Relationships Between Breast Edema and Axillary Lymph Node Metastasis in Breast Cancer
Abstract
1. Introduction and Aim
2. Materials and Methods
2.1. MRI Evaluation Protocol
2.2. Histopathological Evaluation of Tissue Samples
2.3. Statistical Analysis
3. Results
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, J.; Ma, W.; Jiang, X.; Cui, C.; Wang, H.; Chen, J.; Nie, R.; Wu, Y.; Li, L. Development and Validation of Nomograms Predictive of Axillary Nodal Status to Guide Surgical Decision-Making in Early-Stage Breast Cancer. J. Cancer 2019, 10, 1263–1274. [Google Scholar] [CrossRef]
- Maeseele, N.; Faes, J.; Van de Putte, T.; Vlasselaer, J.; de Jonge, E.; Schobbens, J.C.; Deraedt, K.; Debrock, G.; Van de Putte, G. Axillary lymph node dissection on the run? Facts Views Vis. Obgyn. 2017, 9, 45–49. [Google Scholar]
- D’Orsi, C.J.; Sickles, E.A.; Bassett, L.W.; Appleton, C.M.; Berg, W.A.; Burnside, E.S. ACR BI-RADS® Atlas, Breast Imaging Reporting and Data System; American College of Radiology: Reston, VA, USA, 2013; pp. 39–48. [Google Scholar]
- Spak, D.A.; Plaxco, J.S.; Santiago, L.; Dryden, M.J.; Dogan, B. BI-RADS® fifth edition: A summary of changes. Diagn. Interv. Imaging 2017, 98, 179–190. [Google Scholar] [CrossRef]
- Xue, M.; Che, S.; Tian, Y.; Xie, L.; Huang, L.; Zhao, L.; Guo, N.; Li, J. Nomogram Based on Breast MRI and Clinicopathologic Features for Predicting Axillary Lymph Node Metastasis in Patients with Early-Stage Invasive Breast Cancer: A Retrospective Study. Clin. Breast Cancer 2022, 22, e428–e437. [Google Scholar] [CrossRef]
- Shu, C.; Hung, T.; Lai, W. Diagnostic accuracy of pre-operative breast magnetic resonance imaging (MRI) in predicting axillary lymph node metastasis: Variations in intrinsic subtypes, and strategy to improve negative predictive value-an analysis of 2473 invasive breast cancer patients. Breast Cancer 2023, 30, 976–985. [Google Scholar] [CrossRef]
- Uematsu, T.; Kasami, M.; Watanabe, J. Is evaluation of the presence of prepectoral edema on T2-weighted with fat-suppression 3 T breast MRI a simple and readily available noninvasive technique for estimation of prognosis in patients with breast cancer? Breast Cancer 2014, 21, 684–692. [Google Scholar] [CrossRef]
- Uematsu, T. Focal breast edema associated with malignancy on T2-weighted images of breast MRI: Peritumoral edema, prepectoral edema, and subcutaneous edema. Breast Cancer 2015, 22, 66–70. [Google Scholar] [CrossRef]
- Zhao, S.; Li, Y.; Ning, N.; Liang, H.; Wu, Y.; Wu, Q.; Wang, Z.; Tian, J.; Yang, J.; Gao, X.; et al. Associated of peritumoral region features assessed on breast MRI and prognosis of breast cancer: A systematic review and meta-analysis. Eur. Radiol. 2024, 34, 6108–6120. [Google Scholar] [CrossRef]
- Rickham, P.P. Human experimentation. Code of ethics of the World Medical Association. Declaration of Helsinki. Br. Med. J. 1964, 2, 177. [Google Scholar]
- Burstein, H.J.; Curigliano, G.; Thürlimann, B.; Weber, W.P.; Poortmans, P.; Regan, M.M.; Senn, H.J.; Winer, E.P.; Gnant, M.; Panelists of the St Gallen Consensus Conference. Customizing local and systemic therapies for women with early breast cancer: The St. Gallen International Guidelines for treatment of early breast cancer 2021. Ann. Oncol. 2021, 32, 1216–1235. [Google Scholar] [CrossRef]
- Sardanelli, F.; Boetes, C.; Borisch, B.; Decker, T.; Federico, M.; Gilbert, F.J.; Helbich, T.; Heywang-Köbrunner, S.H.; Kaiser, W.A.; Kerin, M.J.; et al. Magnetic resonance imaging of the breast: Recommendations from the EUSOMA working group. Eur. J. Cancer 2010, 46, 1296–1316. [Google Scholar] [CrossRef] [PubMed]
- Olena Weaver Jessica, W.T. Leung Biomarkers and Imaging of Breast Cancer. AJR Am. J. Roentgenol. 2018, 210, 271–278. [Google Scholar]
- Surov, A.; Chang, Y.W.; Li, L.; Martincich, L.; Partridge, S.C.; Kim, J.Y.; Wienke, A. Apparent diffusion coefficient cannot predict molecular subtype and lymph node metastases in invasive breast cancer: A multicenter analysis. BMC Cancer 2019, 19, 1043. [Google Scholar] [CrossRef]
- Belli, P.; Costantini, M.; Bufi, E.; Giardina, G.G.; Rinaldi, P.; Franceschini, G.; Bonomo, L. Diffusion magnetic resonance imaging in breast cancer characterisation: Correlations between the apparent diffusion coefficient and major prognostic factors. Radiol. Med. 2015, 120, 268–276. [Google Scholar] [CrossRef] [PubMed]
- Dietzel, M.; Baltzer, P.A.; Vag, T.; Gröschel, T.; Gajda, M.; Camara, O.; Kaiser, W.A. Application of breast MRI for prediction of lymph node metastases—Systematic approach using 17 individual descriptors and a dedicated decision tree. Acta Radiol. 2010, 51, 885–894. [Google Scholar] [CrossRef]
- Oeffinger, K.C.; Fontham, E.T.; Etzioni, R.; Herzig, A.; Michaelson, J.S.; Shih, Y.-C.T.; Walter, L.C.; Church, T.R.; Flowers, C.R.; LaMonte, S.J.; et al. Breast Cancer Screening for Women at Average Risk: 2015 Guideline Update From the American Cancer Society. JAMA 2015, 314, 1599–1614, Erratum in JAMA 2016, 315, 1406. [Google Scholar] [CrossRef]
- Baltzer, P.A.; Dietzel, M.; Kaiser, W.A. A simple and robust classification tree for differentiation between benign and malignant lesions in MR-mammography. Eur. Radiol. 2013, 23, 2051–2060. [Google Scholar] [CrossRef]
- Kaiser, C.G.; Herold, M.; Krammer, J.; Baltzer, P.; Gajda, M.; Camara, O.; Schoenberg, S.; Kaiser, W.A.; Dietzel, M. Prognostic Value of “Prepectoral Edema” in MR-mammography. Anticancer Res. 2017, 37, 1989–1995. [Google Scholar]
- Cheon, H.; Kim, H.J.; Kim, T.H.; Ryeom, H.-K.; Lee, J.; Kim, G.C.; Yuk, J.-S.; Kim, W.H. Invasive Breast Cancer: Prognostic Value of Peritumoral Edema Identified at Preoperative MR Imaging. Radiology 2018, 287, 68–75. [Google Scholar] [CrossRef]
- Harada, T.L.; Uematsu, T.; Nakashima, K.; Sugino, T.; Nishimura, S.; Takahashi, K.; Hayashi, T.; Tadokoro, Y.; Watanabe, J.; Nakamoto, S.; et al. Is the presence of edema and necrosis on T2WI pretreatment breast MRI the key to predict pCR of triple negative breast cancer? Eur. Radiol. 2020, 30, 3363–3370. [Google Scholar] [CrossRef]
- Hasebe, T.; Yamauchi, C.; Iwasaki, M.; Ishii, G.; Wada, N.; Imoto, S. Grading system for lymph vessel tumor emboli for prediction of the outcome of invasive ductal carcinoma of the breast. Hum. Pathol. 2008, 39, 427–436. [Google Scholar] [CrossRef] [PubMed]
- Panzironi, G.; Moffa, G.; Galati, F.; Marzocca, F.; Rizzo, V.; Pediconi, F. Peritumoral edema as a biomarker of the aggressiveness of breast cancer: Results of a retrospective study on a 3 T scanner. Breast Cancer Res. Treat. 2020, 181, 53–60. [Google Scholar] [CrossRef]
- Baltzer, P.A.; Yang, F.; Dietzel, M.; Herzog, A.; Simon, A.; Vag, T.; Gajda, M.; Camara, O.; Kaiser, W.A. Sensitivity and specificity of unilateral edema on T2w-TSE sequences in MR-Mammography considering 974 histologically verified lesions. Breast J. 2010, 16, 233–239. [Google Scholar] [CrossRef] [PubMed]
- Blumgart, E.I.; Uren, R.F.; Nielsen, P.M.; Nash, M.P.; Reynolds, H.M. Predicting lymphatic drainage patterns and primary tumour location in patients with breast cancer. Breast Cancer Res. Treat. 2011, 130, 699–705. [Google Scholar] [CrossRef] [PubMed]
- Renz, D.M.; Baltzer, P.A.; Böttcher, J.; Thaher, F.; Gajda, M.; Camara, O.; Runnebaum, I.B.; Kaiser, W.A. Magnetic resonance imaging of inflammatory breast carcinoma and acute mastitis. A comparative study. Eur. Radiol. 2008, 18, 2370–2380. [Google Scholar] [CrossRef]
- Uematsu, T.; Kasami, M.; Watanabe, J. Can T2-weighted 3-T breast MRI predict clinically occult inflammatory breast cancer before pathological examination? A single-center experience. Breast Cancer 2014, 21, 115–121. [Google Scholar] [CrossRef]
- Santucci, D.; Faiella, E.; Cordelli, E.; Calabrese, A.; Landi, R.; de Felice, C.; Zobel, B.B.; Grasso, R.F.; Iannello, G.; Soda, P. The Impact of Tumor Edema on T2-Weighted 3T-MRI Invasive Breast Cancer Histological Characterization: A Pilot Radiomics Study. Cancers 2021, 13, 4635. [Google Scholar] [CrossRef]
- Akdoğan Gemici, A.; Tokgoz Ozal, S.; Hocaoğlu, E.; Arslan, G.; Sen, E.; Altınay, S.; İnci, E. Relation of peritumoral, prepectoral and diffuse edema with histopathologic findings of breast cancer in preoperative 3T magnetic resonance imaging. J. Surg. Med. 2019, 3, 49–53. [Google Scholar]
- Houssami, N.; Ciatto, S.; Macaskill, P.; Lord, S.J.; Warren, R.M.; Dixon, J.M.; Irwig, L. Accuracy and surgical impact of magnetic resonance imaging in breast cancer staging: Systematic review and meta-analysis in detection of multifocal and multicentric cancer. J. Clin. Oncol. 2008, 26, 3248–3258. [Google Scholar] [CrossRef]
- Girardi, V.; Carbognin, G.; Camera, L.; Baglio, I.; Bucci, A.; Bonetti, F.; Mucelli, R.P. Multifocal, multicentric and contralateral breast cancers: Breast MR imaging in the preoperative evaluation of patients with newly diagnosed breast cancer. Radiol. Med. 2011, 116, 1226–1238. [Google Scholar] [CrossRef]
- Coombs, N.J.; Boyages, J. Multifocal and multicentric breast cancer: Does each focus matter? J. Clin. Oncol. 2005, 23, 7497–7502. [Google Scholar] [CrossRef] [PubMed]
- Jain, S.; Rezo, A.; Shadbolt, B.; Dahlstrom, J.E. Synchronous multiple ipsilateral breast cancers: Implications for patient management. Pathology 2009, 41, 57–67. [Google Scholar] [CrossRef]
- Esserman, L.; Hylton, N.; Yassa, L.; Barclay, J.; Frankel, S.; Sickles, E. Utility of magnetic resonance imaging in the management of breast cancer: Evidence for improved preoperative staging. J. Clin. Oncol. 1999, 17, 110–119. [Google Scholar] [CrossRef] [PubMed]
- Miller, S.; Causey, M.W.; Cahanding, N.; Brown, T.; Smith, D. Management of Multifocal/Multicentric Breast Cancer: Current Perspective. Prog. Sci. 2015, 2, e04. [Google Scholar]
- Koscielny, S.; Tubiana, M.; Lê, M.G.; Valleron, A.J.; Mouriesse, H.; Contesso, G.; Sarrazin, D. Breast cancer: Relationship between the size of the primary tumour and the probability of metastatic dissemination. Br. J. Cancer 1984, 49, 709–715. [Google Scholar] [CrossRef]
- Chua, B.; Ung, O.; Taylor, R.; Boyages, J. Frequency and predictors of axillary lymph node metastases in invasive breast cancer. ANZ J. Surg. 2001, 71, 723–728. [Google Scholar] [CrossRef]
- Weissenbacher, T.M.; Zschage, M.; Janni, W.; Jeschke, U.; Dimpfl, T.; Mayr, D.; Rack, B.; Schindlbeck, C.; Friese, K.; Dian, D. Multicentric and multifocal versus unifocal breast cancer: Is the tumor-node-metastasis classification justified? Breast Cancer Res. Treat. 2010, 122, 27–34. [Google Scholar] [CrossRef]
- Cabioglu, N.; Ozmen, V.; Kaya, H.; Tuzlali, S.; Igci, A.; Muslumanoglu, M.; Kecer, M.; Dagoglu, T. Increased lymph node positivity in multifocal and multicentric breast cancer. J. Am. Coll. Surg. 2009, 208, 67–74. [Google Scholar] [CrossRef]
- Molnar, C.; Stolnicu, S. Multifocal/multicentric breast carcinomas showing intertumoural heterogeneity: A comparison of histological tumour type and Nottingham histological grade of primary tumour and lymph node metastasis. Pol. J. Pathol. 2015, 66, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Avera, E.; Valentic, L.; Bui, L. Current understanding and distinct features of multifocal and multicentric breast cancers. Cancer Rep. 2023, 6, e1851. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
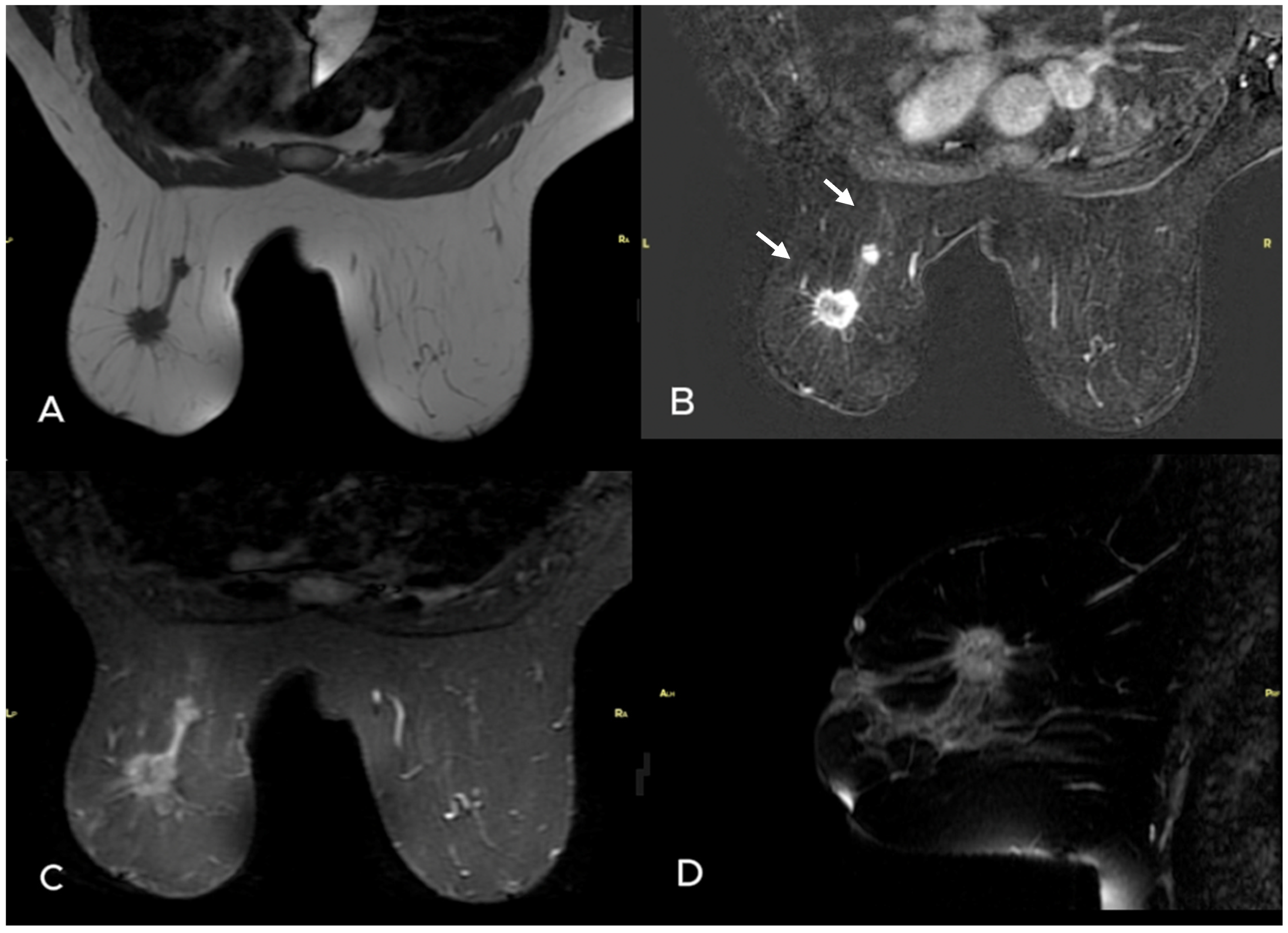
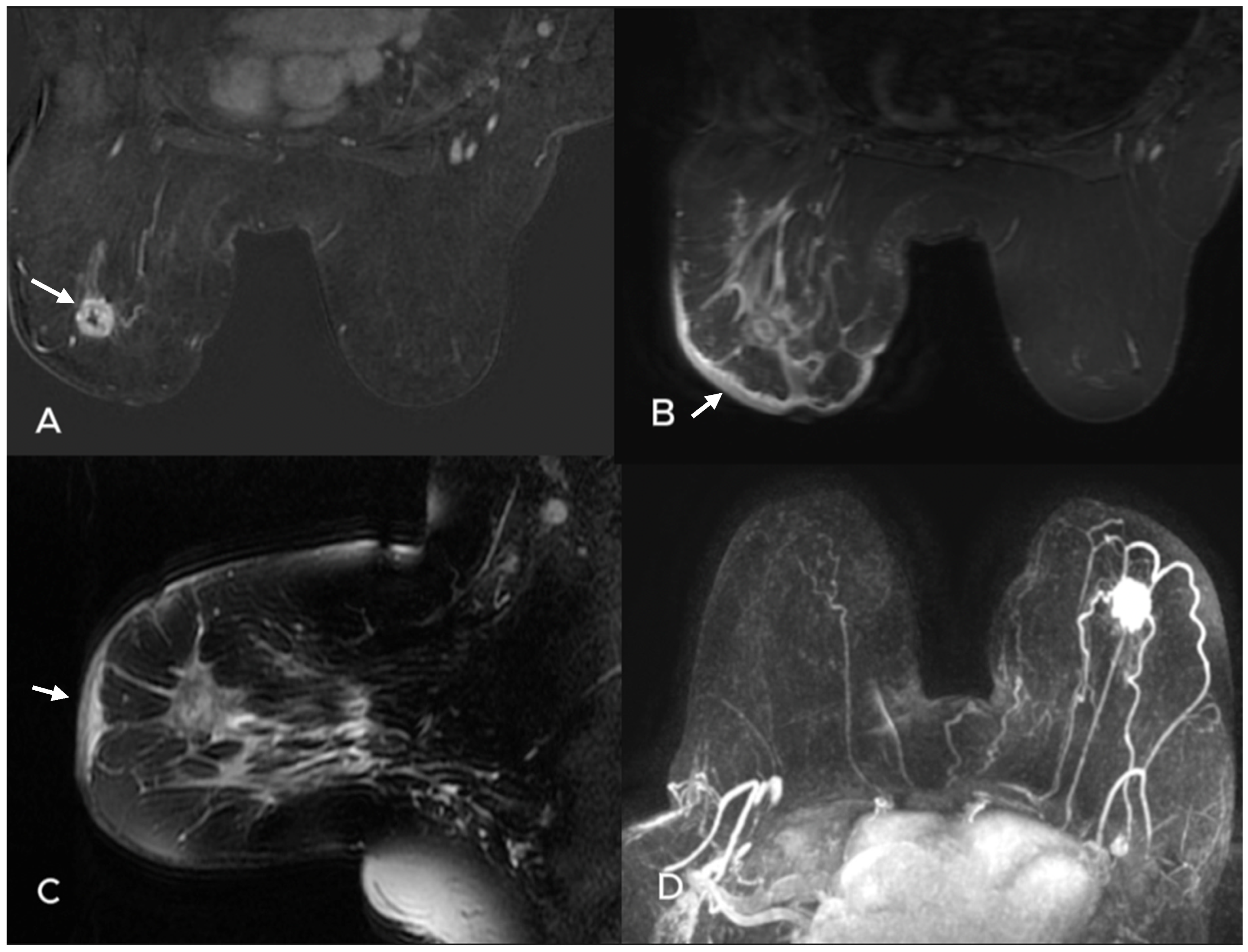
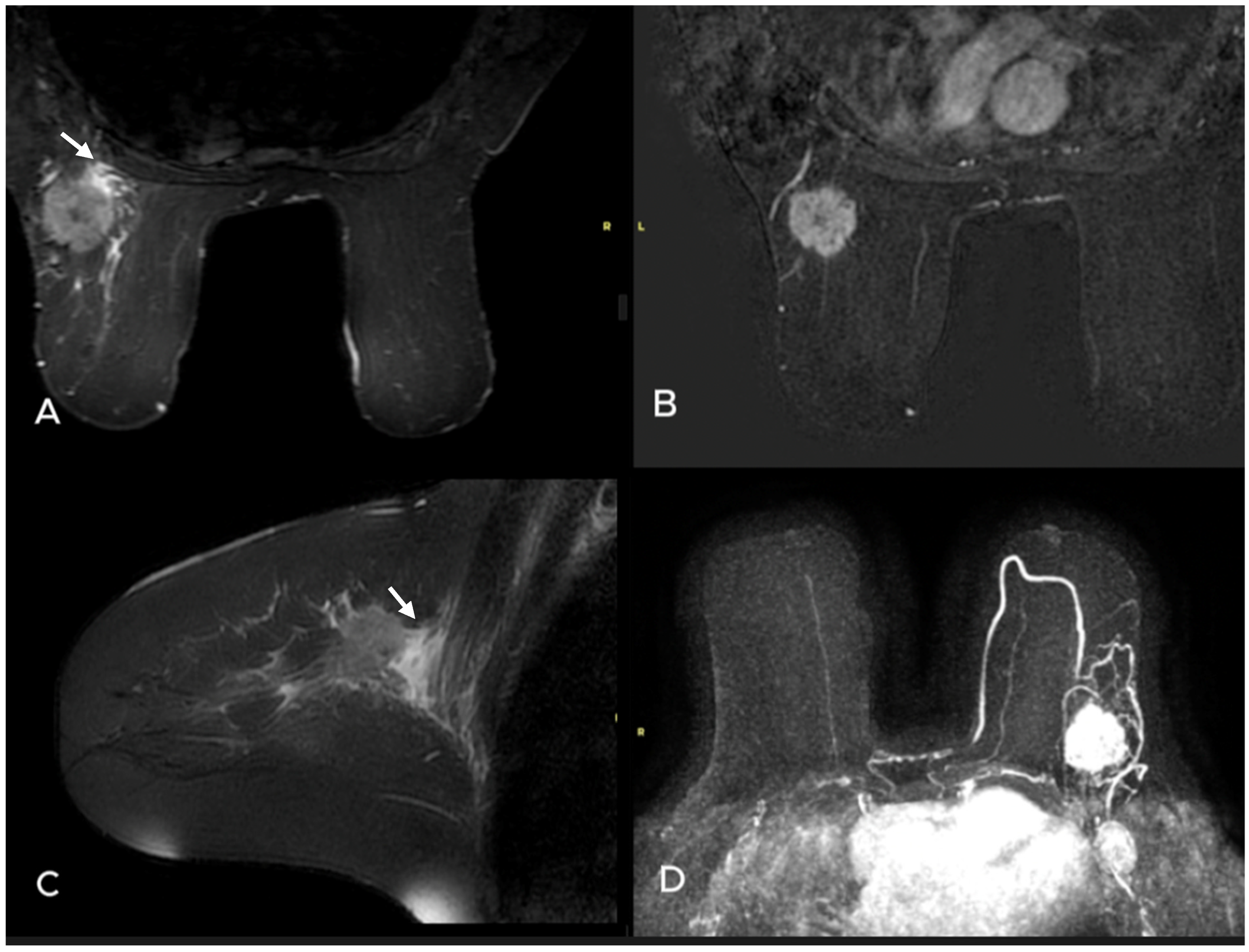
| Variables | Metastatic ALN (−) n = 35 | Metastatic ALN(+) n = 88 | p Value |
|---|---|---|---|
| Age (IQR) | 48 (41–63) | 45 (39–54) | 0.042 * |
| Distance from skin (IQR) (mm) | 14 (10–20) | 12 (5–18) | 0.36 |
| Lesion Side (right vs. left) n (%) | 13 (37.1) | 51 (58) | 0.037 * |
| MRI | |||
| Number of lesions n (%) | 0.001 * | ||
| Single | 26 (74.3) | 37 (42.0) | |
| Multifocal | 6 (17.1) | 22 (25.0) | |
| Multicentric | 2 (5.7) | 21 (23.9) | |
| Diffuse | 1 (2.9) | 8 (9.1) | |
| Tumor size in MRI (IQR) (mm) | 20 (17–30) | 32 (21–43) | 0.001 * |
| Necrosis n (%) | 5 (14.3) | 28 (31.8) | 0.05 * |
| Diffusion restriction n (%) | 26 (74.3) | 75 (84.2) | 0.13 |
| Presence of edema n (%) | 0.001 * | ||
| None | 17 (48.6) | 14 (15.9) | |
| Peritumoral edema | 14 (40.0) | 43 (48.9) | |
| Diffuse edema | 4 (11.4) | 31 (35.2) | |
| Background enhancement n (%) | 0.30 | ||
| Minimal | 4 (11.4) | 4 (4.5) | |
| Mild | 6 (17.1) | 24 (27.3) | |
| Moderate | 20 (57.1) | 35 (39.8) | |
| Marked | 5 (14.3) | 25 (28.4) | |
| Fibroglandular tissue n (%) | 0.36 | ||
| Type A | 2 (5.7) | 1 (1.1) | |
| Type B | 11 (31.4) | 27 (30.7) | |
| Type C | 11 (31.4) | 27 (30.7) | |
| TypeD | 11 (31.4) | 33 (37.5) | |
| Ki n (%) | 15 (10–25) | 30 (15–43) | 0.002 * |
| Molecular subtype n (%) | 0.016 * | ||
| Luminal A | 14 (40.0) | 17 (19.3) | |
| Luminal B | 17 (48.6) | 46 (52.3) | |
| Her-2 enriched | 3 (8.6) | 13 (14.8) | |
| Triple negative | 1 (2.9) | 12 (13.6) |
| Right Breast n = 64 | Left Breast n = 59 | p Value | |
|---|---|---|---|
| Quadrant 1 n (%) | 0.58 | ||
| Inner | 11 (17.2) | 15 (25.4) | |
| Outer | 38 (59.4) | 31 (52.5) | |
| Central | 15 (23.4) | 13 (22.0) | |
| Quadtrant 2 n (%) | 0.44 | ||
| Upper | 34 (53.1) | 24 (40.7) | |
| Lower | 9 (14.1) | 14 (23.7) | |
| Central | 21 (32.8) | 21 (35.6) | |
| Depth n (%) | 0.35 | ||
| Anterior | 12 (18.8) | 12 (20.3) | |
| Central | 22 (34.4) | 18 (30.5) | |
| Posterior | 18 (28.1) | 20 (33.9) | |
| Extensive | 12 (18.8) | 9 (15.3) |
| Variables | Univariate | Multivariate | ||||
|---|---|---|---|---|---|---|
| Unadjusted OR | %95 CI | p Value | Adjusted OR | %95 CI | p Value | |
| Age | 0.96 | 0.93–0.99 | 0.023 | 0.95 | 0.92–1.01 | 0.051 |
| Presence of edema (none vs. perilesional-diffuse) | 3.19 | 1.71–5.95 | <0.001 | 2.46 | 1.11–5.48 | 0.027 |
| Number of lesions (single vs. multicentric-multifocal-diffuse) | 2.31 | 1.34–3.98 | 0.003 | 1.86 | 1.01–3.43 | 0.046 |
| Molecular subtype | 2.05 | 1.19–3.51 | 0.009 | 1.97 | 0.89–2.48 | 0.12 |
| Tumor size (MRI, mm) | 1.05 | 1.01–1.09 | 0.004 | 1.03 | 0.97–1.06 | 0.23 |
| Kİ-67 | 1.02 | 1.01–1.04 | 0.022 | 1.02 | 1.00–1.04 | 0.62 |
| Side (right vs. left) | 0.42 | 0.19–0.96 | 0.039 | 0.54 | 0.26–1.18 | 0.42 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Deniz Altıntaş, D.; Esen Icten, G.; Taşkın, F.; Uras, C. Relationships Between Breast Edema and Axillary Lymph Node Metastasis in Breast Cancer. Diagnostics 2025, 15, 1300. https://doi.org/10.3390/diagnostics15111300
Deniz Altıntaş D, Esen Icten G, Taşkın F, Uras C. Relationships Between Breast Edema and Axillary Lymph Node Metastasis in Breast Cancer. Diagnostics. 2025; 15(11):1300. https://doi.org/10.3390/diagnostics15111300
Chicago/Turabian StyleDeniz Altıntaş, Derya, Gul Esen Icten, Füsun Taşkın, and Cihan Uras. 2025. "Relationships Between Breast Edema and Axillary Lymph Node Metastasis in Breast Cancer" Diagnostics 15, no. 11: 1300. https://doi.org/10.3390/diagnostics15111300
APA StyleDeniz Altıntaş, D., Esen Icten, G., Taşkın, F., & Uras, C. (2025). Relationships Between Breast Edema and Axillary Lymph Node Metastasis in Breast Cancer. Diagnostics, 15(11), 1300. https://doi.org/10.3390/diagnostics15111300






