The Potential Morphological Stenosis Pattern of the Arcuate Foramen
Abstract
1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Standring, S.; Anand, N.; Birch, R.; Collins, P.; Crossman, A.; Gleeson, M.; Jawaheer, G.; Smith, A.; Spratt, J.; Stringer, M.; et al. Gray’s Anatomy: The Anatomical Basis of Clinical Practice, 41st ed.; Elsevier: London, UK, 2016. [Google Scholar]
- Ahn, J.; Duran, M.; Syldort, S.; Rizvi, A.; D’Antoni, A.V.; Johal, J.; Iwanaga, J.; Oskouian, R.J.; Tubbs, R.S. Arcuate Foramen: Anatomy, Embryology, Nomenclature, Pathology, and Surgical Considerations. World Neurosurg. 2018, 118, 197–202. [Google Scholar] [CrossRef] [PubMed]
- Tubbs, R.S.; Johnson, P.C.; Shoja, M.M.; Loukas, M.; Oakes, W.J. Foramen Arcuale: Anatomical Study and Review of the Literature. J. Neurosurg. Spine 2007, 6, 31–34. [Google Scholar] [CrossRef]
- Gul, E.; Atik, I. Does Ponticulus Posticus Affect Vertebral Artery Diameter. Surg. Radiol. Anat. 2024, 46, 1517–1524. [Google Scholar] [CrossRef]
- Pękala, P.A.; Henry, B.M.; Pękala, J.R.; Saganiak, K.; Taterra, D.; Walocha, J.A.; Tubbs, R.S.; Tomaszewski, K.A. Lateral and Posterolateral Foraminal Variations of the Atlas: A Meta-Analysis. J. Clin. Neurosci. 2017, 40, 74–82. [Google Scholar] [CrossRef]
- Al-Redouan, A.; Hudak, R.; Nanka, O.; Kachlik, D. The Morphological Stenosis Pattern of the Suprascapular Notch Is Revealed Yielding Higher Incidence in the Discrete Type and Elucidating the Inevitability of Osteoplasty in Horizontally Oriented Stenosis. Knee Surg. Sports Traumatol. Arthrosc. 2021, 29, 2272–2280. [Google Scholar] [CrossRef] [PubMed]
- Paschopoulos, I.; Triantafyllou, G.; Papadopoulos-Manolarakis, P.; Luzzi, S.; Karangeli, N.; Tsakotos, G.; Galzio, R.; Piagkou, M. The Morphological Stenosis Pattern of the Caroticoclinoid Foramen. Diagnostics 2024, 15, 76. [Google Scholar] [CrossRef] [PubMed]
- Brenner, E.; Bleys, R.L.A.W.; de Caro, R.; Catereniuc, I.; Chirculescu, A.R.M.; Destrieux, C.; Eppler, E.; Filgueira, L.; Kachlik, D.; Kiss, P.; et al. The Legal and Ethical Framework Governing Body Donation in Europe—2nd Update on Current Practice. Ann. Anat. -Anat. Anz. 2024, 252, 152195. [Google Scholar] [CrossRef]
- Triantafyllou, G.; Papadopoulos-Manolarakis, P.; Tsakotos, G.; Piagkou, M. The Posterior Petroclinoid Fold and Petroclival Ligament Ossification Patterns. Surg. Radiol. Anat. 2025, 47, 53. [Google Scholar] [CrossRef]
- Karangeli, N.; Triantafyllou, G.; Duparc, F.; Vassiou, K.; Vlychou, M.; Tsakotos, G.; Piagkou, M. Retrostyloid and Retromandibular Courses of the External Carotid Artery. Surg. Radiol. Anat. 2024, 47, 23. [Google Scholar] [CrossRef]
- Pȩkala, P.A.; Henry, B.M.; Pȩkala, J.R.; Hsieh, W.C.; Vikse, J.; Sanna, B.; Walocha, J.A.; Tubbs, R.S.; Tomaszewski, K.A. Prevalence of Foramen Arcuale and Its Clinical Significance: A Meta-Analysis of 55,985 Subjects. J. Neurosurg. Spine 2017, 27, 276–290. [Google Scholar] [CrossRef]
- Saunders, S.R.; Popovich, F. A Family Study of Two Skeletal Variants: Atlas Bridging and Clinoid Bridging. Am. J. Phys. Anthr. 1978, 49, 193–203. [Google Scholar] [CrossRef] [PubMed]
- Zaborowski, Z. The Sagittal Foramen of the Atlas. Folia Morphol. 1975, 45, 375–381. [Google Scholar]
- Sato, E. Studies on the Ponticulus Posterior and Ponticulus Lateralis of the Human First Cervical Vertebra. Sapporo Med. J. 1978, 47, 599–617. [Google Scholar]
- Stubbs, D.M. The Arcuate Foramen. Spine 1992, 17, 1502–1504. [Google Scholar] [CrossRef]
- Mitchell, J. The Incidence and Dimensions of the Retroarticular Canal of the Atlas Vertebra. Cells Tissues Organs 1998, 163, 113–120. [Google Scholar] [CrossRef]
- Wysocki, J.; Bubrowski, M.; Reymond, J.; Kwiatkowski, J. Anatomical Variants of the Cervical Vertebrae and the First Thoracic Vertebra in Man. Folia Morphol. 2003, 62, 357–363. [Google Scholar]
- Beck, R.W.; Holt, K.R.; Fox, M.A.; Hurtgen-Grace, K.L. Radiographic Anomalies That May Alter Chiropractic Intervention Strategies Found in a New Zealand Population. J. Manip. Physiol. Ther. 2004, 27, 554–559. [Google Scholar] [CrossRef]
- Lee, M.J.; Cassinelli, E.; Riew, K.D. The Feasibility of Inserting Atlas Lateral Mass Screws via the Posterior Arch. Spine 2006, 31, 2798–2801. [Google Scholar] [CrossRef]
- Krishnamurthy, A.; Nayak, S.R.; Khan, S.; Prabhu, L.V.; Ramanathan, L.A.; Ganesh Kumar, C.; Prasad Sinha, A. Arcuate Foramen of Atlas: Incidence, Phylogenetic and Clinical Significance. Rom. J. Morphol. Embryol. 2007, 48, 263–266. [Google Scholar]
- Simsek, S.; Yigitkanli, K.; Comert, A.; Acar, H.I.; Seckin, H.; Er, U.; Belen, D.; Tekdemir, I.; Elhan, A. Posterior Osseous Bridging of C1. J. Clin. Neurosci. 2008, 15, 686–688. [Google Scholar] [CrossRef]
- Hong, J.T.; Lee, S.W.; Son, B.C.; Sung, J.H.; Yang, S.H.; Kim, I.S.; Park, C.K. Analysis of Anatomical Variations of Bone and Vascular Structures around the Posterior Atlantal Arch Using Three-Dimensional Computed Tomography Angiography. J. Neurosurg. Spine 2008, 8, 230–236. [Google Scholar] [CrossRef] [PubMed]
- Sharma, V.; Chaudhary, D.; Mitra, R. Prevalence of Ponticulus Posticus in Indian Orthodontic Patients. Dentomaxillofacial Radiol. 2010, 39, 277–283. [Google Scholar] [CrossRef] [PubMed]
- Baeesa, S.S.; Bokhari, R.F.; Bajunaid, K.M.; Al-Sayyad, M.J. Prevalence of the Foramen Arcuale of the Atlas in a Saudi Population. Neurosciences 2012, 17, 345–351. [Google Scholar] [PubMed]
- Bayrakdar, I.S.; Miloglu, O.; Altun, O.; Gumussoy, I.; Durna, D.; Yilmaz, A.B. Cone Beam Computed Tomography Imaging of Ponticulus Posticus: Prevalence, Characteristics, and a Review of the Literature. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2014, 118, e210–e219. [Google Scholar] [CrossRef]
- Geist, J.R.; Geist, S.-M.R.Y.; Lin, L.-M. A Cone Beam CT Investigation of Ponticulus Posticus and Lateralis in Children and Adolescents. Dentomaxillofacial Radiol. 2014, 43, 20130451. [Google Scholar] [CrossRef]
- Mudit, G.; Srinivas, K.; Sateesha, R. Retrospective Analysis of Ponticulus Posticus in Indian Orthodontic Patients—A Lateral Cephalometric Study. Ethiop. J. Health Sci. 2014, 24, 285. [Google Scholar] [CrossRef][Green Version]
- Pérez, I.E.; Chávez, A.K. Frequency of Ponticulus Posticus, Sella Turcica Bridge and Clinoid Enlargement in Cleft Lip and Palate Peruvian Patients: A Comparative Study with Non-Cleft Patients. Int. J. Morphol. 2015, 33, 895–901. [Google Scholar] [CrossRef]
- Chen, C.-H.; Chen, Y.-K.; Wang, C.-K. Prevalence of Ponticuli Posticus among Patients Referred for Dental Examinations by Cone-Beam CT. Spine J. 2015, 15, 1270–1276. [Google Scholar] [CrossRef]
- Stropus, R.; Naujokaitė, E.; Sakalauskaitė, I. The Prevalence of Skeletotopy Variation of the Atlas and Vertebral Arteries Among Lithuanians and Its Significance for Physical Medicine Procedures and Rehabilitation. Reabil. Moksl. Slauga Kineziter. Ergoter. 2019, 12, 1. [Google Scholar] [CrossRef]
- Gibelli, D.; Cappella, A.; Cerutti, E.; Spagnoli, L.; Dolci, C.; Sforza, C. Prevalence of Ponticulus Posticus in a Northern Italian Orthodontic Population: A Lateral Cephalometric Study. Surg. Radiol. Anat. 2016, 38, 309–312. [Google Scholar] [CrossRef]
- Natsis, K.; Piperaki, E.T.; Fratzoglou, M.; Lazaridis, N.; Tsitsopoulos, P.P.; Samolis, A.; Kostares, M.; Piagkou, M. Atlas Posterior Arch and Vertebral Artery’s Groove Variants: A Classification, Morphometric Study, Clinical and Surgical Implications. Surg. Radiol. Anat. 2019, 41, 985–1001. [Google Scholar] [CrossRef] [PubMed]
- Paraskevas, G.; Papaziogas, B.; Tsonidis, C.; Kapetanos, G. Gross Morphology of the Bridges over the Vertebral Artery Groove on the Atlas. Surg. Radiol. Anat. 2005, 27, 129–136. [Google Scholar] [CrossRef] [PubMed]
- Pękala, P.A.; Henry, B.M.; Phan, K.; Pękala, J.R.; Taterra, D.; Walocha, J.A.; Tubbs, R.S.; Tomaszewski, K.A. Presence of a Foramen Arcuale as a Possible Cause for Headaches and Migraine: Systematic Review and Meta-Analysis. J. Clin. Neurosci. 2018, 54, 113–118. [Google Scholar] [CrossRef] [PubMed]

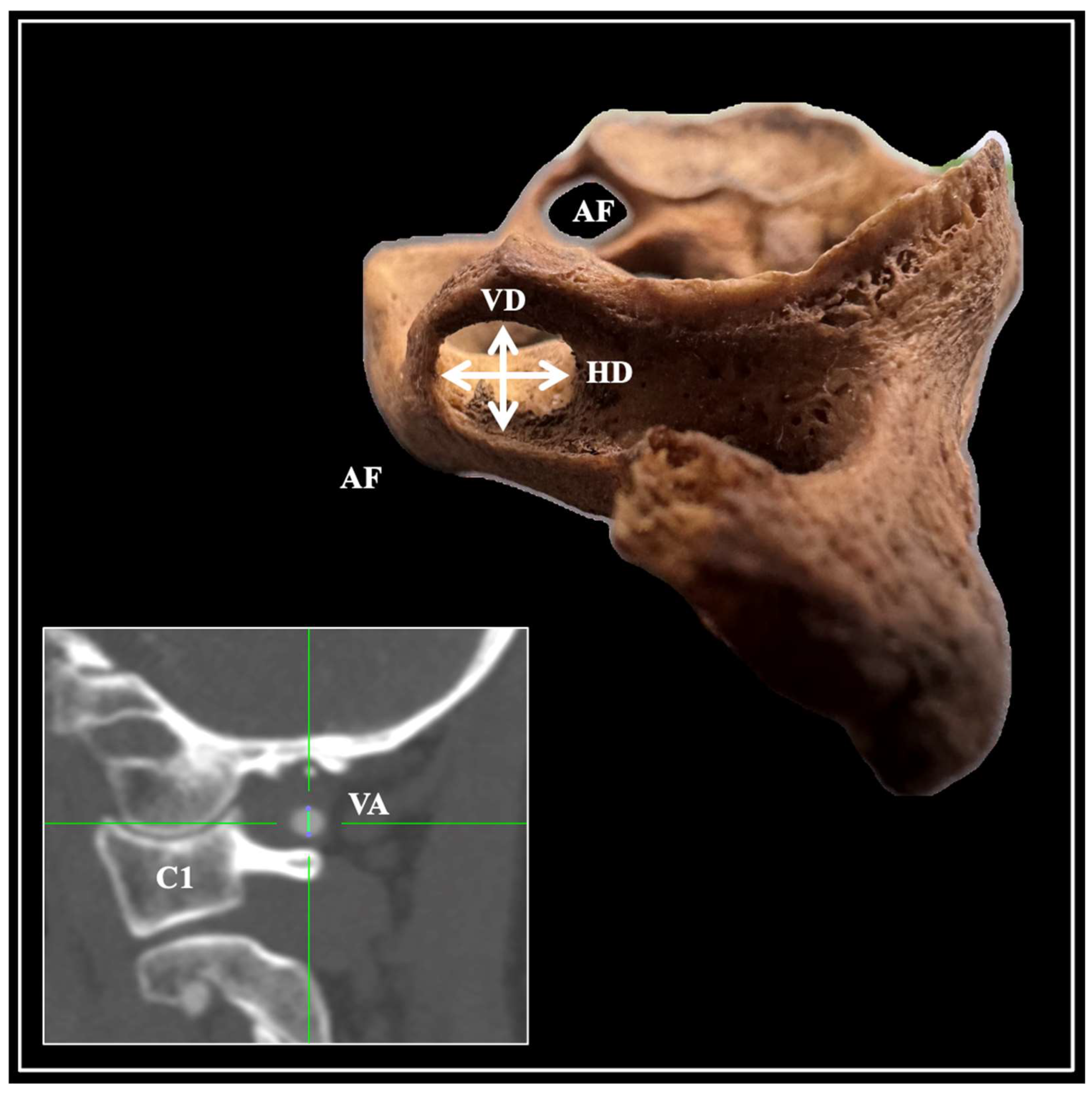
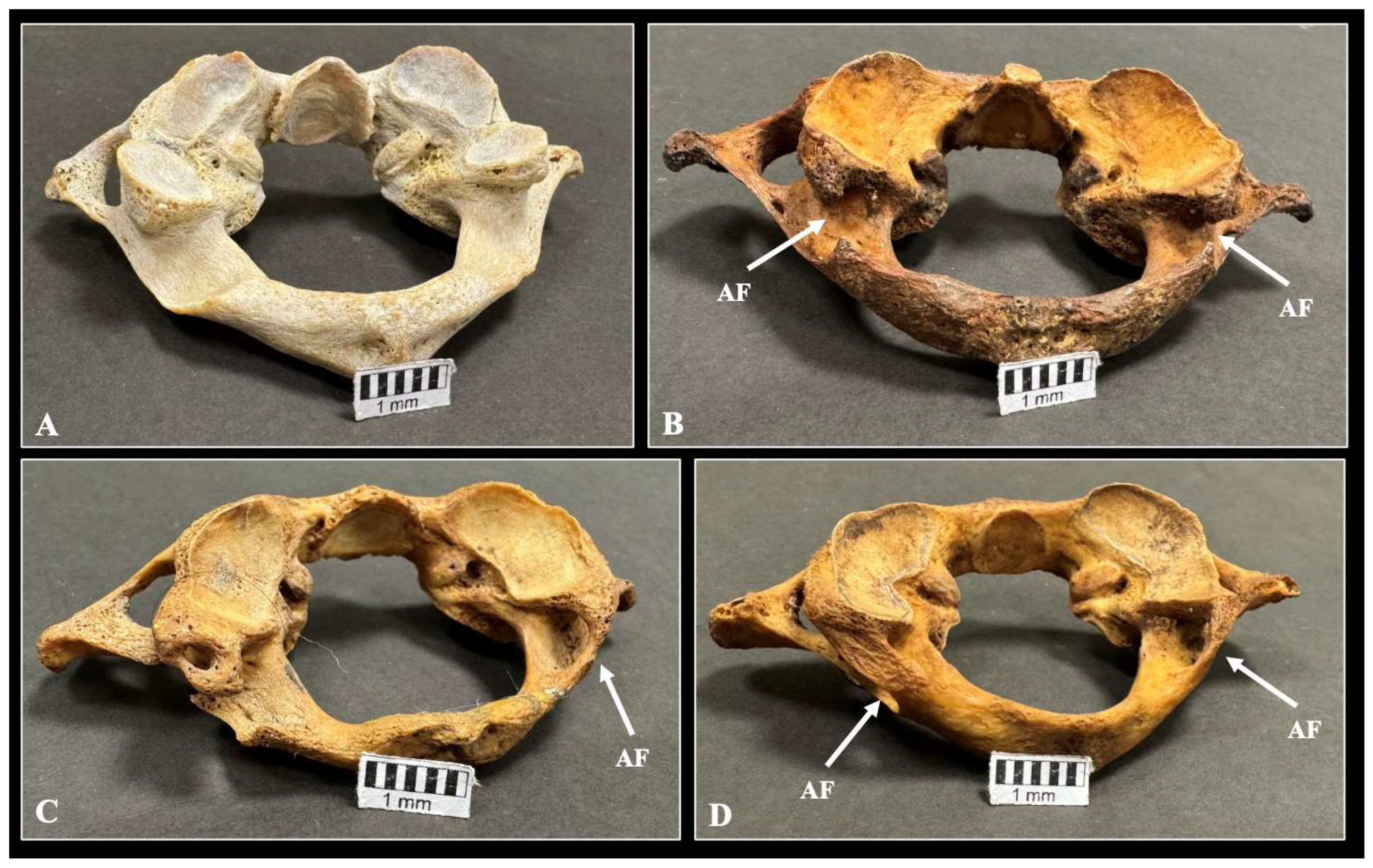
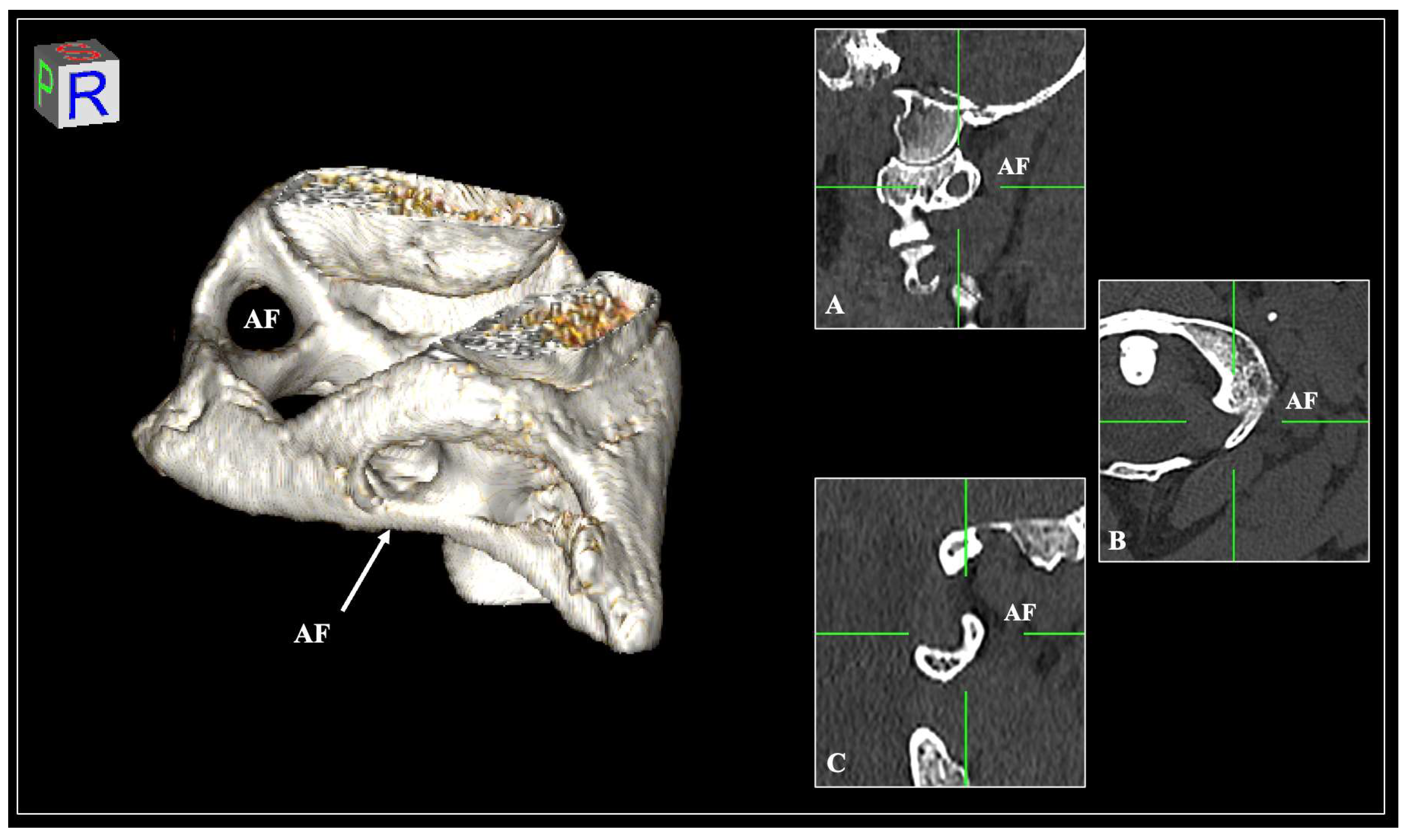
| Presence of AF | Total n (%) | Left n (%) | Right n (%) | p-Value | Female n (%) | Male n (%) | p-Value |
|---|---|---|---|---|---|---|---|
| Incomplete type | 111 (18.5) | 58 (19.3) | 53 (17.6) | 0.899 | 57 (33.5) | 37 (28.4) | 0.238 |
| Complete type | 67 (11.2) | 33 (11) | 34 (11.3) | 0.897 | 14 (8.2) | 19 (14.6) | 0.133 |
| AF Morphometry (Diameters) | Total Mean (SD) | Left Mean (SD) | Right Mean (SD) | p-Value | Female Mean (SD) | Male Mean (SD) | p-Value |
|---|---|---|---|---|---|---|---|
| Vertical (VD)-n = 149 AFs | 6.41 (1.12) | 6.42 (1.09) | 6.40 (1.15) | 0.911 | 6.56 (1.01) | 6.46 (1.03) | 0.591 |
| Horizontal (HD)-n = 149 AFs | 6.20 (1.02) | 6.33 (1.04) | 6.06 (0.98) | 0.126 | 6.12 (1.02) | 6.30 (1.02) | 0.320 |
| Parameters | Possible Risk Calculation | ||
|---|---|---|---|
| Low Risk (>6.0 mm) | Intermediate Risk (5.0–6.0 mm) | High Risk (<5.0 mm) | |
| Total-n (%) | 109 (61.2%) | 52 (29.2%) | 17 (9.6%) |
| By side | |||
| Left-n (%) | 58 (32.5%) | 25 (14.04%) | 8 (4.4%) |
| Right-n (%) | 51 (28.6%) | 27 (15.16%) | 9 (5.0%) |
| p-value | 0.780 | ||
| By sex | |||
| Female-n (%) | 48 (26.9%) | 19 (10.6%) | 4 (2.2%) |
| Male-n (%) | 36 (20.2%) | 16 (8.9%) | 4 (2.2%) |
| p-value | 0.124 | ||
| By the AF morphology presence | |||
| AF incomplete type-n (%) | 74 (41.57%) | 29 (16.2%) | 8 (4.4%) |
| AF complete type-n (%) | 35 (19.66%) | 23 (12.9%) | 9 (5.0%) |
| p-value | 0.130 | ||
| Parameters | Possible Risk Calculation | ||
|---|---|---|---|
| Low Risk (>6.0 mm) | Intermediate Risk (5.0–6.0 mm) | High Risk (<5.0 mm) | |
| Total-n (%) | 98 (55.1%) | 58 (32.6%) | 22 (12.4%) |
| By side | |||
| Left-n (%) | 52 (29.2%) | 28 (15.7%) | 11 (6.2%) |
| Right-n (%) | 46 (25.8%) | 30 (16.9%) | 11 (6.2%) |
| p-value | 0.841 | ||
| By sex | |||
| Female-n (%) | 38 (21.3%) | 24 (13.5%) | 9 (5.1%) |
| Male-n (%) | 35 (19.7%) | 17 (9.6%) | 4 (2.2%) |
| p-value | 0.485 | ||
| By the AF morphology presence | |||
| AF incomplete type-n (%) | 66 (37.1%) | 30 (16.9%) | 15 (8.4%) |
| AF complete type-n (%) | 32 (18.0%) | 28 (15.7%) | 7 (3.9%) |
| p-value | 0.125 | ||
| Author | Year | Country | No. of Subjects | AF Frequency | |
|---|---|---|---|---|---|
| Complete% | Incomplete% | ||||
| Zaborowski [13] | 1975 | Poland | 4046 | 8.7 | 2.9 |
| Sato and Noriyasu [14] | 1978 | Japan | 1428 | 5.5 | NR |
| Stubbs et al. [15] | 1991 | USA | 1000 | 13.5 | 5.2 |
| Mitchel [16] | 1998 | South Africa | 1354 | 13.3 | NR |
| Wysocki et al. [17] | 2003 | Poland | 95 | 13.7 | 17.9 |
| Beck et al. [18] | 2004 | Zew Zealand | 847 | 13.6 | NR |
| Lee et al. [19] | 2006 | USA | 709 | 22.1 | 4.8 |
| Krishnamurthy et al. [20] | 2007 | India | 1044 | 8.3 | 5.5 |
| Simsek et al. [21] | 2007 | Turkey | 158 | 3.8 | 5.7 |
| Tubbs et al. [3] | 2007 | USA | 60 | 5.0 | NR |
| Hong et al. [22] | 2008 | South Korea | 1013 | 6.5 | 9.1 |
| Sharma et al. [23] | 2010 | India | 858 | 4.3 | NR |
| Baeesa et al. [24] | 2012 | Saudi Arabia | 453 | 16.1 | 31.8 |
| Bayrakdar et al. [25] | 2014 | Turkey | 730 | 9.5 | 11.1 |
| Geist et al. [26] | 2014 | Taiwan | 576 | 10.4 | 15.8 |
| Mudit et al. [27] | 2014 | India | 650 | 2.9 | 8.0 |
| Chavez and Perez [28] | 2015 | Peru | 1219 | 8.4 | 11.1 |
| Chen et al. [29] | 2015 | Taiwan | 500 | 4.6 | 2.8 |
| Stropus et al. [30] | 2015 | Lithuania | 706 | 7.5 | 24.9 |
| Gibeli et al. [31] | 2016 | Italy | 221 | 7.7 | 9.0 |
| Natsis et al. [32] | 2019 | Greece | 244 | 15.98 | 18.03 |
| Present study | 2025 | Greece | 300 | 11.2 | 18.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paschopoulos, I.; Piagkou, M.; Triantafyllou, G.; Papadopoulos-Manolarakis, P.; Duparc, F.; Demetriou, F.; Tsakotos, G.; Tudose, R.-C.; Rusu, M.C.; Toader, O.D. The Potential Morphological Stenosis Pattern of the Arcuate Foramen. Diagnostics 2025, 15, 1203. https://doi.org/10.3390/diagnostics15101203
Paschopoulos I, Piagkou M, Triantafyllou G, Papadopoulos-Manolarakis P, Duparc F, Demetriou F, Tsakotos G, Tudose R-C, Rusu MC, Toader OD. The Potential Morphological Stenosis Pattern of the Arcuate Foramen. Diagnostics. 2025; 15(10):1203. https://doi.org/10.3390/diagnostics15101203
Chicago/Turabian StylePaschopoulos, Ioannis, Maria Piagkou, George Triantafyllou, Panagiotis Papadopoulos-Manolarakis, Fabrice Duparc, Fotis Demetriou, George Tsakotos, Rǎzvan-Costin Tudose, Mugurel Constantin Rusu, and Oana Daniela Toader. 2025. "The Potential Morphological Stenosis Pattern of the Arcuate Foramen" Diagnostics 15, no. 10: 1203. https://doi.org/10.3390/diagnostics15101203
APA StylePaschopoulos, I., Piagkou, M., Triantafyllou, G., Papadopoulos-Manolarakis, P., Duparc, F., Demetriou, F., Tsakotos, G., Tudose, R.-C., Rusu, M. C., & Toader, O. D. (2025). The Potential Morphological Stenosis Pattern of the Arcuate Foramen. Diagnostics, 15(10), 1203. https://doi.org/10.3390/diagnostics15101203








