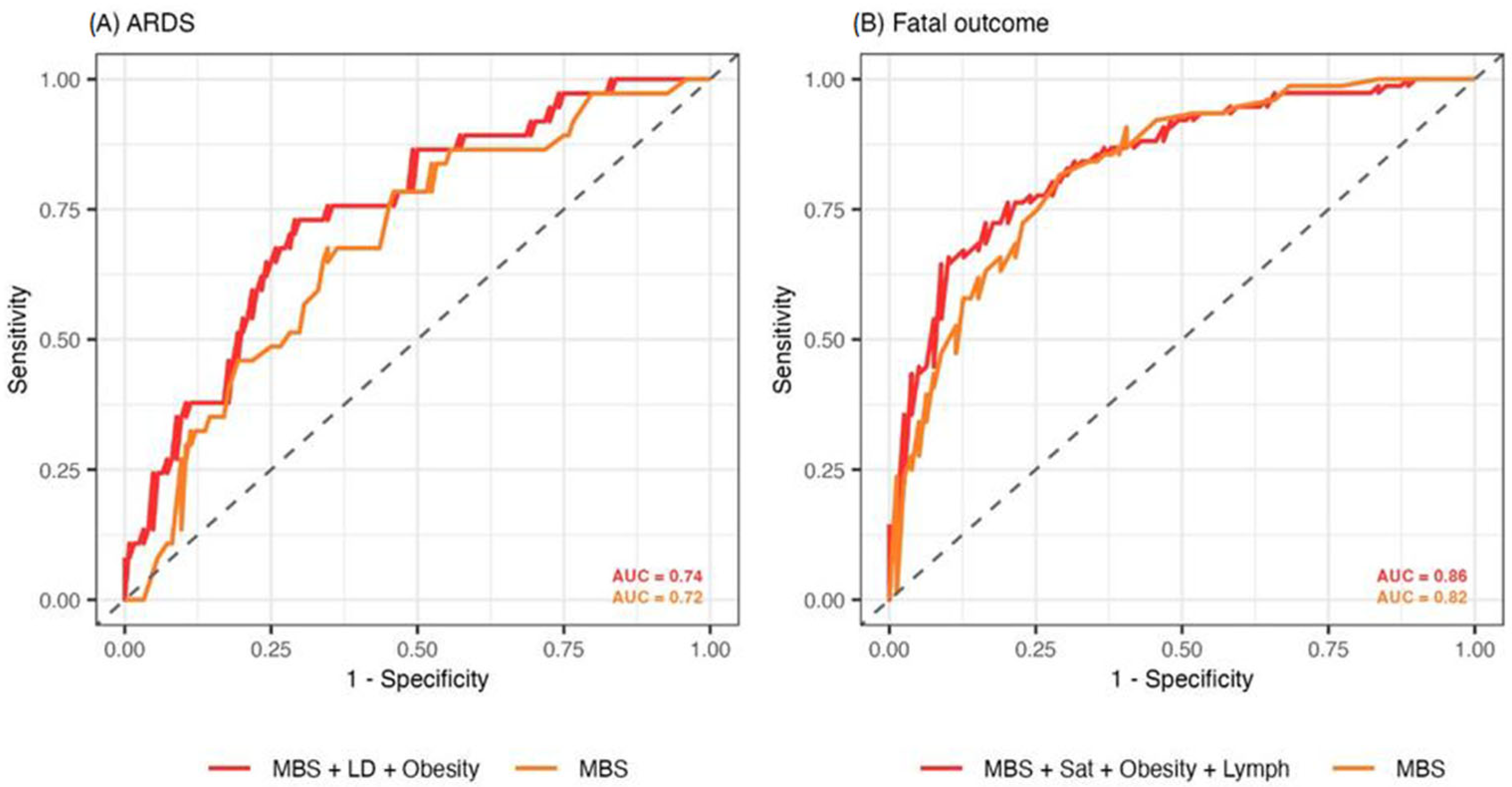
3.3. Predictive Value of the Modified Brixia Score in ARDS and Fatal Outcome Prognosis
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
- The following abbreviations are used in this manuscript:
| COVID-19 | Coronavirus disease of 2019 |
| MBS | Modified Brixia score |
| ARDS | Acute respiratory distress syndrome |
| CT | Computed tomography |
| ICU | Intensive care unit |
| CXR | Chest X-ray |
| ICR | Interquartile range |
| AUC | Area under the curve |
| CI | Confidence interval |
| ROC | Receiver operating characteristics |
References
- Zhu, H.; Wei, L.; Niu, P. The novel coronavirus outbreak in Wuhan, China. Glob. Health Res. Policy 2020, 5, 6. [Google Scholar] [CrossRef] [PubMed]
- Ai, T.; Yang, Z.; Hou, H.; Zhan, C.; Chen, C.; Lv, W.; Tao, Q.; Sun, Z.; Xia, L. Correlation of chest CT and RT-PCR testing for coronavirus disease 2019 (COVID-19) in China: A report of 1014 cases. Radiology 2020, 296, E32–E40. [Google Scholar] [CrossRef]
- Borghesi, A.; Maroldi, R. COVID-19 outbreak in Italy: Experimental chest X-ray scoring system for quantifying and monitoring disease progression. Radiol. Med. 2020, 125, 509–513. [Google Scholar] [CrossRef]
- Manna, S.; Wruble, J.; Maron, S.Z.; Toussie, D.; Voutsinas, N.; Finkelstein, M.; Cedillo, M.A.; Diamond, J.; Eber, C.; Jacobi, A.; et al. COVID-19: A multimodality review of radiologic techniques, clinical utility, and imaging features. Radiol. Cardiothorac. Imaging 2020, 2, e200210. [Google Scholar] [CrossRef] [PubMed]
- Stefanidis, K.; Konstantelou, E.; Yusuf, G.T.; Oikonomou, A.; Tavernaraki, K.; Karakitsos, D.; Loukides, S.; Vlahos, I. Radiological, epidemiological and clinical patterns of pulmonary viral infections. Eur. J. Radiol. 2021, 136, 109548. [Google Scholar] [CrossRef]
- Jeličić, K.; Đaković Rode, O.; Višković, K.; Mehmedović, A.; Kurolt, I. COVID-19 Microbiological and radiological diagnostics. Infektol. Glasn. 2021, 40, 97–106. [Google Scholar] [CrossRef]
- Borghesi, A.; Zigliani, A.; Golemi, S.; Carapella, N.; Maculotti, P.; Farina, D.; Maroldi, R. Chest X-ray severity index as a predictor of in-hospital mortality in coronavirus disease 2019: A study of 302 patients from Italy. Int. J. Infect. Dis. 2020, 96, 291–293. [Google Scholar] [CrossRef] [PubMed]
- Monaco, C.G.; Zaottini, F.; Schiaffino, S.; Villa, A.; Della Pepa, G.; Carbonaro, L.A.; Menicagli, L.; Cozzi, A.; Carriero, S.; Arpaia, F.; et al. Chest x-ray severity score in COVID-19 patients on emergency department admission: A two-centre study. Eur. Radiol. Exp. 2020, 4, 68. [Google Scholar] [CrossRef]
- Warren, M.A.; Zhao, Z.; Koyama, T.; Bastarache, J.A.; Shaver, C.M.; Semler, M.W.; Rice, T.W.; Matthay, M.A.; Calfee, C.S.; Ware, L.B. Severity scoring of lung oedema on the chest radiograph is associated with clinical outcomes in ARDS. Thorax 2018, 73, 840–846. [Google Scholar] [CrossRef]
- Hasan, S.S.; Capstick, T.; Ahmed, R.; Kow, C.S.; Mazhar, F.; Merchant, H.A.; Zaidi, S.T.R. Mortality in COVID-19 patients with acute respiratory distress syndrome and corticosteroids use: A systematic review and meta-analysis. Expert. Rev. Respir. Med. 2020, 14, 1149–1163. [Google Scholar] [CrossRef]
- Pranskunas, A.; Zaveckiene, J.; Baranauskas, T.; Zakarauskaite, B.; Zykute, D.; Tamosuitis, T. Early association between respiratory mechanics and radiological changes in mechanically ventilated critically ill patients with COVID-19. Intern. Emerg. Med. 2024, 19, 1081–1088. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, I.D.A.; Satoto, B.; Handoyono, T.; Santoso, A.G.; Sukmaningtyas, H.; Ningrum, F.H. Significant relationship between Brixia score and the degree of acute respiratory distress syndrome in COVID-19 patients. Med. Hosp. 2024, 11, 183–187. [Google Scholar] [CrossRef]
- ARDS Definition of Task Force; Ranieri, V.M.; Rubenfeld, G.D.; Thompson, B.T.; Ferguson, N.D.; Caldwell, E.; Fan, E.; Camporota, L.; Slutsky, A.S. Acute respiratory distress syndrome. JAMA 2012, 307, 2526–2533. [Google Scholar] [CrossRef]
- Cano, E.J.; Fuentes, X.F.; Campioli, C.C.; O’horo, J.C.; Abu Saleh, O.; Odeyemi, Y.; Yadav, H.; Temesgen, Z. Impact of Corticosteroids in Coronavirus Disease 2019 Outcomes: Systematic Review and Meta-analysis. Chest 2021, 159, 1019–1040. [Google Scholar] [CrossRef]
- Mehmedović, A.; Bodulić, K.; Hrabak Paar, M.; Višković, K. Use of modified Brixia score in predicting mortality in hospitalized patients with COVID-19 pneumonia. In Proceedings of the 4th Edition of World Congress in Infectious Diseases/Hybrid Event, Rome, Italy, 21–22 June 2023. [Google Scholar]
- World Health Organization. Clinical Management of COVID-19: Interim Guidance, 27 May 2020; World Health Organization: Geneva, Switzerland, 2020. Available online: https://iris.who.int/bitstream/handle/10665/332196/WHO-2019-nCoV-clinical-2020.5-eng.pdf (accessed on 1 March 2025).
- Singer, M.; Deutschman, C.S.; Seymour, C.W.; Shankar-Hari, M.; Annane, D.; Bauer, M.; Bellomo, R.; Bernard, G.R.; Chiche, J.-D.; Coopersmith, C.M.; et al. The third international consensus definitions for sepsis and septic shock (sepsis-3). JAMA 2016, 315, 801–810. [Google Scholar] [CrossRef] [PubMed]
- Cheung, A.K.; Chang, T.I.; Cushman, W.C.; Furth, S.L.; Hou, F.F.; Ix, J.H.; Knoll, G.A.; Muntner, P.; Pecoits-Filho, R.; Sarnak, M.J.; et al. KDIGO 2021 clinical practice guideline for the management of blood pressure in chronic kidney disease. Kidney Int. 2021, 99, S1–S87. [Google Scholar] [CrossRef] [PubMed]
- Gamer, M.; Lemon, J.; Singh, I.F.P. irr: Various Coefficients of Interrater Reliability and Agreement. R Package Version 0.84.1. 2012. Available online: https://CRAN.R-project.org/package=irr (accessed on 10 March 2025).
- Signorell, A. DescTools: Tools for Descriptive Statistics. R Package Version 0.99.60. 2024. Available online: https://cran.r-project.org/package=DescTools (accessed on 10 March 2025).
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2023; Available online: https://www.R-project.org/ (accessed on 5 February 2025).
- Hanley, M.; Brosnan, C.; O’Neill, D.; Ni Mhuircheartaigh, N.; Logan, M.; Morrin, M.M.; Hurley, K.; Sulaiman, I.; O’Brien, E.; Morgan, R.; et al. Modified Brixia chest X-ray severity scoring system and correlation with intubation, non-invasive ventilation and death in a hospitalised COVID-19 cohort. J. Med. Imaging Radiat. Oncol. 2022, 66, 761–767. [Google Scholar] [CrossRef]
- Bain, W.; Yang, H.; Shah, F.A.; Suber, T.; Drohan, C.; Al-Yousif, N.; DeSensi, R.S.; Bensen, N.; Schaefer, C.; Rosborough, B.R.; et al. COVID-19 versus non–COVID-19 acute respiratory distress syndrome: Comparison of demographics, physiologic parameters, inflammatory biomarkers, and clinical outcomes. Ann. Am. Thorac. Soc. 2021, 18, 1202. [Google Scholar] [CrossRef]
- Jensen, C.M.; Costa, J.C.; Nørgaard, J.C.; Zucco, A.G.; Neesgaard, B.; Niemann, C.U.; Ostrowski, S.R.; Reekie, J.; Holten, B.; Kalhauge, A.; et al. Chest x-ray imaging score is associated with severity of COVID-19 pneumonia: The MBrixia score. Sci. Rep. 2022, 12, 21019. [Google Scholar] [CrossRef]
- Nevola, R.; Russo, A.; Scuotto, S.; Imbriani, S.; Aprea, C.; Abitabile, M.; Beccia, D.; Brin, C.; Carusone, C.; Cinone, F.; et al. Non-invasive respiratory support in SARS-CoV-2 related acute respiratory distress syndrome: When is it most appropriate to start treatment? Respir. Res. 2022, 23, 327. [Google Scholar] [CrossRef]
- Au-Yong, I.; Higashi, Y.; Giannotti, E.; Fogarty, A.; Morling, J.R.; Grainge, M.; Race, A.; Juurlink, I.; Simmonds, M.; Briggs, S.; et al. Chest Radiograph Scoring Alone or Combined with Other Risk Scores for Predicting Outcomes in COVID-19. Radiology 2022, 302, 460–469. [Google Scholar] [CrossRef] [PubMed]
- Mruk, B.; Walecki, J.; Wasilewski, P.G.; Paluch, Ł.; Sklinda, K. Interobserver Agreement in Semi-Quantitative Scale-Based Interpretation of Chest Radiographs in COVID-19 Patients. Med. Sci. Monit. 2021, 27, e931277. [Google Scholar] [CrossRef]
- Haber, R.; Ghezzawi, M.; Puzantian, H.; Haber, M.; Saad, S.; Ghandour, Y.; El Bachour, J.; Yazbeck, A.; Hassanieh, G.; Mehdi, C.; et al. Mortality risk in patients with obesity and COVID-19 infection: A systematic review and meta-analysis. Metabolism 2024, 155, 155812. [Google Scholar] [CrossRef]
- Uppot, R.N.; Sahani, D.V.; Hahn, P.F.; Gervais, D.; Mueller, P.R. Impact of obesity on medical imaging and image-guided intervention. Am. J. Roentgenol. 2007, 188, 433–440. [Google Scholar] [CrossRef]
- Al-Murshedi, S.H.; Hogg, P.; England, A. The impact of being overweight on image quality when undertaking adult chest X-ray examinations using routine protocols. In Proceedings of the European Congress of Radiology (ECR), Vienna, Austria, 27 February–3 March 2019. [Google Scholar] [CrossRef]
- Kodikara, I.; Galabada, B.A.; Hettiarachchi, N.S. The mortality predicting ability of chest X-ray severity scoring systems in Covid-19 pneumonia. Ceylon Med. J. 2021, 66, 168–176. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.; Sollee, J.; Hsieh, C.; Yue, H.; Vandal, N.; Shanahan, J.; Choi, J.W.; Tran, T.M.L.; Halsey, K.; Iheanacho, F.; et al. COVID-19 mortality prediction in the intensive care unit with deep learning based on longitudinal chest X-rays and clinical data. Eur. Radiol. 2022, 32, 4446–4456. [Google Scholar] [CrossRef] [PubMed]
- Sjoding, M.W.; Taylor, D.; Motyka, J.; Lee, E.; Co, I.; Claar, D.; McSparron, J.; Ansari, S.; Kerlin, M.P.; Reilly, J.P.; et al. Deep learning to detect acute respiratory distress syndrome on chest radiographs: A retrospective study with external validation. Lancet Digit. Health 2021, 3, e340–e348. [Google Scholar] [CrossRef]
- Lai, K.L.; Hu, F.C.; Wen, F.Y.; Chen, J.J. Lymphocyte count is a universal predictor of health outcomes in COVID-19 patients before mass vaccination: A meta-analytical study. J. Glob. Health 2022, 12, 05041. [Google Scholar] [CrossRef]
- Zhao, W.; Li, H.; Li, J.; Xu, B.; Xu, J. The mechanism of multiple organ dysfunction syndrome in patients with COVID-19. J. Med. Virol. 2022, 94, 1886–1892. [Google Scholar] [CrossRef]
- Benatti, S.V.; Venturelli, S.; Crotti, G.; Ghirardi, A.; Binda, F.; Savardi, M.; Previtali, G.; Seghezzi, M.; Marozzi, R.; Corsi, A.; et al. Clinical variables associated with late-onset thrombotic and cardiovascular events, after SARS-CoV-2 infection, in a cohort of patients from the first epidemic wave: An 18-month analysis on the “Surviving-COVID” cohort from Bergamo, Italy. Front. Cardiovasc. Med. 2023, 10, 1280584. [Google Scholar] [CrossRef]
- Li, T.; Li, W.; Chen, F.; Xu, Q.; Du, G.; Fu, Y.; Yuan, L.; Zhang, S.; Wu, W.; He, P.; et al. The chest X-ray score baseline in predicting continuous oxygen therapy failure in low-risk aged patients after thoracic surgery. J. Thorac. Dis. 2024, 16, 1885–1899. [Google Scholar] [CrossRef] [PubMed]
- Limratana, P.; Maisat, W.; Tsai, A.; Yuki, K. Perioperative Factors and Radiographic Severity Scores for Predicting the Duration of Mechanical Ventilation After Arterial Switch Surgery. J. Cardiothorac. Vasc. Anesth. 2024, 38, 992–1005. [Google Scholar] [CrossRef] [PubMed]
| Characteristics | N (%)/Median (IQR) |
|---|---|
| Sex (male) | 178 (61.0%) |
| Age (years) | 74 (63–82) |
| COVID-19-vaccinated (two doses) | 78 (28.9%) |
| SARS-CoV-2 variant | |
| B.1.1.7 (Alpha) | 49 (16.8%) |
| P.1. (Gamma) | 76 (26.0%) |
| B.1.617.2 (Delta) | 167 (57.2%) |
| Comorbidities (No.) | 3 (1–4) |
| Hypertension | 197 (67.5%) |
| Heart disease | 111 (38.0%) |
| Diabetes | 70 (24.0%) |
| Obesity | 65 (22.3%) |
| Malignant disease | 33 (11.3%) |
| Day of disease on admission | 8 (6–10) |
| Oxygen saturation on admission (%) | 90 (84–93) |
| Hospitalization length (days) | 10.5 (7–17) |
| Disease severity | |
| Mild | 26 (8.9%) |
| Moderate | 96 (32.9%) |
| Severe | 63 (21.6%) |
| Critical | 107 (36.6%) |
| HFNC | 123 (42.1%) |
| Mechanical ventilation | 88 (30.4%) |
| Complications | |
| Acute kidney failure | 96 (32.9%) |
| Decompensated heart failure | 65 (22.3%) |
| Septic shock | 55 (18.8%) |
| Pleural effusion | 36 (12.3%) |
| Cytokine release syndrome | 28 (9.6%) |
| ICU admission | 95 (32.5%) |
| ARDS | 73 (25.0%) |
| Death | 150 (51.4%) |
| Parameter | N | MBS Median (IQR) | p-Value | Adjusted p-Value |
|---|---|---|---|---|
| Sex | ||||
| Male | 178 | 9 (6–12) | 0.059 | 0.087 |
| Female | 114 | 10 (6–14) | ||
| Vaccination status | ||||
| Vaccinated | 78 | 8 (5–11) | 0.005 | 0.009 |
| Unvaccinated | 214 | 10 (6–14) | ||
| SARS-CoV-2 variant | ||||
| B.1.1.7 (Alpha) | 48 | 10 (6–12) | 0.201 | 0.249 |
| P.1. (Gamma) | 76 | 10 (6–16) | ||
| B.1.617.2 (Delta) | 167 | 9 (6–13) | ||
| Hypertension | ||||
| Yes | 197 | 9 (6–13) | 0.171 | 0.231 |
| No | 95 | 9 (6–13) | ||
| Heart disease | ||||
| Yes | 111 | 10 (7–12) | 0.082 | 0.116 |
| No | 181 | 9 (6–13) | ||
| Diabetes | ||||
| Yes | 70 | 10 (7–14) | 0.184 | 0.238 |
| No | 222 | 9 (6–12) | ||
| Obesity | ||||
| Yes | 65 | 10 (6–14) | 0.498 | 0.532 |
| No | 227 | 9 (6–12) | ||
| Malignant disease | ||||
| Yes | 33 | 9 (7–14) | 0.475 | 0.526 |
| No | 259 | 9 (6–13) | ||
| COVID-19 severity | ||||
| Mild | 26 | 5 (4–8) | <0.001 | <0.001 |
| Moderate | 96 | 7 (5–10) | ||
| Severe | 63 | 11 (7–14) | ||
| Critical | 107 | 12 (9–16) | ||
| Acute kidney failure | ||||
| Yes | 96 | 11 (6–15) | 0.019 | 0.031 |
| No | 196 | 9 (6–12) | ||
| Decompensated heart failure | ||||
| Yes | 65 | 12 (9–16) | <0.001 | <0.001 |
| No | 227 | 8 (6–12) | ||
| Septic shock | ||||
| Yes | 55 | 12 (10–17) | <0.001 | <0.001 |
| No | 237 | 8 (6–12) | ||
| Pleural effusion | ||||
| Yes | 36 | 10 (6–11) | 0.398 | 0.457 |
| No | 256 | 9 (6–13) | ||
| Cytokine release syndrome | ||||
| Yes | 28 | 12 (9–16) | 0.009 | 0.016 |
| No | 264 | 9 (6–12) | ||
| ARDS | ||||
| Yes | 73 | 12 (9–18) | <0.001 | <0.001 |
| No | 219 | 8 (6–12) | ||
| ICU admission | ||||
| Yes | 95 | 12 (10–17) | <0.001 | <0.001 |
| No | 197 | 8 (5–11) | ||
| Mechanical ventilation | ||||
| Yes | 88 | 12 (9–17) | <0.001 | <0.001 |
| No | 204 | 8 (6–12) | ||
| COVID-19 outcome | ||||
| Died | 150 | 12 (9–16) | <0.001 | <0.001 |
| Survived | 142 | 6 (5–9) |
| Parameter | Correlation Coefficient with MBS (95% CI) | p-Value | Adjusted p-Value |
|---|---|---|---|
| Age | 0.06 [−0.05, 0.17] | 0.296 | 0.353 |
| Number of comorbidities | 0.01 [−0.11, 0.12] | 0.900 | 0.930 |
| Day of disease on admission | 0.02 [−0.11, 0.12] | 0.974 | 0.986 |
| Oxygen saturation on admission | −0.47 [−0.56, −0.36] | <0.001 | <0.001 |
| Hospitalization length | 0.12 [0.00, 0.23] | 0.049 | 0.076 |
| Laboratory parameters | |||
| LD | 0.45 [0.35, 0.55] | <0.001 | <0.001 |
| D-dimers | 0.43 [0.33, 0.52] | <0.001 | <0.001 |
| Lactate | 0.42 [0.31, 0.52] | <0.001 | <0.001 |
| Albumins | −0.39 [−0.50, −0.27] | <0.001 | <0.001 |
| Lymphocytes (%) | −0.39 [−0.48, −0.28] | <0.001 | <0.001 |
| Neutrophils (%) | 0.38 [0.27, 0.47] | <0.001 | <0.001 |
| Urea | 0.37 [0.26, 0.48] | <0.001 | <0.001 |
| CRP | 0.35 [0.23, 0.45] | <0.001 | <0.001 |
| The best model predicting ARDS | |||
| Predictor | aOR (95% CI) | p-value | Adjusted p-value |
| MBS | 1.21 [1.10, 1.32] | <0.001 | <0.001 |
| LD on admission | 1.03 [1.01, 1.06] | 0.003 | 0.005 |
| Obesity | 2.58 [1.05, 6.37] | 0.039 | 0.039 |
| The best model predicting fatal outcome | |||
| Predictor | aOR (95% CI) | p-value | Adjusted p-value |
| MBS | 1.40 [1.24, 1.61] | <0.001 | <0.001 |
| Oxygen saturation on admission | 0.90 [0.84, 0.96] | 0.002 | 0.004 |
| Obesity | 3.72 [1.38, 10.84] | 0.009 | 0.012 |
| Percentage of lymphocytes on admission | 0.73 [0.55, 0.96] | 0.032 | 0.032 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mehmedović, A.; Bodulić, K.; Višković, K.; Rakušić, N.; Markotić, A.; Hrabak Paar, M. The Use of the Modified Brixia Score for Predicting Mortality and Acute Respiratory Distress Syndrome in Patients with COVID-19 Pneumonia: What Have We Learned? Diagnostics 2025, 15, 1409. https://doi.org/10.3390/diagnostics15111409
Mehmedović A, Bodulić K, Višković K, Rakušić N, Markotić A, Hrabak Paar M. The Use of the Modified Brixia Score for Predicting Mortality and Acute Respiratory Distress Syndrome in Patients with COVID-19 Pneumonia: What Have We Learned? Diagnostics. 2025; 15(11):1409. https://doi.org/10.3390/diagnostics15111409
Chicago/Turabian StyleMehmedović, Armin, Kristian Bodulić, Klaudija Višković, Nevena Rakušić, Alemka Markotić, and Maja Hrabak Paar. 2025. "The Use of the Modified Brixia Score for Predicting Mortality and Acute Respiratory Distress Syndrome in Patients with COVID-19 Pneumonia: What Have We Learned?" Diagnostics 15, no. 11: 1409. https://doi.org/10.3390/diagnostics15111409
APA StyleMehmedović, A., Bodulić, K., Višković, K., Rakušić, N., Markotić, A., & Hrabak Paar, M. (2025). The Use of the Modified Brixia Score for Predicting Mortality and Acute Respiratory Distress Syndrome in Patients with COVID-19 Pneumonia: What Have We Learned? Diagnostics, 15(11), 1409. https://doi.org/10.3390/diagnostics15111409









