The Prevalence and Significance of Incidental Positron Emission Tomography Findings in the Brain Using Radiotracers Other than [18F]FDG: A Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Review Protocol, Working Group, and Review Question
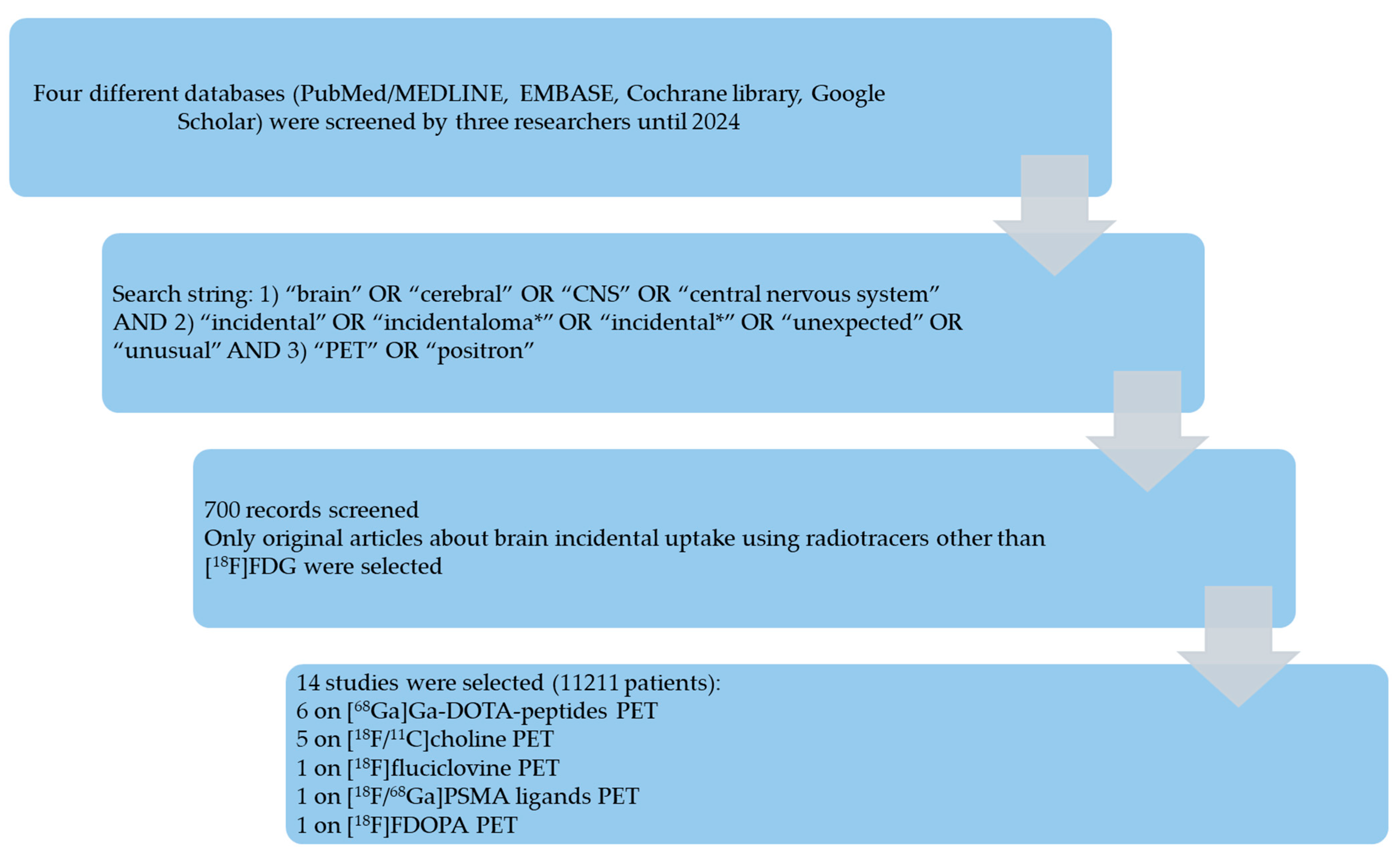
2.2. Search Strategy
2.3. Study Selection
2.4. Data Extraction and Quality Assessment
2.5. Statistical Analysis
3. Results
3.1. Literature Search
3.2. Qualitative Synthesis
3.2.1. [68Ga]Ga-DOTA-Peptides (Somatostatin Receptor PET)
3.2.2. Radiolabeled Choline ([18F/11C]Choline)
3.2.3. Radiolabeled PSMA Ligands ([18F/68Ga]PSMA Ligands)
3.2.4. [18F]Fluciclovine
3.2.5. [18F]FDOPA
3.3. Quantitative Synthesis
4. Discussion
4.1. Literature Data
4.2. Limitations and Suggestions for Future Research
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- O’Sullivan, J.W.; Muntinga, T.; Grigg, S.; Ioannidis, J.P.A. Prevalence and outcomes of incidental imaging findings: Umbrella review. BMJ 2018, 361, k2387. [Google Scholar] [CrossRef] [PubMed]
- Alabousi, M.; Wilson, E.; Al-Ghetaa, R.K.; Patlas, M.N. General Review on the Current Management of Incidental Findings on Cross-Sectional Imaging: What Guidelines to Use, How to Follow Them, and Management and Medical-Legal Considerations. Radiol. Clin. N. Am. 2021, 59, 501–509. [Google Scholar] [CrossRef] [PubMed]
- Juweid, M.E.; Al-Qasem, S.F.; Khuri, F.R.; Gallamini, A.; Lohmann, P.; Ziellenbach, H.J.; Mottaghy, F.M. Beyond fluorodeoxyglucose: Molecular imaging of cancer in precision medicine. CA Cancer J. Clin. 2025. [Google Scholar] [CrossRef] [PubMed]
- Skotting, M.B.; Holst, A.V.; Munch, T.N. Incidental findings in MRI of the brain. Ugeskr. Læger 2024, 186, V12230770. [Google Scholar] [CrossRef]
- Wangaryattawanich, P.; Rutman, A.M.; Petcharunpaisan, S.; Mossa-Basha, M. Incidental findings on brain magnetic resonance imaging (MRI) in adults: A review of imaging spectrum, clinical significance, and management. Br. J. Radiol. 2023, 96, 20220108. [Google Scholar] [CrossRef]
- Sunny, D.E.; Amoo, M.; Al Breiki, M.; Teng, E.D.W.; Henry, J.; Javadpour, M. Prevalence of incidental intracranial findings on magnetic resonance imaging: A systematic review and meta-analysis. Acta Neurochir. 2022, 164, 2751–2765. [Google Scholar] [CrossRef]
- Treglia, G.; Sadeghi, R.; Del Sole, A.; Giovanella, L. Diagnostic performance of PET/CT with tracers other than F-18-FDG in oncology: An evidence-based review. Clin. Transl. Oncol. 2014, 16, 770–775. [Google Scholar] [CrossRef]
- Signore, G.; Meyer, M.; Albano, D.; Bertagna, F.; Nicod-Lalonde, M.; Schaefer, N.; Giovanella, L.; Prior, J.O.; Treglia, G. Prevalence and clinical significance of incidental 18F-FDG uptake in the pituitary. Clin. Transl. Imaging 2020, 8, 237–242. [Google Scholar] [CrossRef]
- Sadeghi, R.; Treglia, G. Systematic reviews and meta-analyses of diagnostic studies: A practical guideline. Clin. Transl. Imaging 2017, 5, 83–87. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. J. Clin. Epidemiol. 2021, 134, 178–189. [Google Scholar] [CrossRef]
- Study Quality Assessment Tools. Available online: https://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools (accessed on 31 December 2024).
- McLaughlin, L.A.; Yildirim, O.; Rosenblum, M.K.; Imber, B.S.; Haseltine, J.M.; Zelefsky, M.J.; Schöder, H.; Morris, M.J.; Rafelson, W.M.; Krebs, S.; et al. Identification of incidental brain tumors in prostate cancer patients via PSMA PET/CT. J. Neurooncol. 2023, 163, 455–462. [Google Scholar] [CrossRef]
- Ensign, S.F.; Agarwal, M.; Klanderman, M.; Badawy, M.; Halfdanarson, T.R.; Johnson, D.R.; Sonbol, M.B.; Kendi, A.T. Clinical utility of somatostatin receptor positron emission tomography imaging biomarkers for characterization of meningioma amongincidental central nervous system lesions. Nucl. Med. Commun. 2023, 44, 663–670. [Google Scholar] [CrossRef]
- Umana, G.E.; Ferini, G.; Harikar, M.M.; Venkataram, T.; Costanzo, R.; Scalia, G.; Palmisciano, P.; Brunasso, L.; Paolini, F.; Sciortino, A.; et al. Detection of “Incidentalomas” on Brain and Body 68Ga-DOTATOC-PET Scans: A Retrospective Study and Case Illustration. Anticancer Res. 2022, 42, 5867–5873. [Google Scholar] [CrossRef]
- Albano, D.; Treglia, G.; Dondi, F.; Bertagna, F. Prevalence of Brain Incidental Lesions Detected by 68Ga-DOTA Peptides PET/CT. Medicina 2022, 58, 916. [Google Scholar] [CrossRef]
- Baiomy, A.; Schellingerhout, D.; Chapin, B.F.; Weinberg, J.S.; Raza, S.M.; Macapinlac, H.; Ravizzini, G. Rate of incidental central nervous system meningioma detected in patients undergoing 18F-fluciclovine PET/CT imaging for evaluation of prostate cancer. Nucl. Med. Commun. 2021, 42, 755–762. [Google Scholar] [CrossRef]
- Roland, A.; Drouet, C.; Boulahdour, H.; Cochet, A.; De Bari, B. Unusual uptakes on 18F-fluorocholine positron emission tomography/computed tomography (PET/CT): A retrospective study of 368 prostate cancer patients referred for a biochemical recurrence or an initial staging. Quant. Imaging Med. Surg. 2021, 11, 172–182. [Google Scholar] [CrossRef]
- Parghane, R.V.; Talole, S.; Basu, S. Prevalence of hitherto unknown brain meningioma detected on 68Ga-DOTATATE positron-emission tomography/computed tomography in patients with metastatic neuroendocrine tumor and exploring potential of 177Lu-DOTATATE peptide receptor radionuclide therapy as single-shot treatment approach targeting both tumors. World J. Nucl. Med. 2019, 18, 160–170. [Google Scholar] [CrossRef]
- Calabria, F.; Chiaravalloti, A.; Cicciò, C.; Gangemi, V.; Gullà, D.; Rocca, F.; Gallo, G.; Cascini, G.L.; Schillaci, O. PET/CT with 18F-choline: Physiological whole bio-distribution in male and female subjects and diagnostic pitfalls on 1000 prostate cancer patients: 18F-choline PET/CT bio-distribution and pitfalls. A southern Italian experience. Nucl. Med. Biol. 2017, 51, 40–54. [Google Scholar] [CrossRef]
- Calabria, F.F.; Chiaravalloti, A.; Jaffrain-Rea, M.L.; Zinzi, M.; Sannino, P.; Minniti, G.; Rubello, D.; Schillaci, O. 18F-DOPA PET/CT Physiological Distribution and Pitfalls: Experience in 215 Patients. Clin. Nucl. Med. 2016, 41, 753–760. [Google Scholar] [CrossRef]
- Cleary, J.O.; Yeung, J.; McMeekin, H.; Wilhelm, T.; Wagner, T. The significance of incidental brain uptake on 68Ga-DOTATATE PET-CT in neuroendocrine tumour patients. Nucl. Med. Commun. 2016, 37, 1197–1205. [Google Scholar] [CrossRef]
- Calabria, F.; Chiaravalloti, A.; Schillaci, O. 18F-choline PET/CT pitfalls in image interpretation: An update on 300 examined patients with prostate cancer. Clin. Nucl. Med. 2014, 39, 122–130. [Google Scholar] [CrossRef] [PubMed]
- Kuyumcu, S.; Özkan, Z.G.; Sanli, Y.; Yilmaz, E.; Mudun, A.; Adalet, I.; Unal, S. Physiological and tumoral uptake of 68Ga-DOTATATE: Standardized uptake values and challenges in interpretation. Ann. Nucl. Med. 2013, 27, 538–545. [Google Scholar] [CrossRef]
- Schillaci, O.; Calabria, F.; Tavolozza, M.; Cicciò, C.; Carlani, M.; Caracciolo, C.R.; Danieli, R.; Orlacchio, A.; Simonetti, G. 18F-choline PET/CT physiological distribution and pitfalls in image interpretation: Experience in 80 patients with prostate cancer. Nucl. Med. Commun. 2010, 31, 39–45. [Google Scholar] [CrossRef]
- Fallanca, F.; Giovacchini, G.; Picchio, M.; Bettinardi, V.; Messa, C.; Fazio, F. Incidental detection by [11C]choline PET/CT of meningiomas in prostate cancer patients. Q. J. Nucl. Med. Mol. Imaging 2009, 53, 417–421. [Google Scholar]
- Randles, R.; Finnegan, A. Guidelines for writing a systematic review. Nurse Educ. Today 2023, 125, 105803. [Google Scholar] [CrossRef]
- Hope, T.A.; Allen-Auerbach, M.; Bodei, L.; Calais, J.; Dahlbom, M.; Dunnwald, L.K.; Graham, M.M.; Jacene, H.A.; Heath, C.L.; Mittra, E.S.; et al. SNMMI Procedure Standard/EANM Practice Guideline for SSTR PET: Imaging Neuroendocrine Tumors. J. Nucl. Med. 2023, 64, 204–210. [Google Scholar] [CrossRef]
- Bentestuen, M.; Gossili, F.; Almasi, C.E.; Zacho, H.D. Prevalence and significance of incidental findings on 68 Ga-DOTA-conjugated somatostatin receptor-targeting peptide PET/CT: A systematic review of the literature. Cancer Imaging 2022, 22, 44. [Google Scholar] [CrossRef]
- Galldiks, N.; Albert, N.L.; Sommerauer, M.; Grosu, A.L.; Ganswindt, U.; Law, I.; Preusser, M.; Le Rhun, E.; Vogelbaum, M.A.; Zadeh, G.; et al. PET imaging in patients with meningioma-report of the RANO/PET Group. Neuro-Oncology 2017, 19, 1576–1587. [Google Scholar] [CrossRef]
- Albert, N.L.; Preusser, M.; Traub-Weidinger, T.; Tolboom, N.; Law, I.; Palmer, J.D.; Guedj, E.; Furtner, J.; Fraioli, F.; Huang, R.Y.; et al. Joint EANM/EANO/RANO/SNMMI practice guideline/procedure standards for diagnostics and therapy (theranostics) of meningiomas using radiolabeled somatostatin receptor ligands: Version 1.0. Eur. J. Nucl. Med. Mol. Imaging 2024, 51, 3662–3679. [Google Scholar] [CrossRef]
- Giovacchini, G.; Giovannini, E.; Leoncini, R.; Riondato, M.; Ciarmiello, A. PET and PET/CT with radiolabeled choline in prostate cancer: A critical reappraisal of 20 years of clinical studies. Eur. J. Nucl. Med. Mol. Imaging 2017, 44, 1751–1776. [Google Scholar] [CrossRef]
- Treglia, G.; Giovannini, E.; Di Franco, D.; Calcagni, M.L.; Rufini, V.; Picchio, M.; Giordano, A. The role of positron emission tomography using carbon-11 and fluorine-18 choline in tumors other than prostate cancer: A systematic review. Ann. Nucl. Med. 2012, 26, 451–461. [Google Scholar] [CrossRef] [PubMed]
- Calabria, F.F.; Barbarisi, M.; Gangemi, V.; Grillea, G.; Cascini, G.L. Molecular imaging of brain tumors with radiolabeled choline PET. Neurosurg. Rev. 2018, 41, 67–76. [Google Scholar] [CrossRef] [PubMed]
- Fendler, W.P.; Eiber, M.; Beheshti, M.; Bomanji, J.; Calais, J.; Ceci, F.; Cho, S.Y.; Fanti, S.; Giesel, F.L.; Goffin, K.; et al. PSMA PET/CT: Joint EANM procedure guideline/SNMMI procedure standard for prostate cancer imaging 2.0. Eur. J. Nucl. Med. Mol. Imaging 2023, 50, 1466–1486. [Google Scholar] [CrossRef] [PubMed]
- Rizzo, A.; Dall’Armellina, S.; Pizzuto, D.A.; Perotti, G.; Zagaria, L.; Lanni, V.; Treglia, G.; Racca, M.; Annunziata, S. PSMA Radioligand Uptake as a Biomarker of Neoangiogenesis in Solid Tumours: Diagnostic or Theragnostic Factor? Cancers 2022, 14, 4039. [Google Scholar] [CrossRef]
- Lauri, C.; Chiurchioni, L.; Russo, V.M.; Zannini, L.; Signore, A. PSMA Expression in Solid Tumors beyond the Prostate Gland: Ready for Theranostic Applications? J. Clin. Med. 2022, 11, 6590. [Google Scholar] [CrossRef]
- Malik, D.; Sood, A.; Mittal, B.R.; Singh, H.; Basher, R.K.; Shukla, J.; Bhattacharya, A.; Singh, S.K. Nonspecific Uptake of 68Ga-Prostate-Specific Membrane Antigen in Diseases other than Prostate Malignancy on Positron Emission Tomography/Computed Tomography Imaging: A Pictorial Assay and Review of Literature. Indian J. Nucl. Med. 2018, 33, 317–325. [Google Scholar] [CrossRef]
- Sheikhbahaei, S.; Afshar-Oromieh, A.; Eiber, M.; Solnes, L.B.; Javadi, M.S.; Ross, A.E.; Pienta, K.J.; Allaf, M.E.; Haberkorn, U.; Pomper, M.G.; et al. Pearls and pitfalls in clinical interpretation of prostate-specific membrane antigen (PSMA)-targeted PET imaging. Eur. J. Nucl. Med. Mol. Imaging 2017, 44, 2117–2136. [Google Scholar] [CrossRef]
- Hofman, M.S.; Hicks, R.J.; Maurer, T.; Eiber, M. Prostate-specific Membrane Antigen PET: Clinical Utility in Prostate Cancer, Normal Patterns, Pearls, and Pitfalls. RadioGraphics 2018, 38, 200–217. [Google Scholar] [CrossRef]
- Malan, N.; Vangu, M.D. Normal Variants, Pitfalls, and Artifacts in Ga-68 Prostate Specific Membrane Antigen (PSMA) PET/CT Imaging. Front. Nucl. Med. 2022, 2, 825512. [Google Scholar] [CrossRef]
- Voter, A.F.; Werner, R.A.; Savas, H.; Gafita, A.; Ross, A.E.; Gorin, M.A.; Solnes, L.B.; Pomper, M.G.; Rowe, S.P.; Sheikhbahaei, S. A Practical Guide to the Pearls and Pitfalls of PSMA PET Imaging. Semin. Nucl. Med. 2024, 54, 119–131. [Google Scholar] [CrossRef]
- Keidar, Z.; Gill, R.; Goshen, E.; Israel, O.; Davidson, T.; Morgulis, M.; Pirmisashvili, N.; Ben-Haim, S. 68Ga-PSMA PET/CT in prostate cancer patients—Patterns of disease, benign findings and pitfalls. Cancer Imaging 2018, 18, 39. [Google Scholar] [CrossRef] [PubMed]
- Mondia, M.W.L.; Batchala, P.P.; Dreicer, R.; Devitt, M.E.; McCord, M.R.; Mut, M.; Sheehan, J.P.; Schiff, D.; Fadul, C.E. Incidental Brain Metastases From Prostate Cancer Diagnosed With PSMA PET/CT and MRI: A Case Series and Literature Review. Prostate 2025. [Google Scholar] [CrossRef] [PubMed]
- Stopa, B.M.; Crowley, J.; Juhász, C.; Rogers, C.M.; Witcher, M.R.; Kiser, J.W. Prostate-Specific Membrane Antigen as Target for Neuroimaging of Central Nervous System Tumors. Mol. Imaging 2022, 2022, 5358545. [Google Scholar] [CrossRef]
- Nanni, C.; Zanoni, L.; Bach-Gansmo, T.; Minn, H.; Willoch, F.; Bogsrud, T.V.; Edward, E.P.; Savir-Baruch, B.; Teoh, E.; Ingram, F.; et al. [18F]Fluciclovine PET/CT: Joint EANM and SNMMI procedure guideline for prostate cancer imaging-version 1.0. Eur. J. Nucl. Med. Mol. Imaging 2020, 47, 579–591. [Google Scholar] [CrossRef]
- Schuster, D.M.; Nanni, C.; Fanti, S.; Oka, S.; Okudaira, H.; Inoue, Y.; Sörensen, J.; Owenius, R.; Choyke, P.; Turkbey, B.; et al. Anti-1-amino-3-18F-fluorocyclobutane-1-carboxylic acid: Physiologic uptake patterns, incidental findings, and variants that may simulate disease. J. Nucl. Med. 2014, 55, 1986–1992. [Google Scholar] [CrossRef]
- Stormezand, G.N.; de Meyer, E.; Koopmans, K.P.; Brouwers, A.H.; Luurtsema, G.; Dierckx, R.A.J.O. Update on the Role of [18F]FDOPA PET/CT. Semin. Nucl. Med. 2024, 54, 845–855. [Google Scholar] [CrossRef]
- Treglia, G.; Muoio, B.; Trevisi, G.; Mattoli, M.V.; Albano, D.; Bertagna, F.; Giovanella, L. Diagnostic Performance and Prognostic Value of PET/CT with Different Tracers for Brain Tumors: A Systematic Review of Published Meta-Analyses. Int. J. Mol. Sci. 2019, 20, 4669. [Google Scholar] [CrossRef]
- Franceschi, A.M.; Matthews, R.; Bangiyev, L.; Relan, N.; Chaudhry, A.; Franceschi, D. Added Value of Including Entire Brain on Body Imaging With FDG PET/MRI. Am. J. Roentgenol. 2018, 211, 176–184. [Google Scholar] [CrossRef]
- Nia, E.S.; Garland, L.L.; Eshghi, N.; Nia, B.B.; Avery, R.J.; Kuo, P.H. Incidence of Brain Metastases on Follow-up 18F-FDG PET/CT Scans of Non-Small Cell Lung Cancer Patients: Should We Include the Brain? J. Nucl. Med. Technol. 2017, 45, 193–197. [Google Scholar] [CrossRef]
- Kung, B.T.; Auyong, T.K.; Tong, C.M. Prevalence of detecting unknown cerebral metastases in fluorodeoxyglucose positron emission tomography/computed tomography and its potential clinical impact. World J. Nucl. Med. 2014, 13, 108–111. [Google Scholar] [CrossRef]
- Abdelmalik, A.G.; Alenezi, S.; Muzaffar, R.; Osman, M.M. The Incremental Added Value of Including the Head in 18F-FDG PET/CT Imaging for Cancer Patients. Front. Oncol. 2013, 3, 71. [Google Scholar] [CrossRef]
- Toriihara, A.; Yamaga, E.; Nakadate, M.; Oyama, J.; Tateishi, U. Detection of unexpected emergency diseases using FDG-PET/CT in oncology patients. Jpn. J. Radiol. 2017, 35, 539–545. [Google Scholar] [CrossRef] [PubMed]
- Tseng, J.R.; Su, Y.Y.; Lee, M.H.; Chen, K.Y.; Tsai, S.F.; Yen, T.C. Clinical usefulness of FDG PET/CT in the detection of unusual central nervous system infections. J. Neurol. Sci. 2014, 345, 244–247. [Google Scholar] [CrossRef] [PubMed]
- Chung, K.J.; Abdelhafez, Y.G.; Spencer, B.A.; Jones, T.; Tran, Q.; Nardo, L.; Chen, M.S., Jr.; Sarkar, S.; Medici, V.; Lyo, V.; et al. Quantitative PET imaging and modeling of molecular blood-brain barrier permeability. Nat. Commun. 2025, 16, 3076. [Google Scholar] [CrossRef] [PubMed]
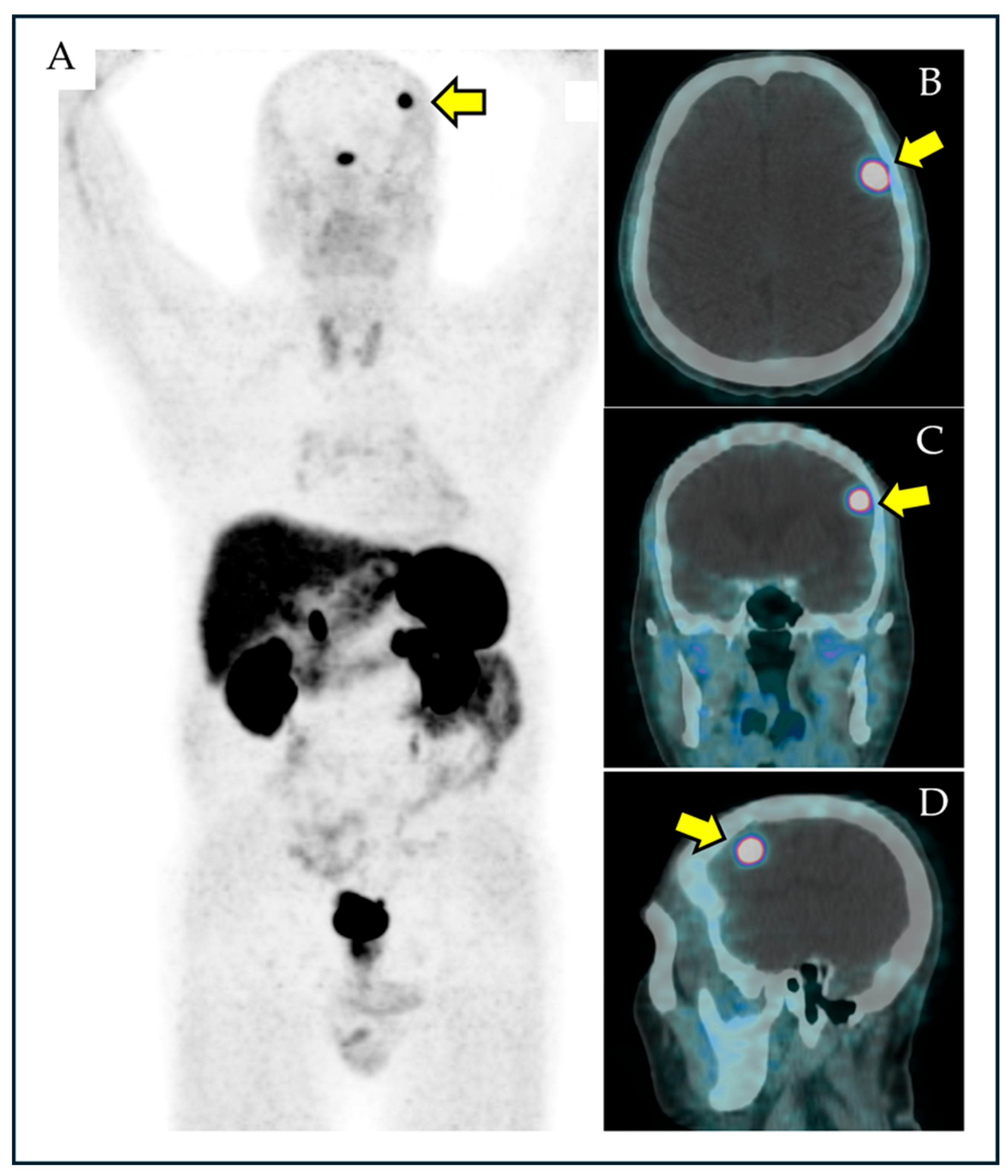
| PET Tracer | Authors | Year | Country | Patients with PET Scans | Patients with BIPs | Patients with Suspicious Meningioma Among Those with BIPs | Prevalence of BIPs | Pooled Prevalence of BIPs |
|---|---|---|---|---|---|---|---|---|
| [68Ga]Ga-DOTA-peptides | Ensign et al. [13] | 2023 | USA | 3154 | 145 | 123 (85%) | 4.6% | 4.6% |
| Albano et al. [15] | 2022 | Italy/Switzerland | 430 | 48 | 38 (79%) | 11.2% | ||
| Umana et al. [14] | 2022 | Italy | 1000 | 18 | 10 (56%) | 1.8% | ||
| Parghane et al. [18] | 2019 | India | 500 | 12 | 6 (50%) | 2.4% | ||
| Cleary et al. [21] | 2016 | UK/Australia | 313 | 22 | 21 (95%) | 7.0% | ||
| Kuyumcu et al. [23] | 2013 | Turkey | 120 | 3 | 1 (33%) | 2.5% | ||
| [68Ga/18F]PSMA ligands | McLaughlin et al. [12] | 2023 | USA | 2763 | 33 | 16 (48%) | 1.2% | 1.2% |
| [18F/11C]Choline | Roland et al. [17] | 2020 | France | 368 | 2 | 2 (100%) | 0.5% | 1% |
| Calabria et al. [19] | 2017 | Italy | 1000 | 14 | 12 (86%) | 1.4% | ||
| Calabria et al. [22] * | 2014 | Italy | 300 | 6 | 6 (100%) | 2.0% | ||
| Schillaci et al. [24] * | 2010 | Italy | 80 | 2 | 1 (50%) | 2.5% | ||
| Fallanca et al. [25] | 2009 | Italy | 402 | 4 | 4 (100%) | 1% | ||
| [18F]Fluciclovine | Baiomy et al. [16] | 2021 | USA | 566 | 14 | 10 (71%) | 2.5% | 2.5% |
| [18F]FDOPA | Calabria et al. [20] | 2016 | Italy | 215 (77 **) | 3 | 0 (0%) | 3.9% | 3.9% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iacovitti, C.M.; Muoio, B.; Albano, D.; Rizzo, A.; Cuzzocrea, M.; Paone, G.; Treglia, G. The Prevalence and Significance of Incidental Positron Emission Tomography Findings in the Brain Using Radiotracers Other than [18F]FDG: A Systematic Review and Meta-Analysis. Diagnostics 2025, 15, 1204. https://doi.org/10.3390/diagnostics15101204
Iacovitti CM, Muoio B, Albano D, Rizzo A, Cuzzocrea M, Paone G, Treglia G. The Prevalence and Significance of Incidental Positron Emission Tomography Findings in the Brain Using Radiotracers Other than [18F]FDG: A Systematic Review and Meta-Analysis. Diagnostics. 2025; 15(10):1204. https://doi.org/10.3390/diagnostics15101204
Chicago/Turabian StyleIacovitti, Cesare Michele, Barbara Muoio, Domenico Albano, Alessio Rizzo, Marco Cuzzocrea, Gaetano Paone, and Giorgio Treglia. 2025. "The Prevalence and Significance of Incidental Positron Emission Tomography Findings in the Brain Using Radiotracers Other than [18F]FDG: A Systematic Review and Meta-Analysis" Diagnostics 15, no. 10: 1204. https://doi.org/10.3390/diagnostics15101204
APA StyleIacovitti, C. M., Muoio, B., Albano, D., Rizzo, A., Cuzzocrea, M., Paone, G., & Treglia, G. (2025). The Prevalence and Significance of Incidental Positron Emission Tomography Findings in the Brain Using Radiotracers Other than [18F]FDG: A Systematic Review and Meta-Analysis. Diagnostics, 15(10), 1204. https://doi.org/10.3390/diagnostics15101204








