Left Atrial Strain—Current Review of Clinical Applications
Abstract
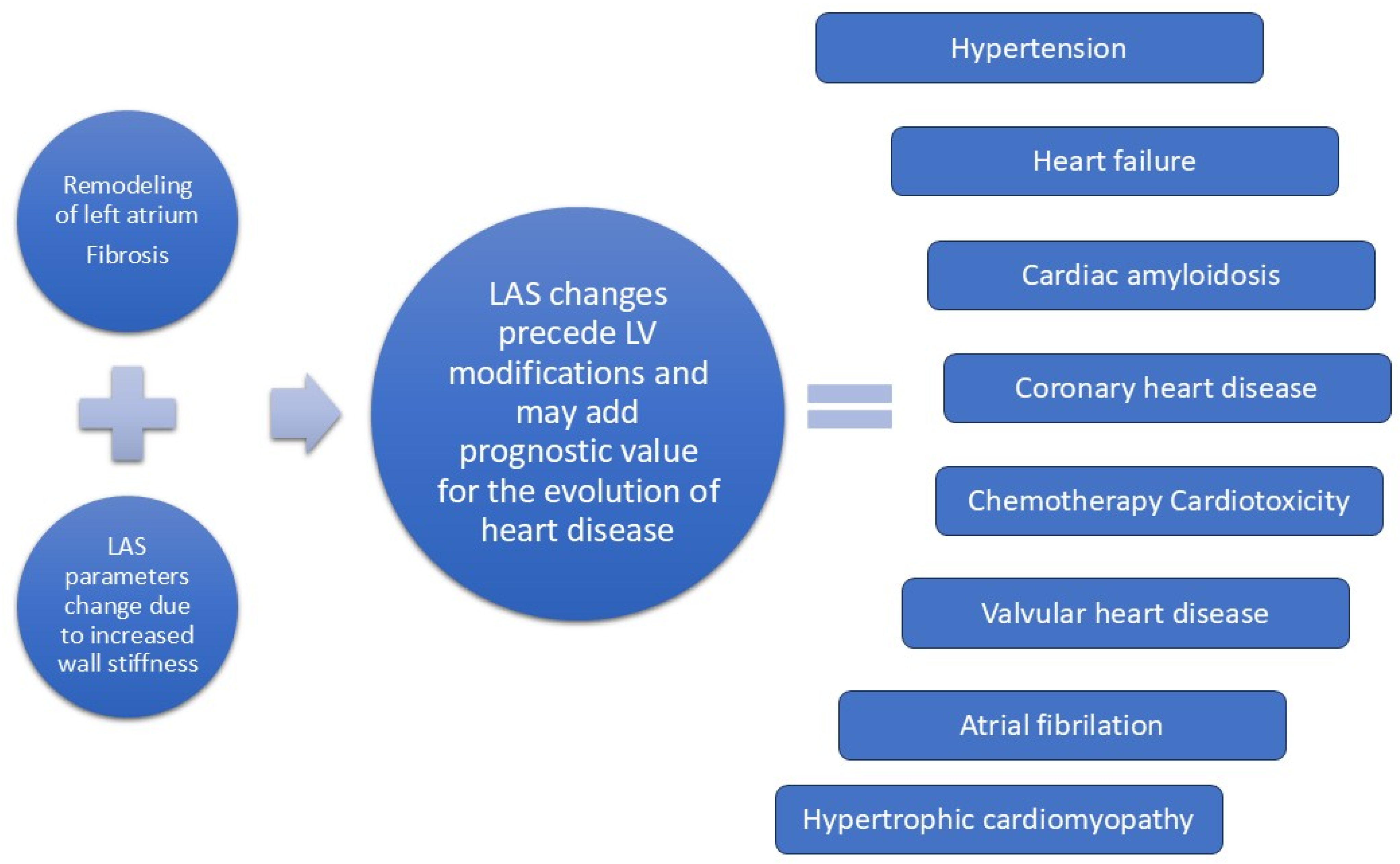
1. Introduction
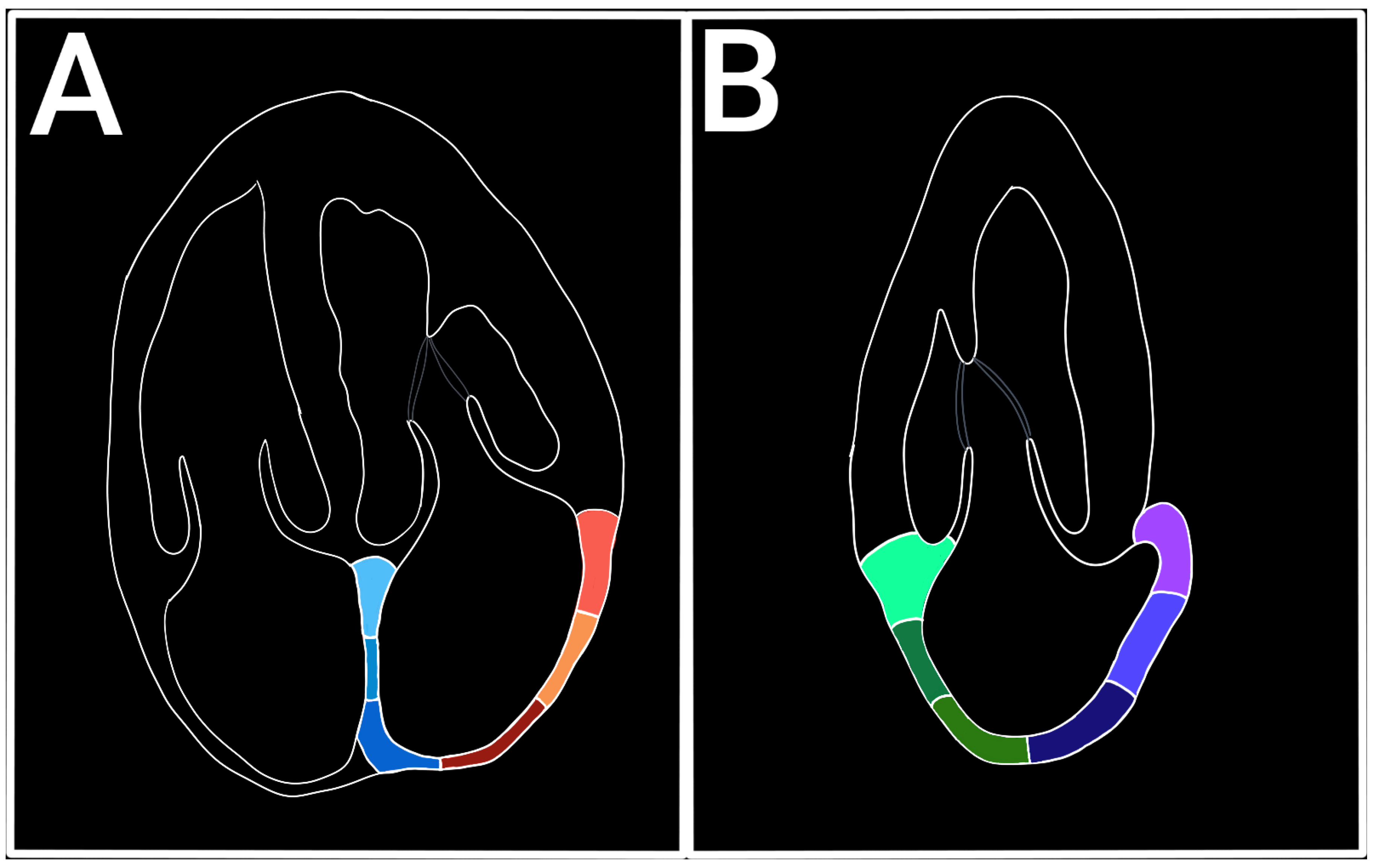
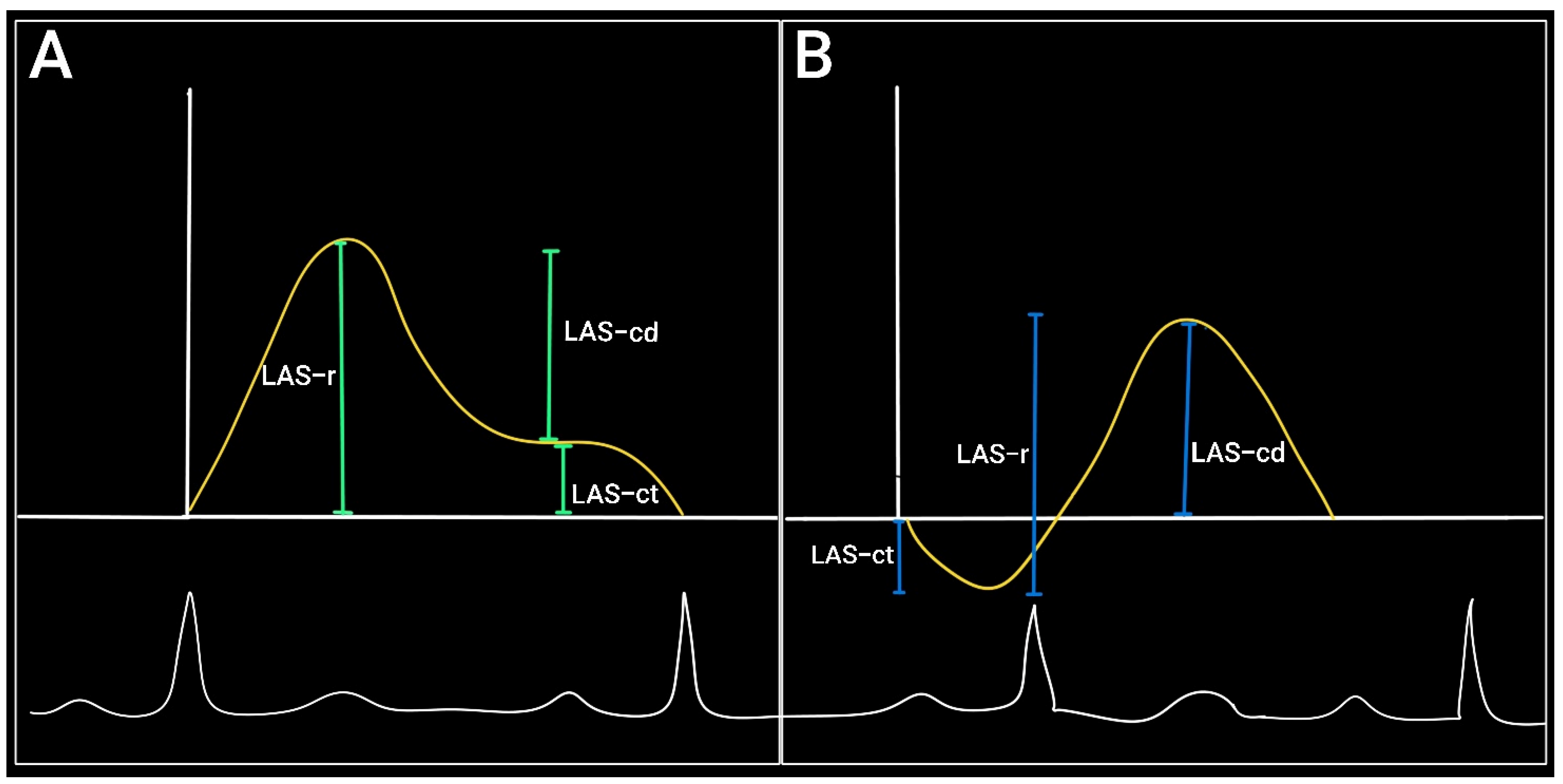
2. Assessment of LA Strain (LAS) by Echocardiography
Normal Values of Left Atrial Strain
3. Left Atrial Strain in Hypertension
4. Left Atrial Strain and Heart Failure with Preserved Ejection Fraction
5. Left Atrial Strain in Heart Failure with Mildly Reduced Ejection Fraction
6. Left Atrial Strain in Heart Failure with Reduced Ejection Fraction
7. Left Atrial Strain in Cardiac Amyloidosis
8. Left Atrial Strain and Coronary Heart Disease
9. Left Atrial Strain in Atrial Fibrillation
10. Left Atrial Strain in the Assessment of Chemotherapy-Induced Cardiotoxicity
11. LAS in Valvular Heart Disease
11.1. LAS in Mitral Regurgitation (MR)
11.2. LAS in Mitral Stenosis (MS)
11.3. LAS in Aortic Stenosis (AS)
12. Method Limitations
13. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| A2C | Apical two chamber view |
| A4C | Apical four chamber view |
| AF | Atrial fibrillation |
| CA | Cardiac amyloidosis |
| CCT | Cardiac computed tomography |
| CMR | Cardiac magnetic resonance |
| DOAJ | Directory of open access journals |
| GPALS | Global peak atrial longitudinal strain |
| HFmrEF | Heart failure with mildly reduced ejection fraction |
| HFpEF | Heart failure with preserved ejection fraction |
| HFrEF | Heart failure with reduced ejection fraction |
| LA | Left atrium |
| LAP | Left atrial pressure |
| LAS | Left atrium strain |
| LAS-cd | Conduit function of LA |
| LAS-ct | Pump function of LA |
| LASR | Left atrial strain rate |
| LAS-r | Reservoir function of LA |
| LAV | Left atrial volume |
| LAVI | Left atrial volume index |
| LV | Left ventricle |
| LVEF | Left ventricle ejection fraction |
| LVGLS | Left ventricle global longitudinal strain |
| MDPI | Multidisciplinary Digital Publishing Institute |
| NP | Natriuretic peptides |
| PACS | Peak atrial contraction strain |
| PALS | Peak atrial longitudinal strain |
| STE | Speckle tracking echocardiography |
| STEMI | ST-segment elevation myocardial infarction |
| TTE | Transthoracic echocardiography |
| VHD | Valvular heart disease |
References
- Kebed, K.Y.; Addetia, K.; Lang, R.M. Importance of the Left Atrium: More Than a Bystander? Heart Fail. Clin. 2019, 15, 191–204. [Google Scholar] [CrossRef] [PubMed]
- Hoit, B.D. Left atrial size and function: Role in prognosis. JACC J. 2014, 63, 493–505. [Google Scholar] [CrossRef] [PubMed]
- Thomas, L.; Levett, K.; Boyd, A.; Hons, B.; Leung, D.Y.C.; Schiller, N.B.; Ross, D.L.; Sydney, A.; Francisco, S. Compensatory Changes in Atrial Volumes with Normal Aging: Is Atrial Enlargement Inevitable? JACC J. 2002, 40, 1630–1635. [Google Scholar] [CrossRef]
- Pathan, F.; D’Elia, N.; Nolan, M.T.; Marwick, T.H.; Negishi, K. Left atrial strain: A multi-modality, multi-vendor comparison study. Eur. Heart J. Cardiovasc. Imaging 2021, 22, 102–110. [Google Scholar] [CrossRef]
- Benjamin, M.M.; Munir, M.S.; Shah, P.; Kinno, M.; Rabbat, M.; Sanagala, T.; Syed, M.A. Comparison of left atrial strain by feature-tracking cardiac magnetic resonance with speckle-tracking transthoracic echocardiography. Int. J. Cardiovasc. Imaging 2021, 38, 1383–1389. [Google Scholar] [CrossRef]
- Hosokawa, T.; Kawakami, H.; Tanabe, Y.; Yoshida, K.; Endo, Y.; Tamai, F.; Nishiyama, H.; Fukuyama, N.; Inoue, K.; Yamaguchi, O.; et al. Feasibility of left atrial strain assessment using cardiac computed tomography in patients with paroxysmal atrial fibrillation. Int. J. Cardiovasc. Imaging 2024, 40, 1725–1734. [Google Scholar] [CrossRef]
- Hosokawa, T.; Kawakami, H.; Tanabe, Y.; Fukuyama, N.; Yoshida, K.; Ohara, K.; Kitamura, T.; Kawaguchi, N.; Kido, T.; Nagai, T.; et al. Left atrial strain assessment using cardiac computed tomography in patients with hypertrophic cardiomyopathy. Jpn. J. Radiol. 2023, 41, 843–853. [Google Scholar] [CrossRef]
- Thomas, L.; Marwick, T.H.; Popescu, B.A.; Donal, E.; Badano, L.P. Left Atrial Structure and Function, and Left Ventricular Diastolic Dysfunction: JACC State-of-the-Art Review. JACC J. 2019, 73, 1961–1977. [Google Scholar] [CrossRef]
- Marincheva, G.; Iakobishvili, Z.; Valdman, A.; Laish-Farkash, A. Left Atrial Strain: Clinical Use and Future Applications-A Focused Review Article. Rev. Cardiovasc. Med. 2022, 23, 154. [Google Scholar] [CrossRef]
- Mor-Avi, V.; Lang, R.M.; Badano, L.P.; Belohlavek, M.; Cardim, N.M.; Derumeaux, G.; Galderisi, M.; Marwick, T.; Nagueh, S.F.; Sengupta, P.P.; et al. Current and Evolving Echocardiographic Techniques for the Quantitative Evaluation of Cardiac Mechanics: ASE/EAE Consensus Statement on Methodology and Indications. J. Am. Soc. Echocardiogr. 2011, 24, 277–313. [Google Scholar] [CrossRef]
- Cameli, M.; Lisi, M.; Focardi, M.; Reccia, R.; Natali, B.M.; Sparla, S.; Mondillo, S. Left Atrial Deformation Analysis by Speckle Tracking Echocardiography for Prediction of Cardiovascular Outcomes. Am. J. Cardiol. 2012, 110, 264–269. [Google Scholar] [CrossRef] [PubMed]
- Cameli, M.; Miglioranza, M.H.; Magne, J.; Mandoli, G.E.; Benfari, G.; Ancona, R.; Sibilio, G.; Reskovic Luksic, V.; Dejan, D.; Griseli, L.; et al. Multicentric Atrial Strain COmparison between Two Different Modalities: MASCOT HIT Study. Diagnostics 2020, 10, 946. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.; Liu, H.; Sun, J.; Zhang, J.; Li, L.; Tang, Q.; Liu, Y.; Deng, Y. Evaluation of left atrial and ventricular remodeling in atrial fibrillation subtype by using speckle tracking echocardiography. Front. Cardiovasc. Med. 2023, 10, 1208577. [Google Scholar] [CrossRef]
- Hayashi, S.; Yamada, H.; Bando, M.; Saijo, Y.; Nishio, S.; Hirata, Y.; Klein, A.L.; Sata, M. Optimal Analysis of Left Atrial Strain by Speckle Tracking Echocardiography: P-wave versus R-wave Trigger. Echocardiography 2015, 32, 1241–1249. [Google Scholar] [CrossRef]
- Figueiredo, F.A.; Costa Filho, A.L.; Figueiredo, F.A.; Chavez, L.M.T.; Teixeira, M.F.A.; Barbosa, W.S.; Bronzatto, P.H.; Cintra, P.R.; Nunes, M.C.P. Left Atrial Strain: Clinical Applications and Prognostic Implications. ABC Imagem Cardiovasc. 2024, 37, e20240003. [Google Scholar] [CrossRef]
- Cameli, M.; Lisi, M.; Righini, F.M.; Massoni, A.; Natali, B.M.; Focardi, M.; Tacchini, D.; Geyer, A.; Curci, V.; Di Tommaso, C.; et al. Usefulness of Atrial Deformation Analysis to Predict Left Atrial Fibrosis and Endocardial Thickness in Patients Undergoing Mitral Valve Operations for Severe Mitral Regurgitation Secondary to Mitral Valve Prolapse. Am. J. Cardiol. 2013, 111, 595–601. [Google Scholar] [CrossRef]
- Saraiva, R.M.; Demirkol, S.; Buakhamsri, A.; Greenberg, N.; Popović, Z.B.; Thomas, J.D.; Klein, A.L. Left Atrial Strain Measured by Two-Dimensional Speckle Tracking Represents a New Tool to Evaluate Left Atrial Function. J. Am. Soc. Echocardiogr. 2010, 23, 172–180. [Google Scholar] [CrossRef]
- Voigt, J.-U.; Mălăescu, G.-G.; Haugaa, K.; Badano, L. How to do LA strain. Eur. Heart J. Cardiovasc. Imaging 2020, 21, 715–717. [Google Scholar] [CrossRef]
- Badano, L.P.; Kolias, T.J.; Muraru, D.; Abraham, T.P.; Aurigemma, G.; Edvardsen, T.; D’Hooge, J.; Donal, E.; Fraser, A.G.; Marwick, T. Standardization of left atrial, right ventricular, and right atrial deformation imaging using two-dimensional speckle tracking echocardiography: A consensus document of the EACVI/ASE/Industry Task Force to standardize deformation imaging. Eur. Heart J. Cardiovasc. Imaging 2018, 19, 591–600. [Google Scholar] [CrossRef]
- Svartstein, A.-S.W.; Lassen, M.H.; Skaarup, K.G.; Grove, G.L.; Vyff, F.; Ravnkilde, K.; Pedersen, S.; Galatius, S.; Modin, D.; Biering-Sørensen, T. Predictive value of left atrial strain in relation to atrial fibrillation following acute myocardial infarction. Int. J. Cardiol. 2022, 364, 52–59. [Google Scholar] [CrossRef]
- Bo, K.; Gao, Y.; Zhou, Z.; Gao, X.; Liu, T.; Zhang, H.; Li, Q.; Wang, H.; Xu, L. Incremental prognostic value of left atrial strain in patients with heart failure. ESC Heart Fail. 2022, 9, 3942–3953. [Google Scholar] [CrossRef] [PubMed]
- Jia, F.; Chen, A.; Zhang, D.; Fang, L.; Chen, W. Prognostic Value of Left Atrial Strain in Heart Failure: A Systematic Review and Meta-Analysis. Front. Cardiovasc. Med. 2022, 9, 935103. [Google Scholar] [CrossRef] [PubMed]
- Modin, D.; Biering-Sørensen, S.R.; Møgelvang, R.; Alhakak, A.S.; Jensen, J.S.; Biering-Sørensen, T. Prognostic value of left atrial strain in predicting cardiovascular morbidity and mortality in the general population. Eur. Heart J. Cardiovasc. Imaging 2019, 20, 804–815. [Google Scholar] [CrossRef]
- Hsu, P.-C.; Lee, W.-H.; Chu, C.-Y.; Lee, H.-H.; Lee, C.-S.; Yen, H.-W.; Lin, T.-H.; Voon, W.-C.; Lai, W.-T.; Sheu, S.-H.; et al. Prognostic role of left atrial strain and its combination index with transmitral E-wave velocity in patients with atrial fibrillation. Sci. Rep. 2016, 6, 17318. [Google Scholar] [CrossRef]
- Kowallick, J.T.; Kutty, S.; Edelmann, F.; Chiribiri, A.; Villa, A.; Steinmetz, M.; Sohns, J.M.; Staab, W.; Bettencourt, N.; Unterberg-Buchwald, C.; et al. Quantification of left atrial strain and strain rate using Cardiovascular Magnetic Resonance myocardial feature tracking: A feasibility study. J. Cardiovasc. Magn. Reson. 2014, 16, 60. [Google Scholar] [CrossRef]
- Sonaglioni, A.; Lombardo, M.; Nicolosi, G.L.; Rigamonti, E.; Anzà, C. Incremental diagnostic role of left atrial strain analysis in thrombotic risk assessment of nonvalvular atrial fibrillation patients planned for electrical cardioversion. Int. J. Cardiovasc. Imaging 2021, 37, 1539–1550. [Google Scholar] [CrossRef]
- Popescu, A.; Bézy, S.; Voigt, J.-U. Translating High-Frame-Rate Imaging into Clinical Practice: Where Do We Stand? Rom. J. Cardiol. 2023, 33, 35–46. [Google Scholar] [CrossRef]
- Voigt, J.-U.; Pedrizzetti, G.; Lysyansky, P.; Marwick, T.H.; Houle, H.; Baumann, R.; Pedri, S.; Ito, Y.; Abe, Y.; Metz, S.; et al. Definitions for a common standard for 2D speckle tracking echocardiography: Consensus document of the EACVI/ASE/Industry Task Force to standardize deformation imaging. Eur. Heart J. Cardiovasc. Imaging 2015, 16, 1–11. [Google Scholar] [CrossRef]
- Sun, B.J.; Park, J.-H.; Lee, M.; Choi, J.-O.; Lee, J.-H.; Shin, M.-S.; Kim, M.-J.; Jung, H.O.; Park, J.R.; Sohn, I.S.; et al. Normal Reference Values for Left Atrial Strain and Its Determinants from a Large Korean Multicenter Registry. J. Cardiovasc. Imaging 2020, 28, 186. [Google Scholar] [CrossRef]
- Sugimoto, T.; Robinet, S.; Dulgheru, R.; Bernard, A.; Ilardi, F.; Contu, L.; Addetia, K.; Caballero, L.; Kacharava, G.; Athanassopoulos, G.D.; et al. Echocardiographic reference ranges for normal left atrial function parameters: Results from the EACVI NORRE study. Eur. Heart J. Cardiovasc. Imaging 2018, 19, 630–638. [Google Scholar] [CrossRef]
- Pathan, F.; D’Elia, N.; Nolan, M.T.; Marwick, T.H.; Negishi, K. Normal Ranges of Left Atrial Strain by Speckle-Tracking Echocardiography: A Systematic Review and Meta-Analysis. J. Am. Soc. Echocardiogr. 2017, 30, 59–70.e8. [Google Scholar] [CrossRef] [PubMed]
- Yafasov, M.; Olsen, F.J.; Skaarup, K.G.; Lassen, M.C.H.; Johansen, N.D.; Lindgren, F.L.; Jensen, G.B.; Schnohr, P.; Møgelvang, R.; Søgaard, P.; et al. Normal values for left atrial strain, volume, and function derived from 3D echocardiography: The Copenhagen City Heart Study. Eur. Heart J. Cardiovasc. Imaging 2024, 25, 602–612. [Google Scholar] [CrossRef] [PubMed]
- Mondillo, S.; Cameli, M.; Caputo, M.L.; Lisi, M.; Palmerini, E.; Padeletti, M.; Ballo, P. Early Detection of Left Atrial Strain Abnormalities by Speckle-Tracking in Hypertensive and Diabetic Patients with Normal Left Atrial Size. J. Am. Soc. Echocardiogr. 2011, 24, 898–908. [Google Scholar] [CrossRef]
- Taamallah, K.; Yaakoubi, W.; Haggui, A.; Hajlaoui, N.; Fehri, W. Early detection of left atrial dysfunction in hypertensive patients: Role of Speckle Tracking imaging. Tunis. Med. 2022, 100, 788–799. [Google Scholar]
- Xu, T.-Y.; Sun, J.P.; Lee, A.P.-W.; Yang, X.S.; Ji, L.; Zhang, Z.; Li, Y.; Yu, C.-M.; Wang, J.-G. Left Atrial Function as Assessed by Speckle-Tracking Echocardiography in Hypertension. Medicine 2015, 94, e526. [Google Scholar] [CrossRef]
- Stefani, L.D.; Trivedi, S.J.; Ferkh, A.; Emerson, P.; Marschner, S.; Gan, G.; Altman, M.; Thomas, L. Left atrial mechanics evaluated by two-dimensional strain analysis: Alterations in essential hypertension. J. Hypertens. 2024, 42, 274–282. [Google Scholar] [CrossRef]
- Sun, Q.; Pan, Y.; Zhao, Y.; Liu, Y.; Jiang, Y. Association of Nighttime Systolic Blood Pressure with Left Atrial-Left Ventricular–Arterial Coupling in Hypertension. Front. Cardiovasc. Med. 2022, 9, 814756. [Google Scholar] [CrossRef]
- O’Driscoll, J.M.; McCarthy, F.P.; Giorgione, V.; Jalaludeen, N.; Seed, P.T.; Gill, C.; Sparkes, J.; Poston, L.; Marber, M.; Shennan, A.H.; et al. Left Atrial Mechanics Following Preeclamptic Pregnancy. Hypertension 2024, 81, 1644–1654. [Google Scholar] [CrossRef]
- Mousa, M.; Salam, Z.A.; ElSawye, M.; Omran, A.; Aly, K. Effect of Blood Pressure Control on Left Atrial Function Assessed by 2D Echocardiography in Newly Diagnosed Patients with Systemic Hypertension. Int. J. Cardiovasc. Acad. 2024, 10, 102–114. [Google Scholar] [CrossRef]
- Girard, A.A.; Denney, T.S.; Sharifov, O.F.; Lloyd, S.G. Spironolactone Improves Left Atrial Function and Atrioventricular Coupling in Resistant Hypertension. Circulation 2023, 148 (Suppl. S1), A17781. [Google Scholar] [CrossRef]
- Javadi, N.; Bismee, N.N.; Abbas, M.T.; Scalia, I.G.; Pereyra, M.; Baba Ali, N.; Attaripour Esfahani, S.; Awad, K.; Farina, J.M.; Ayoub, C.; et al. Left Atrial Strain: State of the Art and Clinical Implications. J. Pers. Med. 2024, 14, 1093. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Seo, J.H.; Choi, K.H.; Lee, S.H.; Choi, J.-O.; Jeon, E.-S.; Yang, J.H. Prognostic Implications of Left Atrial Stiffness Index in Heart Failure Patients with Preserved Ejection Fraction. JACC Cardiovasc. Imaging 2023, 16, 435–445. [Google Scholar] [CrossRef] [PubMed]
- Kagami, K.; Harada, T.; Yuasa, N.; Saito, Y.; Sorimachi, H.; Murakami, F.; Naito, A.; Tani, Y.; Kato, T.; Wada, N.; et al. Impaired Left Atrial Reserve Function in Heart Failure with Preserved Ejection Fraction. Circ. Cardiovasc. Imaging 2024, 17, e016549. [Google Scholar] [CrossRef]
- Maffeis, C.; Rossi, A.; Cannata, L.; Zocco, C.; Belyavskiy, E.; Radhakrishnan, A.K.; Feuerstein, A.; Morris, D.A.; Pieske-Kraigher, E.; Pieske, B.; et al. Left atrial strain predicts exercise capacity in heart failure independently of left ventricular ejection fraction. ESC Heart Fail. 2022, 9, 842–852. [Google Scholar] [CrossRef]
- Shin, S.; Claggett, B.; Inciardi, R.M.; Santos, A.B.S.; Shah, S.J.; Zile, M.R.; Pfeffer, M.A.; Shah, A.M.; Solomon, S.D. Prognostic Value of Minimal Left Atrial Volume in Heart Failure with Preserved Ejection Fraction. J. Am. Heart Assoc. 2021, 10, e019545. [Google Scholar] [CrossRef]
- Ye, Z.; Miranda, W.R.; Yeung, D.F.; Kane, G.C.; Oh, J.K. Left Atrial Strain in Evaluation of Heart Failure with Preserved Ejection Fraction. J. Am. Soc. Echocardiogr. 2020, 33, 1490–1499. [Google Scholar] [CrossRef]
- Guțu, A.; Rotari, V. Particularitățile insuficienței cardiace cu fracție de ejecție ușor redusă. Rev. Științe Sănătății Mold. 2022, 3, 46–53. [Google Scholar]
- Goette, A.; Kalman, J.M.; Aguinaga, L.; Akar, J.; Cabrera, J.A.; Chen, S.A.; Chugh, S.S.; Corradi, D.; D’Avila, A.; Dobrev, D.; et al. EHRA/HRS/APHRS/SOLAECE expert consensus on atrial cardiomyopathies: Definition, characterization, and clinical implication. Heart Rhythm 2017, 14, e3–e40. [Google Scholar] [CrossRef]
- Carpenito, M.; Fanti, D.; Mega, S.; Benfari, G.; Bono, M.C.; Rossi, A.; Ribichini, F.L.; Grigioni, F. The Central Role of Left Atrium in Heart Failure. Front. Cardiovasc. Med. 2021, 8, 704762. [Google Scholar] [CrossRef]
- Caminiti, G.; Perrone, M.A.; D’Antoni, V.; Marazzi, G.; Gismondi, A.; Vadalà, S.; Di Biasio, D.; Manzi, V.; Iellamo, F.; Volterrani, M. The Improvement of Left Atrial Function after Twelve Weeks of Supervised Concurrent Training in Patients with Heart Failure with Mid-Range Ejection Fraction: A Pilot Study. J. Cardiovasc. Dev. Dis. 2023, 10, 276. [Google Scholar] [CrossRef]
- Bisbal, F.; Baranchuk, A.; Braunwald, E.; de Luna, A.B.; Bayés-Genís, A. Atrial Failure as a Clinical Entity. J. Am. Coll. Cardiol. 2020, 75, 222–232. [Google Scholar] [CrossRef] [PubMed]
- Al Saikhan, L.; Hughes, A.D.; Chung, W.-S.; Alsharqi, M.; Nihoyannopoulos, P. Left atrial function in heart failure with mid-range ejection fraction differs from that of heart failure with preserved ejection fraction: A 2D speckle-tracking echocardiographic study. Eur. Heart J. Cardiovasc. Imaging 2019, 20, 279–290. [Google Scholar] [CrossRef] [PubMed]
- Maffeis, C.; Morris, D.A.; Belyavskiy, E.; Kropf, M.; Radhakrishnan, A.K.; Zach, V.; Rozados da Conceicao, C.; Trippel, T.D.; Pieske-Kraigher, E.; Rossi, A.; et al. Left atrial function and maximal exercise capacity in heart failure with preserved and mid-range ejection fraction. ESC Heart Fail. 2021, 8, 116–128. [Google Scholar] [CrossRef]
- El-Saied, S.B.; El-Sherbeny, W.S.; El-sharkawy, S.I. Impact of sodium glucose co-transporter-2 inhibitors on left atrial functions in patients with type-2 diabetes and heart failure with mildly reduced ejection fraction. IJC Heart Vasc. 2024, 50, 101329. [Google Scholar] [CrossRef]
- Jin, X.; Nauta, J.F.; Hung, C.-L.; Ouwerkerk, W.; Teng, T.-H.K.; Voors, A.A.; Lam, C.S.P.; van Melle, J.P. Left atrial structure and function in heart failure with reduced (HFrEF) versus preserved ejection fraction (HFpEF): Systematic review and meta-analysis. Heart Fail. Rev. 2022, 27, 1933–1955. [Google Scholar] [CrossRef]
- Lundberg, A.; Johnson, J.; Hage, C.; Bäck, M.; Merkely, B.; Venkateshvaran, A.; Lund, L.H.; Nagy, A.I.; Manouras, A. Left atrial strain improves estimation of filling pressures in heart failure: A simultaneous echocardiographic and invasive haemodynamic study. Clin. Res. Cardiol. 2019, 108, 703–715. [Google Scholar] [CrossRef]
- Aga, Y.S.; Abou Kamar, S.; Chin, J.F.; van den Berg, V.J.; Strachinaru, M.; Bowen, D.; Frowijn, R.; Akkerhuis, M.K.; Constantinescu, A.A.; Umans, V.; et al. Potential role of left atrial strain in estimation of left atrial pressure in patients with chronic heart failure. ESC Heart Fail. 2023, 10, 2345–2353. [Google Scholar] [CrossRef]
- Cameli, M.; Mandoli, G.E.; Loiacono, F.; Sparla, S.; Iardino, E.; Mondillo, S. Left atrial strain: A useful index in atrial fibrillation. Int. J. Cardiol. 2016, 220, 208–213. [Google Scholar] [CrossRef]
- Jin, X.; Tay, W.T.; Soon, D.; Sim, D.; Loh, S.Y.; Lee, S.; Jaufeerally, F.; Ling, L.H.; Richards, A.M.; Voors, A.A.; et al. Patients with chronic heart failure and predominant left atrial versus left ventricular myopathy. Cardiovasc. Ultrasound 2025, 23, 1. [Google Scholar] [CrossRef]
- Bouwmeester, S.; van der Stam, J.A.; van Loon, S.L.M.; van Riel, N.A.W.; Boer, A.-K.; Dekker, L.R.; Scharnhorst, V.; Houthuizen, P. Left atrial reservoir strain as a predictor of cardiac outcome in patients with heart failure: The HaFaC cohort study. BMC Cardiovasc. Disord. 2022, 22, 104. [Google Scholar] [CrossRef]
- Park, J.-H.; Hwang, I.-C.; Park, J.J.; Park, J.-B.; Cho, G.-Y. Prognostic power of left atrial strain in patients with acute heart failure. Eur. Heart J. Cardiovasc. Imaging 2021, 22, 210–219. [Google Scholar] [CrossRef] [PubMed]
- Carluccio, E.; Cameli, M.; Rossi, A.; Dini, F.L.; Biagioli, P.; Mengoni, A.; Jacoangeli, F.; Mandoli, G.E.; Pastore, M.C.; Maffeis, C.; et al. Left Atrial Strain in the Assessment of Diastolic Function in Heart Failure: A Machine Learning Approach. Circ. Cardiovasc. Imaging 2023, 16, e014605. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Pavia, P.; Rapezzi, C.; Adler, Y.; Arad, M.; Basso, C.; Brucato, A.; Burazor, I.; Caforio, A.L.P.; Damy, T.; Eriksson, U.; et al. Diagnosis and treatment of cardiac amyloidosis: A position statement of the ESC Working Group on Myocardial and Pericardial Diseases. Eur. Heart J. 2021, 42, 1554–1568. [Google Scholar] [CrossRef]
- Monte, I.P.; Faro, D.C.; Trimarchi, G.; de Gaetano, F.; Campisi, M.; Losi, V.; Teresi, L.; Di Bella, G.; Tamburino, C.; de Gregorio, C. Left Atrial Strain Imaging by Speckle Tracking Echocardiography: The Supportive Diagnostic Value in Cardiac Amyloidosis and Hypertrophic Cardiomyopathy. J. Cardiovasc. Dev. Dis. 2023, 10, 261. [Google Scholar] [CrossRef]
- Mattig, I.; Steudel, T.; Klingel, K.; Barzen, G.; Frumkin, D.; Spethmann, S.; Romero Dorta, E.; Stangl, K.; Heidecker, B.; Landmesser, U.; et al. Right heart and left atrial strain to differentiate cardiac amyloidosis and Fabry disease. Sci. Rep. 2024, 14, 2445. [Google Scholar] [CrossRef]
- Puwanant, S.; Suksiriworaboot, T.; Kochaiyapatana, P.; Puwatnuttasit, P.; Songmuang, S.B. Left Atrial and Left Ventricular Deformation in Identifying Advanced Stage of Cardiac Amyloidosis. J. Am. Coll. Cardiol. 2023, 81, 741. [Google Scholar] [CrossRef]
- Oike, F.; Usuku, H.; Yamamoto, E.; Yamada, T.; Egashira, K.; Morioka, M.; Nishi, M.; Komorita, T.; Hirakawa, K.; Tabata, N.; et al. Prognostic value of left atrial strain in patients with wild-type transthyretin amyloid cardiomyopathy. ESC Heart Fail. 2021, 8, 5316–5326. [Google Scholar] [CrossRef]
- Edbom, F.; Lindqvist, P.; Wiklund, U.; Pilebro, B.; Anan, I.; Flachskampf, F.A.; Arvidsson, S. Assessing left atrial dysfunction in cardiac amyloidosis using LA–LV strain slope. Eur. Heart J.-Imaging Methods Pract. 2024, 2, qyae100. [Google Scholar] [CrossRef]
- Bandera, F.; Martone, R.; Chacko, L.; Ganesananthan, S.; Gilbertson, J.A.; Ponticos, M.; Lane, T.; Martinez-Naharro, A.; Whelan, C.; Quarta, C.; et al. Clinical Importance of Left Atrial Infiltration in Cardiac Transthyretin Amyloidosis. JACC Cardiovasc. Imaging 2022, 15, 17–29. [Google Scholar] [CrossRef]
- Carvalheiro, R.; Marques Antunes, M.; Cardoso, I.; Ferreira, A.; Viegas, J.; Bras, P.; Almeida, I.; Galrinho, A.; Cruz Ferreira, R.; Aguiar Rosa, S. Left atrial mechanics and left ventricular function in cardiac amyloidosis patients treated with tafamidis. Eur. Heart J. Cardiovasc. Imaging 2025, 26 (Suppl. S1), jeae333.313. [Google Scholar] [CrossRef]
- Lindstrom, M.; DeCleene, N.; Dorsey, H.; Fuster, V.; Johnson, C.O.; LeGrand, K.E.; Mensah, G.A.; Razo, C.; Stark, B.; Varieur Turco, J.; et al. Global Burden of Cardiovascular Diseases and Risks Collaboration, 1990–2021. J. Am. Coll. Cardiol. 2022, 80, 2372–2425. [Google Scholar] [CrossRef]
- Ionescu, M.; Ionescu, P.; Suceveanu, A.; Stoian, A.; Motofei, I.; Ardeleanu, V.; Parepa, I.-R. Cardiovascular risk estimation in young patients with ankylosing spondylitis: A new model based on a prospective study in Constanta County, Romania. Exp. Ther. Med. 2021, 21, 529. [Google Scholar] [CrossRef] [PubMed]
- Pedersson, P.R.; Skaarup, K.G.; Lassen, M.C.H.; Olsen, F.J.; Iversen, A.Z.; Jørgensen, P.G.; Biering-Sørensen, T. Left atrial strain is associated with long-term mortality in acute coronary syndrome patients. Int. J. Cardiovasc. Imaging 2024, 40, 841–851. [Google Scholar] [CrossRef] [PubMed]
- Ricken, K.W.L.M.; Lenselink, C.; Venema, C.S.; van der Horst, I.C.C.; van der Harst, P.; Pundziute-Do Prado, G.; Voors, A.A.; Lipsic, E. Left atrial strain predicts long-term heart failure outcomes after ST-elevation myocardial infarction. Int. J. Cardiol. 2025, 422, 132931. [Google Scholar] [CrossRef]
- Tangen, J.; Nguyen, T.M.; Melichova, D.; Klaeboe, L.G.; Forsa, M.; Andresen, K.; Wazzan, A.; Al Lie, O.; Haugaa, K.; Skulstad, H.; et al. The Prognostic Value of Left Atrial Function in Patients with Acute Myocardial Infarction. Diagnostics 2024, 14, 2027. [Google Scholar] [CrossRef]
- Sikora-Frac, M.; Pilichowska-Paszkiet, E.; Smarz, K.; Maciejewski, P.; Kokowicz, P.; Przadka, M.; Budaj, A.; Zaborska, B. Usefulness of left atrial strain in detection of left ventricular diastolic dysfunction in patients with first acute myocardial infarction. Eur. Heart J. Cardiovasc. Imaging 2025, 26 (Suppl. S1), jeae333.074. [Google Scholar] [CrossRef]
- Tu, Y.; Liu, X.; Li, X.; Xue, N. Left atrial stiffness index—An early marker of left ventricular diastolic dysfunction in patients with coronary heart disease. BMC Cardiovasc. Disord. 2024, 24, 371. [Google Scholar] [CrossRef]
- Serenelli, M.; Cantone, A.; Dal Passo, B.; Di Ienno, L.; Fiorio, A.; Pavasini, R.; Passarini, G.; Bertini, M.; Campo, G. Atrial Longitudinal Strain Predicts New-Onset Atrial Fibrillation. JACC Cardiovasc. Imaging 2023, 16, 392–395. [Google Scholar] [CrossRef]
- Xu, Y.; Zhou, J.; Su, B.; Sun, Y.; Zhou, J.; Liu, Y.; Zhou, B.; Zou, C. Left atrial strain parameters to predicting elevated left atrial pressure in patients with atrial fibrillation. Echocardiography 2024, 41, e15876. [Google Scholar] [CrossRef]
- Uziębło-Życzkowska, B.; Krzesiński, P.; Jurek, A.; Krzyżanowski, K.; Kiliszek, M. Correlations between left atrial strain and left atrial pressures values in patients undergoing atrial fibrillation ablation. Kardiol. Pol. 2021, 79, 1223–1230. [Google Scholar] [CrossRef]
- Granchietti, A.G.; Ciardetti, N.; Mazzoni, C.; Garofalo, M.; Mazzotta, R.; Micheli, S.; Chiostri, M.; Orlandi, M.; Biagiotti, L.; Del Pace, S.; et al. Left atrial strain and risk of atrial fibrillation after coronary artery bypass-grafting. Int. J. Cardiol. 2025, 422, 132981. [Google Scholar] [CrossRef] [PubMed]
- Beyls, C.; Hermida, A.; Nicolas, M.; Debrigode, R.; Vialatte, A.; Peschanski, J.; Bunelle, C.; Fournier, A.; Jarry, G.; Landemaine, T.; et al. Left atrial strain analysis and new-onset atrial fibrillation in patients with ST-segment elevation myocardial infarction: A prospective echocardiography study. Arch. Cardiovasc. Dis. 2024, 117, 266–274. [Google Scholar] [CrossRef] [PubMed]
- Hauser, R.; Nielsen, A.B.; Skaarup, K.G.; Lassen, M.C.H.; Duus, L.S.; Johansen, N.D.; Sengeløv, M.; Marott, J.L.; Jensen, G.; Schnohr, P.; et al. Left atrial strain predicts incident atrial fibrillation in the general population: The Copenhagen City Heart Study. Eur. Heart J. Cardiovasc. Imaging 2021, 23, 52–60. [Google Scholar] [CrossRef]
- Rasmussen, S.M.A.; Olsen, F.J.; Jørgensen, P.G.; Fritz-Hansen, T.; Jespersen, T.; Gislason, G.; Biering-Sørensen, T. Utility of left atrial strain for predicting atrial fibrillation following ischemic stroke. Int. J. Cardiovasc. Imaging 2019, 35, 1605–1613. [Google Scholar] [CrossRef]
- Zeng, D.; Chang, S.; Zhang, X.; Zhong, Y.; Cai, Y.; Huang, T.; Wu, J. The Utility of Speckle Tracking Echocardiographic Parameters in Predicting Atrial Fibrillation Recurrence After Catheter Ablation in Patients with Non-Valvular Atrial Fibrillation. Ther. Clin. Risk Manag. 2024, 20, 719–729. [Google Scholar] [CrossRef]
- Sabanovic-Bajramovic, N.; Iglica, A.; Begic, E.; Begic, A.; Dzubur, A.; Naser, N.; Bajramovic, S. Left atrial strain significance in prediction of early atrial fibrillation recurrence after cardioversion and ablation. EP Europace 2023, 25 (Suppl. S1), euad122.022. [Google Scholar] [CrossRef]
- Li, Y.; Li, Y.; Sun, L.; Ye, X.; Cai, Q.; Zhu, W.; Guo, D.; Ding, X.; Wang, J.; Lv, X. Left atrial strain for predicting recurrence in patients with non-valvular atrial fibrillation after catheter ablation: A single-center two-dimensional speckle tracking retrospective study. BMC Cardiovasc. Disord. 2022, 22, 468. [Google Scholar] [CrossRef]
- Moreno-Ruiz, L.A.; Madrid-Miller, A.; Martínez-Flores, J.E.; González-Hermosillo, J.A.; Arenas-Fonseca, J.; Zamorano-Velázquez, N.; Mendoza-Pérez, B. Left atrial longitudinal strain by speckle tracking as independent predictor of recurrence after electrical cardioversion in persistent and long standing persistent non-valvular atrial fibrillation. Int. J. Cardiovasc. Imaging 2019, 35, 1587–1596. [Google Scholar] [CrossRef]
- Abdelhamid, S.; Biomy, R.; Kabil, H.; Raslan, M.; Mostafa, S. Association of Left Atrial Deformation Analysis by Speckle Tracking Echocardiography with Left Atrial Appendage Thrombus in Patients with Primary Valvular Heart Disease. Cureus 2023, 15, e35151. [Google Scholar] [CrossRef]
- Cameli, M.; Lunghetti, S.; Mandoli, G.; Righini, F.; Lisi, M.; Curci, V.; Tommaso, C.; Solari, M.; Nistor, D.; Gismondi, A.; et al. Left Atrial Strain Predicts Pro-Thrombotic State in Patients with Non-Valvular Atrial Fibrillation. J. Atr. Fibrillation 2017, 10, 1641. [Google Scholar] [CrossRef]
- Kupczynska, K.; Michalski, B.; Miskowiec, D.; Kasprzak, J.; Wejner-Mik, P.; Wdowiak-Okrojek, K.; Lipiec, P. Association between left atrial function assessed by speckle-tracking echocardiography and the presence of left atrial appendage thrombus in patients with atrial fibrillation. Anatol. J. Cardiol. 2017, 18, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, S.; Watanabe, T.; Tamura, H.; Nishiyama, S.; Wanezaki, M.; Sato, C.; Yamaura, G.; Ishino, M.; Arimoto, T.; Takahashi, H.; et al. Left atrial strain as evaluated by two-dimensional speckle tracking predicts left atrial appendage dysfunction in patients with acute ischemic stroke. BBA Clin. 2014, 2, 40–47. [Google Scholar] [CrossRef] [PubMed]
- Miller, K.D.; Nogueira, L.; Mariotto, A.B.; Rowland, J.H.; Yabroff, K.R.; Alfano, C.M.; Jemal, A.; Kramer, J.L.; Siegel, R.L. Cancer treatment and survivorship statistics, 2019. CA Cancer J. Clin. 2019, 69, 363–385. [Google Scholar] [CrossRef] [PubMed]
- Bansal, N.; Adams, M.J.; Ganatra, S.; Colan, S.D.; Aggarwal, S.; Steiner, R.; Amdani, S.; Lipshultz, E.R.; Lipshultz, S.E. Strategies to prevent anthracycline-induced cardiotoxicity in cancer survivors. Cardio-Oncology 2019, 5, 18. [Google Scholar] [CrossRef]
- Nicol, M.; Baudet, M.; Cohen-Solal, A. Subclinical Left Ventricular Dysfunction During Chemotherapy. Card. Fail. Rev. 2019, 5, 31–36. [Google Scholar] [CrossRef]
- Awadalla, M.; Hassan, M.Z.O.; Alvi, R.M.; Neilan, T.G. Advanced imaging modalities to detect cardiotoxicity. Curr. Probl. Cancer 2018, 42, 386–396. [Google Scholar] [CrossRef]
- Negishi, K.; Negishi, T.; Hare, J.L.; Haluska, B.A.; Plana, J.C.; Marwick, T.H. Independent and Incremental Value of Deformation Indices for Prediction of Trastuzumab-Induced Cardiotoxicity. J. Am. Soc. Echocardiogr. 2013, 26, 493–498. [Google Scholar] [CrossRef]
- Nagueh, S.F.; Smiseth, O.A.; Appleton, C.P.; Byrd, B.F.; Dokainish, H.; Edvardsen, T.; Flachskampf, F.A.; Gillebert, T.C.; Klein, A.L.; Lancellotti, P.; et al. Recommendations for the Evaluation of Left Ventricular Diastolic Function by Echocardiography: An Update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J. Am. Soc. Echocardiogr. 2016, 29, 277–314. [Google Scholar] [CrossRef]
- Park, H.; Kim, K.H.; Kim, H.Y.; Cho, J.Y.; Yoon, H.J.; Hong, Y.J.; Park, H.W.; Kim, J.H.; Ahn, Y.; Jeong, M.H.; et al. Left atrial longitudinal strain as a predictor of Cancer therapeutics-related cardiac dysfunction in patients with breast Cancer. Cardiovasc. Ultrasound 2020, 18, 28. [Google Scholar] [CrossRef]
- Emerson, P.; Stefani, L.; Boyd, A.; Richards, D.; Hui, R.; Altman, M.; Thomas, L. Alterations in Left Atrial Strain in Breast Cancer Patients Immediately Post Anthracycline Exposure. Heart Lung Circ. 2024, 33, 684–692. [Google Scholar] [CrossRef]
- Inoue, K.; Machino-Ohtsuka, T.; Nakazawa, Y.; Iida, N.; Sasamura, R.; Bando, H.; Chiba, S.; Tasaka, N.; Ishizu, T.; Murakoshi, N.; et al. Early Detection and Prediction of Anthracycline-Induced Cardiotoxicity—A Prospective Cohort Study. Circ. J. 2024, 88, 751–759. [Google Scholar] [CrossRef] [PubMed]
- Di Lisi, D.; Moreo, A.; Casavecchia, G.; Cadeddu Dessalvi, C.; Bergamini, C.; Zito, C.; Madaudo, C.; Madonna, R.; Cameli, M.; Novo, G. Atrial Strain Assessment for the Early Detection of Cancer Therapy-Related Cardiac Dysfunction in Breast Cancer Women (The STRANO STUDY: Atrial Strain in Cardio-Oncology). J. Clin. Med. 2023, 12, 7127. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Cheng, C.; Fan, L.; Xu, X.; Chen, J.; Feng, Y.; Tang, Y.; Yang, C. Assessment of left heart dysfunction to predict doxorubicin cardiotoxicity in children with lymphoma. Front. Pediatr. 2023, 11, 1163664. [Google Scholar] [CrossRef]
- Laufer-Perl, M.; Arias, O.; Dorfman, S.S.; Baruch, G.; Rothschild, E.; Beer, G.; Hasson, S.P.; Arbel, Y.; Rozenbaum, Z.; Topilsky, Y.; et al. Left Atrial Strain changes in patients with breast cancer during anthracycline therapy. Int. J. Cardiol. 2021, 330, 238–244. [Google Scholar] [CrossRef]
- Meloche, J.; Nolan, M.; Amir, E.; Brezden-Masley, C.; Yan, A.; Thampinathan, B.; Woo, A.; Bernd, W.; Thavendiranathan, P. Temporal Changes in Left Atrial Function in Women with Her2+ Breast Cancer Receiving Sequential Anthracyclines and Trastuzumab Therapy. J. Am. Coll. Cardiol. 2018, 71, A1524. [Google Scholar] [CrossRef]
- Goyal, A.; Abbasi, H.Q.; Yakkali, S.; Khan, A.M.; Tariq, M.D.; Sohail, A.H.; Khan, R. Left Atrial Strain as a Predictor of Early Anthracycline-Induced Chemotherapy-Related Cardiac Dysfunction: A Pilot Systematic Review and Meta-Analysis. J. Clin. Med. 2024, 13, 3904. [Google Scholar] [CrossRef]
- Debonnaire, P.; Leong, D.P.; Witkowski, T.G.; Al Amri, I.; Joyce, E.; Katsanos, S.; Schalij, M.J.; Bax, J.J.; Delgado, V.; Marsan, N.A. Left Atrial Function by Two-Dimensional Speckle-Tracking Echocardiography in Patients with Severe Organic Mitral Regurgitation: Association with Guidelines-Based Surgical Indication and Postoperative (Long-Term) Survival. J. Am. Soc. Echocardiogr. 2013, 26, 1053–1062. [Google Scholar] [CrossRef]
- van Garsse, L.; Gelsomino, S.; Luca, F.; Parise, O.; Cheriex, E.; Rao, C.M.; Gensini, G.F.; Maessen, J.G. Left atrial strain and strain rate before and following restrictive annuloplasty for ischaemic mitral regurgitation evaluated by two-dimensional speckle tracking echocardiography. Eur. Heart J. Cardiovasc. Imaging 2013, 14, 534–543. [Google Scholar] [CrossRef][Green Version]
- Cameli, M.; Pastore, M.C.; Righini, F.M.; Mandoli, G.E.; D’Ascenzi, F.; Lisi, M.; Nistor, D.; Sparla, S.; Curci, V.; Di Tommaso, C.; et al. Prognostic value of left atrial strain in patients with moderate asymptomatic mitral regurgitation. Int. J. Cardiovasc. Imaging 2019, 35, 1597–1604. [Google Scholar] [CrossRef]
- Mehta, V.; Chaudhari, D.; Mehra, P.; Mahajan, S.; Yusuf, J.; Gupta, M.D.; Kathuria, S.; Dabla, P.K.; Sukhija, R.; Mukhopadhyay, S.; et al. Left atrial function by two-dimensional speckle tracking echocardiography in patients with severe rheumatic mitral stenosis and pulmonary hypertension. Indian Heart J. 2022, 74, 63–65. [Google Scholar] [CrossRef]
- Tan, E.S.J.; Jin, X.; Oon, Y.Y.; Chan, S.P.; Gong, L.; Lunaria, J.B.; Liew, O.-W.; Chong, J.P.-C.; Tay, E.L.W.; Soo, W.M.; et al. Prognostic Value of Left Atrial Strain in Aortic Stenosis: A Competing Risk Analysis. J. Am. Soc. Echocardiogr. 2023, 36, 29–37.e5. [Google Scholar] [CrossRef] [PubMed]
- Lacy, S.C.; Thomas, J.D.; Syed, M.A.; Kinno, M. Prognostic value of left atrial strain in aortic stenosis: A systematic review. Echocardiography 2024, 41, e15829. [Google Scholar] [CrossRef] [PubMed]
- Sonaglioni, A.; Nicolosi, G.L.; Rigamonti, E.; Lombardo, M. Incremental prognostic role of left atrial reservoir strain in asymptomatic patients with moderate aortic stenosis. Int. J. Cardiovasc. Imaging 2021, 37, 1913–1925. [Google Scholar] [CrossRef]
- Weber, J.; Bond, K.; Flanagan, J.; Passick, M.; Petillo, F.; Pollack, S.; Robinson, N.; Petrossian, G.; Cao, J.J.; Barasch, E. The Prognostic Value of Left Atrial Global Longitudinal Strain and Left Atrial Phasic Volumes in Patients Undergoing Transcatheter Valve Implantation for Severe Aortic Stenosis. Cardiology 2021, 146, 489–500. [Google Scholar] [CrossRef]
- Butcher, S.C.; Hirasawa, K.; Meucci, M.C.; Stassen, J.; Kuneman, J.H.; Pereira, A.R.; van der Kley, F.; de Weger, A.; van Rosendael, P.J.; Marsan, N.A.; et al. Prognostic implications and alterations in left atrial deformation following transcatheter aortic valve implantation. Eur. Heart J. Cardiovasc. Imaging 2024, 25, 1638–1648. [Google Scholar] [CrossRef]
| Study/Guideline | Population | Reservoir LAS (Mean ± SD) | LAS Conduit (Mean± SD) | LAS Contractile (Mean ± SD) |
|---|---|---|---|---|
| Sun et al. (2020) [29] | Healthy adults (Korea) | 35.9 ± 10.6% | 21.9 ± 9.3% | 13.9% ± 3.6% |
| Pathan et al. (2017) [31] | Meta-analysis (21 studies) | 40 ± 8% | 27 ± 8% | 15 ± 6% |
| Yafasov et al. (2024) [32] | Copenhagen | Abnormal thresholds | ||
| 18.4% | 6.8% | 22.2% | ||
| Sugimoto et al. (2018) [30] | European | Mean ± SD or medial (IQR) | ||
| 42.5 (36.1 to 48.0) | 25.7 (20.4 to 31.8) | 16.3 (12.9 to 19.5) | ||
| Year | Title | No. of Patients | Main Conclusions |
|---|---|---|---|
| 2024 | Impaired Left Atrial Reserve Function in Heart Failure With Preserved Ejection Fraction [43] | 240 | LA reservoir function is reduced in patients with HFpEF and it correlates with effort tolerance |
| 2023 | Prognostic Implications of Left Atrial Stiffness Index in Heart Failure Patients With Preserved Ejection Fraction [42] | 307 | An increased atrial stiffness index is associated with higher mortality risk and number of hospitalization in patients with HfpRF |
| 2022 | Left Atrial Strain Predicts Exercise Capacity in Heart Failure Independently of Left Ventricular Ejection Fraction [44] | 171 | A reduced LA reservoir strain is independently associated with a significantly reduced effort capacity |
| 2021 | Prognostic Value of Minimal Left Atrial Volume in Heart Failure With Preserved Ejection Fraction [45] | 347 | The minimum LA volume is a stronger predictor for cardiovascular effect compared with the maximum indexed volume |
| 2020 | Left Atrial Strain in Evaluation of Heart Failure with Preserved Ejection Fraction [46] | 450 | LASR could identify patients with HFpEF and increased filling pressures in the absence of an effort test |
| Title | Authors | Paper | Year | No. of Subjects | Main Conclusions |
|---|---|---|---|---|---|
| Left Atrial Function in HFmrEF Differs from that of HFpEF [52] | Al Saikhan et al. | Eur Heart J Cardiovasc Imaging | 2019 | 184 | LAS (PALS and PACS) is significantly lower in HFmrEF compared with HFpEF, indicating an intrinsic atrial disfunction. |
| Prognostic Value of Left Atrial Strain in Heart Failure [22] | Jia et al. | Front Cardiovasc Med | 2022 | 7787 | Peak atrial longitudinal strain was identified as an independent predictor of all-cause mortality and cardiac hospitalization across all heart failure subtypes. |
| Left Atrial Function and Maximal Exercise Capacity in HFpEF and HFmrEF [53] | Maffeis et al. | ESC Heart Failure | 2021 | 56 | A reduced LAS (LA reservoir strain) correlates with low decreased exercise capacity in patients with HFmrEF and HFpEF. |
| Impact of SGLT2 Inhibitors on Left Atrial Functions in T2DM and HFmrEF [54] | El-Saied et al. | Int J Cardiol Heart Vasc | 2023 | 45 | SGLT2i significantly increase LAS (LAS reservoir, conduit, and contractile) in patients with type 2 diabetes and HFmrEF. |
| Title | Authors | Publication | Year | No. of Subjects | Main Conclusions |
|---|---|---|---|---|---|
| Prognostic Value of Left Atrial Strain in Heart Failure: A Systematic Review and Meta-Analysis [22] | Jia et al. | Frontiers in Cardiovascular Medicine | 2022 | 7.787 | LASR is an independent predictor of mortality and hospitalization rate in heart failure patients. |
| Left Atrial Reservoir Strain as a Predictor of Cardiac Outcome in Patients with Heart Failure: The HaFaC Cohort Study [60] | Bouwmeester et al. | BMC Cardiovascular Disorders | 2022 | 1.000+ | A reduced LASR is associated with an increased risk of cardiovascular events in patients with HFrEF. |
| Left Atrial Structure and Function in Heart Failure with Reduced (HFrEF) Versus Preserved Ejection Fraction (HFpEF) [55] | Jin et al. | Heart Failure Reviews | 2021 | 8.806 | Atrial disfunction is more severe in HFrEF, comparative with HFpEF, being a sign for intrinsic atrial myopathy. |
| Prognostic Power of Left Atrial Strain in Patients with Acute Heart Failure [61] | Park et al. | European Heart Journal—Cardiovascular Imaging | 2021 | 1.000+ | LASR >16% at discharge is associated with better survival rate without major cardiac adverse effects. |
| Left Atrial Strain in the Assessment of Diastolic Function in Heart Failure [62] | Carluccio et al. | Circulation: Cardiovascular Imaging | 2023 | 864 | PALS improves diastolic disfunction classification and the prediction of acute events in patients with heart failure. |
| Patients with Chronic Heart Failure and Predominant Left Atrial Versus Left Ventricular Myopathy [59] | Jin et al. | Cardiovasc Ultrasound | 2025 | 374 | Most heart failure patients exhibit balanced LA/LV myopathy; however, those with predominant LA myopathy tend to be older, have higher rates of atrial fibrillation and diabetes, elevated cardiac stress and injury biomarkers, and poorer clinical outcomes. |
| Title | Authors | Publication | Year | No. of Subjects | Main Conclusions |
|---|---|---|---|---|---|
| Left Atrial and Left Ventricular Deformation in Identifying Advanced Stage of Cardiac Amyloidosis [66] | Puwanant et al. | JACC | 2023 | 50 | LASR was significantly reduced in patients with advanced stage of cardiac amyloidosis than in patients with less advanced stage, and showed high diagnostic accuracy in discriminating advanced stage cardiac amyloidosis |
| Prognostic Value of Left Atrial Strain in Patients with Wild-Type Transthyretin Amyloid Cardiomyopathy [67] | Oike et al. | ESC Heart Fail | 2021 | 129 | A higher LASR is associated with a significantly higher probability of total cardiovascular death and rate of heart failure related hospitalizations in patients with ATTRwt-CM even after adjusting for conventional predictive factors |
| Assessing Left Atrial Dysfunction in Cardiac Amyloidosis using LA-LV Strain Slope [68] | Edbom et al. | Eur Heart J Imaging Methods Pract | 2024 | 59 | PALS demonstrates an independent ability to differentiate ATTR-CM from LVH |
| Clinical Importance of Left Atrial Infiltration in Cardiac Transthyretin Amyloidosis [69] | Bandera et al. | JACC | 2022 | 906 | In patients with cardiac transthyretin amyloidosis, atrial electromechanical dissociation was associated with poorer prognosis compared with subjects in sinus rhythm and effective mechanical contraction |
| Left Atrial Mechanics and Left Ventricular Function in Cardiac Amyloidosis Patients Treated with Tafamidis [70] | Carvalheiro et al. | European Heart Journal—Cardiovascular Imaging | 2025 | 28 | After 1 year of treatment with tafamidis there was a significant reduction of all three phases of LAS |
| Year | Authors | Study Title | Number of Patients | Main Conclusions |
|---|---|---|---|---|
| LAS in evaluating left atrial pressure (LAP) and function in AF | ||||
| 2024 | Xu Y et al. [79] | Left Atrial Strain Parameters to Predicting Elevated Left Atrial Pressure in Patients with Atrial Fibrillation | 142 | LASR, LA stiffness index, and LA filling index were independently associated with elevated LAP. In patients with AF, LAS parameters could be useful to predict elevated LAP and non-inferior to conventional echocardiographic parameters. |
| 2021 | Uziębło-Życzkowska B et al. [80] | Correlations Between Left Atrial Strain and Left Atrial Pressures Values in Patients Undergoing Atrial Fibrillation Ablation | 172 | LASR correlates with invasively measured LA pressures; lower LASR is associated with higher pressures. LASR may serve as a reliable, non-invasive marker of LAP in AF. |
| LAS in predicting new-onset AF | ||||
| 2025 | Granchietti AG et al. [81] | Left Atrial Strain and Risk of Atrial Fibrillation after Coronary Artery Bypass-grafting | 100 | Low preoperative LAS-ct and LASR are significant predictors of postoperative AF in CABG patients. |
| 2024 | Beyls et al. [82] | Left Atrial Strain Analysis and New-onset Atrial Fibrillation in Patients with ST-segment Elevation Myocardial Infarction (STEMI) | 175 | All LAS parameters were significantly impaired in new-onset AF patients, especially LASR. LASR < 27% is significantly associated with increased risk of new-onset AF during hospitalization for STEMI, with high sensitivity and specificity. |
| 2023 | Serenelli M et al. [78] | Atrial Longitudinal Strain Predicts New-Onset Atrial Fibrillation: A Systematic Review and Meta-Analysis | N/A | Reduced LASR is a significant predictor of new-onset AF, suggesting its utility in early detection and prevention strategies. |
| 2022 | Svartstein AW et al. [20] | Predictive Value of Left Atrial Strain in Relation to Atrial Fibrillation Following Acute Myocardial Infarction | 392 | Lower LASR is an independent predictor of incident AF following STEMI, highlighting its prognostic value in post-MI patients. |
| 2021 | Hauser R et al. [83] | Left Atrial Strain Predicts Incident Atrial Fibrillation in the General Population: the Copenhagen City Heart Study | 4466 | Lower peak atrial longitudinal strain (PALS) and peak atrial contraction strain (PACS) independently predict incident AF in the general population, even among individuals with normal LA size and left ventricle function. |
| 2019 | Rasmussen SMA et al. [84] | Utility of Left Atrial Strain for Predicting Atrial Fibrillation Following Ischemic Stroke | 186 | Reduced LASR is associated with higher risk of developing AF after ischemic stroke, suggesting its utility in post-stroke AF risk assessment. |
| LAS in predicting AF recurrence after cardioversion and ablation | ||||
| 2024 | Zeng D et al. [85] | The Utility of Speckle Tracking Echocardiographic Parameters in Predicting Atrial Fibrillation Recurrence After Catheter Ablation in Patients with Non-Valvular Atrial Fibrillation | 380 | Lower LASR and higher LA stiffness were independent predictors of AF recurrence after catheter ablation. LASR ≤ 24.3% and LA stiffness > 0.55 were associated with higher recurrence rates. |
| 2023 | Sabanovic-Bajramovic N et al. [86] | Left Atrial Strain Significance in Prediction of Early Atrial Fibrillation Recurrence after Cardioversion and Ablation | 94 | Peak atrial longitudinal strain (PALS) ≤ 15% was an independent predictor of early AF recurrence after cardioversion or ablation, even in patients with non-dilated LA. |
| 2022 | Li Y et al. [87] | Left Atrial Strain for Predicting Recurrence in Patients with Non-Valvular Atrial Fibrillation After Catheter Ablation | 95 | Decreased LAS-ct independently predicts AF recurrence post-ablation; LAS may contribute to the risk stratification for AF patients and selection of suitable patients for catheter ablation. |
| 2019 | Moreno-Ruiz LA et al. [88] | Left Atrial Longitudinal Strain by Speckle Tracking as Independent Predictor of Recurrence after Electrical Cardioversion in Persistent and Long-standing Persistent Non-valvular Atrial Fibrillation | 131 | Lower global peak atrial longitudinal strain (GPALS) was significantly associated with AF recurrence at 6 months post-electrical cardioversion. GPALS ≤ 10.75% was an independent predictor of recurrence. |
| LAS in assessing thrombotic risk in AF and detecting LA appendage thrombus | ||||
| 2023 | Abdelhamid S et al. [89] | Association of Left Atrial Deformation Analysis by Speckle Tracking Echocardiography with Left Atrial Appendage Thrombus in Patients with Primary Valvular Heart Disease | 200 | Peak atrial longitudinal strain (PALS) < 10.5% was a significant predictor of LA appendage thrombus in patients with primary valvular heart disease, regardless of rhythm. |
| 2017 | Cameli M et al. [90] | Left Atrial Strain Predicts Pro-Thrombotic State in Patients with Non-Valvular Atrial Fibrillation | 79 | Global peak atrial longitudinal strain (PALS) < 8.1% was a strong predictor of LAA thrombus and/or reduced LAA emptying velocity, with high sensitivity and specificity. |
| 2017 | Kupczynska K et al. [91] | Association Between Left Atrial Function Assessed by Speckle-Tracking Echocardiography and the Presence of Left Atrial Appendage Thrombus in Patients with Atrial Fibrillation | 87 | Impaired LAS is associated with higher risk of LA appendage thrombus presence in AF patients, providing incremental value over the CH2ADS2-VASc Score. |
| 2014 | Sasaki S et al. [92] | Left Atrial Strain as Evaluated by Two-Dimensional Speckle Tracking Predicts Left Atrial Appendage Dysfunction in Patients with Acute Ischemic Stroke | 120 | Decreased LA peak systolic strain was independently associated with LA appendage dysfunction in patients with acute ischemic stroke. LA peak systolic strain < 19% predicted LA appendage dysfunction with high sensitivity and specificity. |
| Year | Authors | Title | Number of Patients | Main Conclusions |
|---|---|---|---|---|
| 2024 | Emerson P et al. [100] | Alterations in Left Atrial Strain in Breast Cancer Patients Immediately Post Anthracycline Exposure | 128 | LA strain is a promising marker of early diastolic dysfunction. Reduction in LASR demonstrated increased sensitivity as a potential marker of cardiotoxicity compared to reduction in LV GLS. |
| 2024 | Inoue K et al. [101] | Early Detection and Prediction of Anthracycline-Induced Cardiotoxicity | 383 | Patients who developed cardiotoxicity had greater reductions in LAS-r and LAS-ct 3 months after initiating anthracyclines. Early decline in LAS-r was independently associated with subsequent cardiotoxicity. |
| 2024 | Goyal A et al. [106] | Left Atrial Strain as a Predictor of Early Anthracycline-Induced Chemotherapy-Related Cardiac Dysfunction: A Pilot Systematic Review and Meta-Analysis | 297 | Anthracycline therapy significantly reduced LAS-r and LAS-cd. LAS may serve as an early indicator of cardiotoxicity. |
| 2023 | Di Lisi et al. [102] | Atrial Strain Assessment for the Early Detection of Cancer Therapy-Related Cardiac Dysfunction in Breast Cancer Women (The STRANO STUDY: Atrial Strain in Cardio-Oncology) | 169 | LAS parameters (PALS and LA stiffness) significantly changed during chemotherapy, correlating with GLS changes. A PALS variation > 20.8% identified patients likely to develop asymptomatic mild cardiotoxicity. |
| 2023 | Chen J et al. [103] | Assessment of Left Heart Dysfunction to Predict Doxorubicin Cardiotoxicity in Children with Lymphoma | 23 | LAS-r and LAS-cd decreased significantly after chemotherapy, correlating with LV GLS. LAS can be used for early detection of cardiotoxicity in pediatric lymphoma patients. |
| 2021 | Laufer-Perl M et al. [104] | Left Atrial Strain Changes in Patients with Breast Cancer During Anthracycline Therapy | 40 | LAS-r and LAS-cd reduction occur early during anthracycline therapy, showing significant correlation to the routine echocardiographic diastolic parameters, which may imply a role in the detection of early cardiotoxicity. |
| 2018 | Meloche J et al. [105] | Temporal Changes in Left Atrial Function in Women with HER2+ Breast Cancer Receiving Sequential Anthracyclines and Trastuzumab Therapy | 51 | Intrinsic LA compliance and contractile properties were reduced early with cancer therapy. Patients who developed cardiotoxicity had lower baseline LAS-r and LAS-cd. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rusali, C.A.; Lupu, I.C.; Rusali, L.M.; Cojocaru, L. Left Atrial Strain—Current Review of Clinical Applications. Diagnostics 2025, 15, 1347. https://doi.org/10.3390/diagnostics15111347
Rusali CA, Lupu IC, Rusali LM, Cojocaru L. Left Atrial Strain—Current Review of Clinical Applications. Diagnostics. 2025; 15(11):1347. https://doi.org/10.3390/diagnostics15111347
Chicago/Turabian StyleRusali, Constantin Andrei, Ioana Caterina Lupu, Lavinia Maria Rusali, and Lucia Cojocaru. 2025. "Left Atrial Strain—Current Review of Clinical Applications" Diagnostics 15, no. 11: 1347. https://doi.org/10.3390/diagnostics15111347
APA StyleRusali, C. A., Lupu, I. C., Rusali, L. M., & Cojocaru, L. (2025). Left Atrial Strain—Current Review of Clinical Applications. Diagnostics, 15(11), 1347. https://doi.org/10.3390/diagnostics15111347







