Osteotomies: Indications, Imaging Appearance, Surgical Techniques, and Complications
Abstract
:1. Introduction
1.1. Anatomic and Mechanical Axes in Deformity Correction

1.2. Basic Osteotomy Techniques, Tools, and Hardware
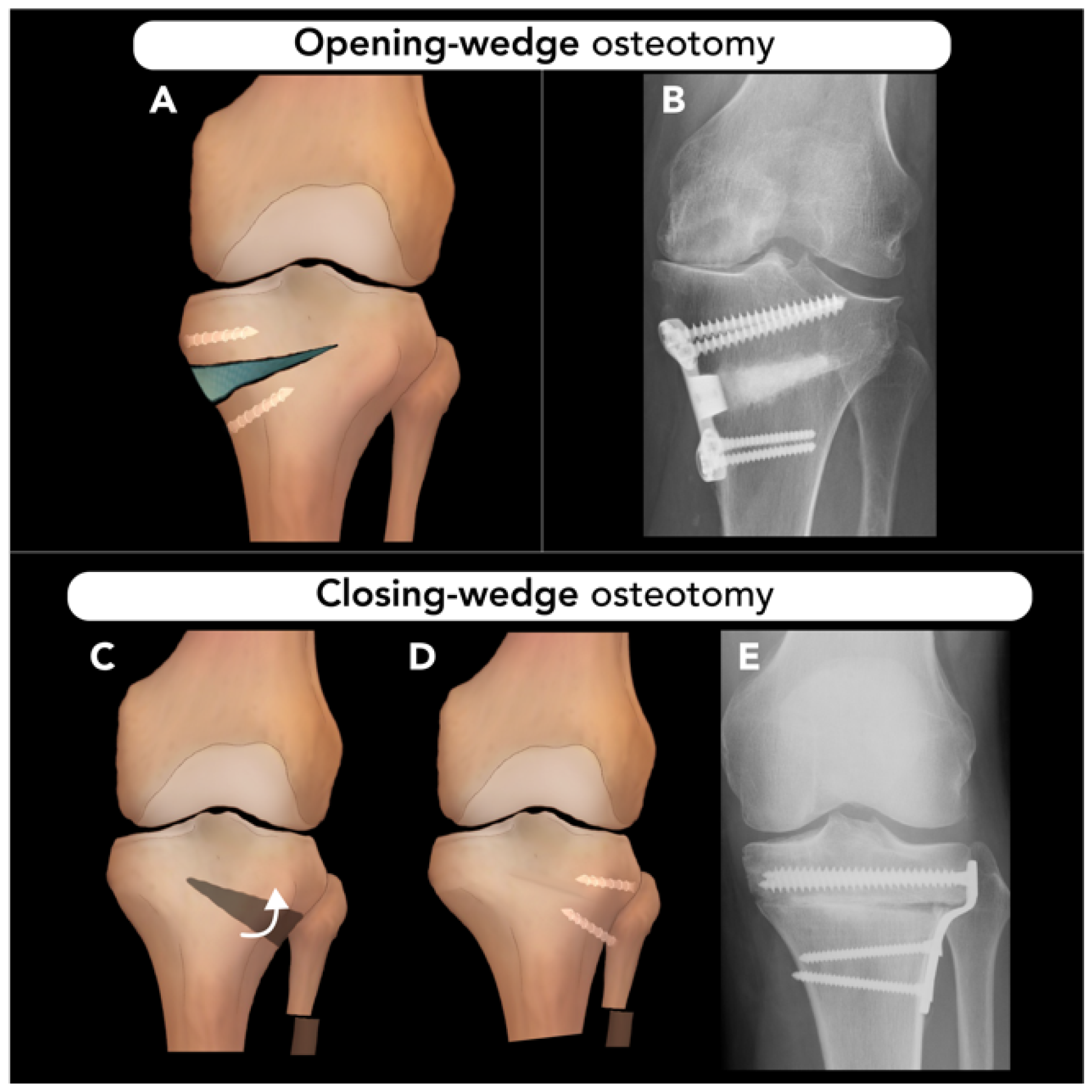
1.2.1. Osteotomy Techniques: Opening-Wedge, Closing-Wedge, and Dome Osteotomies
1.2.2. Osteotomes and Fixation Methods
1.3. Radiographic Assessment and Monitoring
1.4. Post-Operative Complications
2. Osteotomy Approaches
2.1. Skull and Maxillofacial Osteotomies
2.1.1. Craniotomy
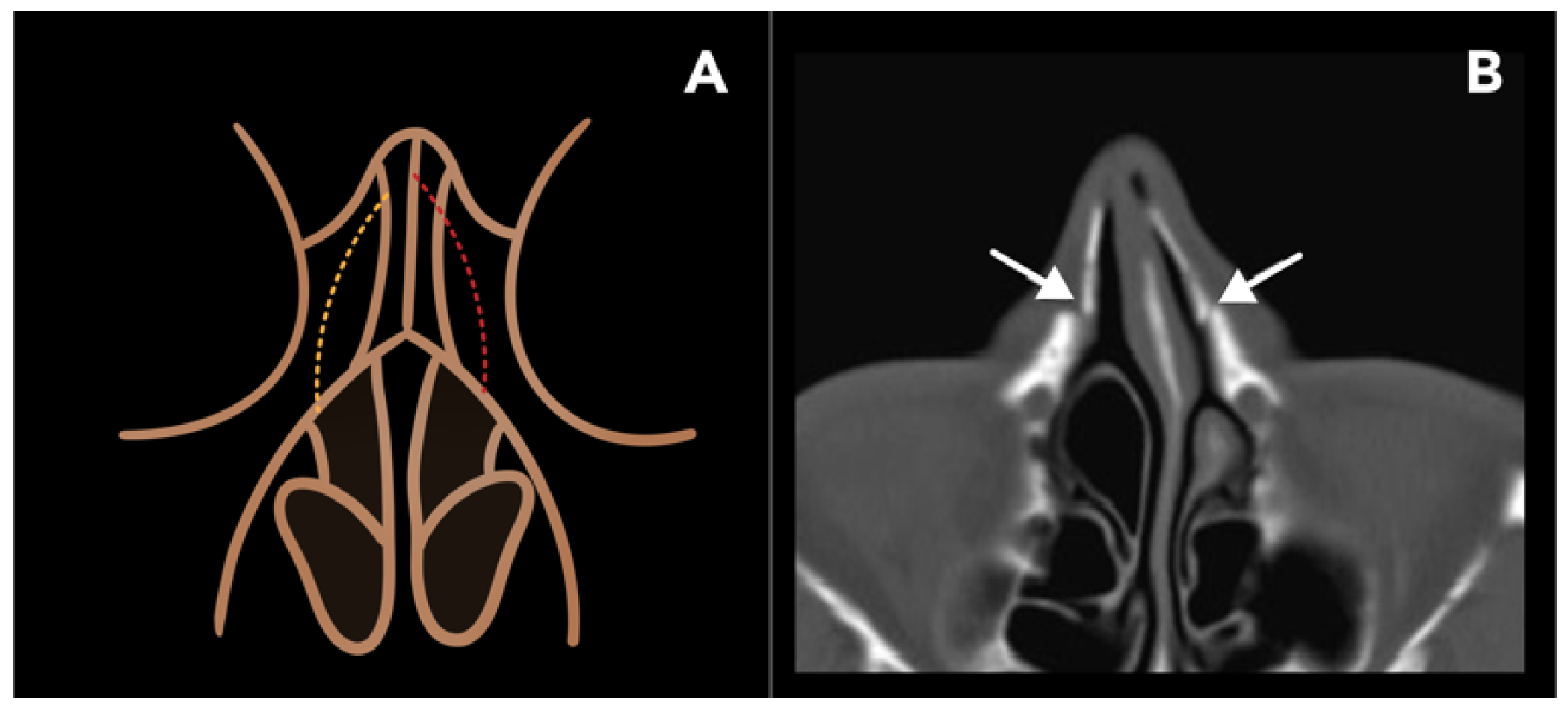
2.1.2. Lateral Nasal Osteotomy
2.1.3. Reduction Malarplasty
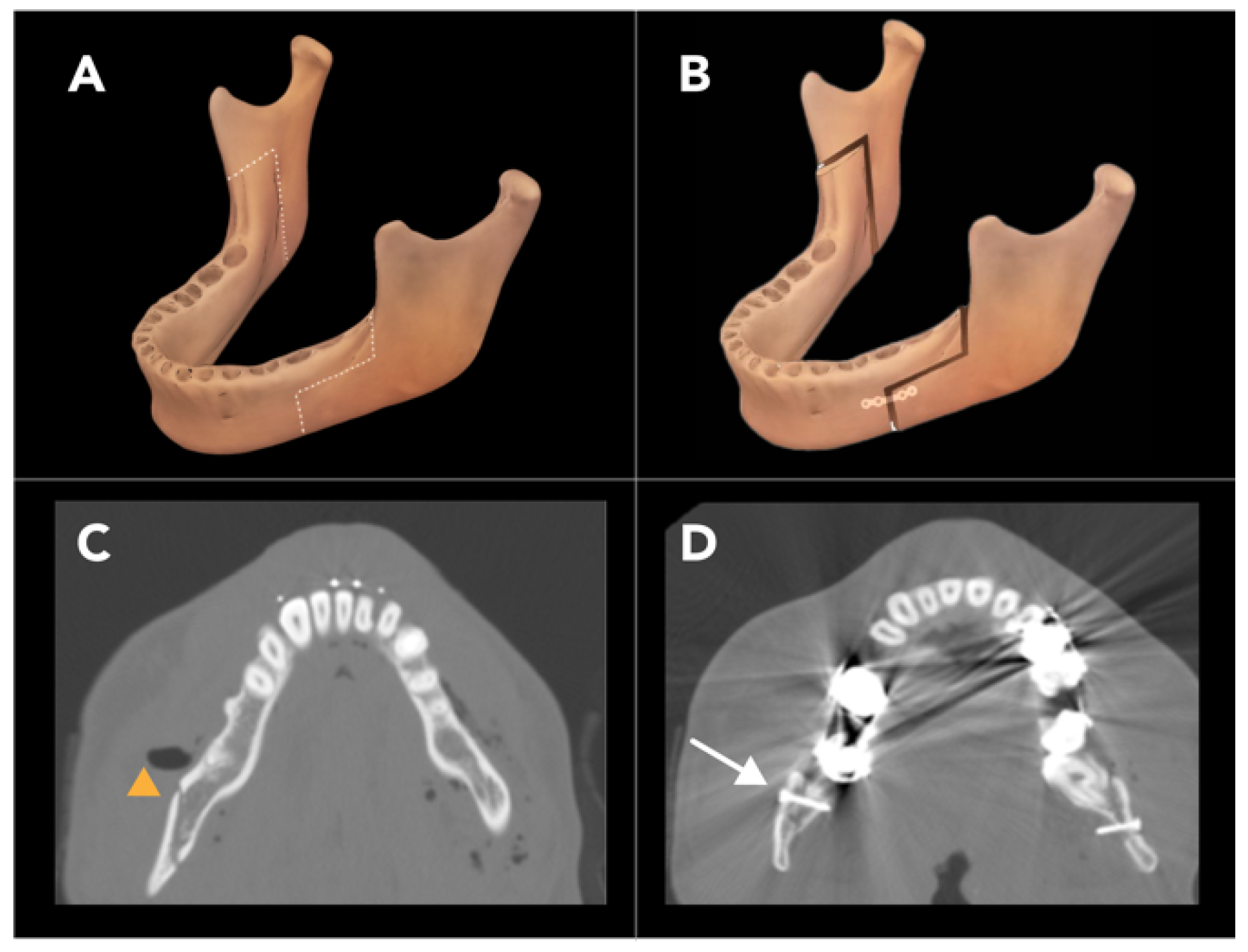
2.1.4. Bilateral Sagittal Split Osteotomy
2.2. Upper Extremity Osteotomies
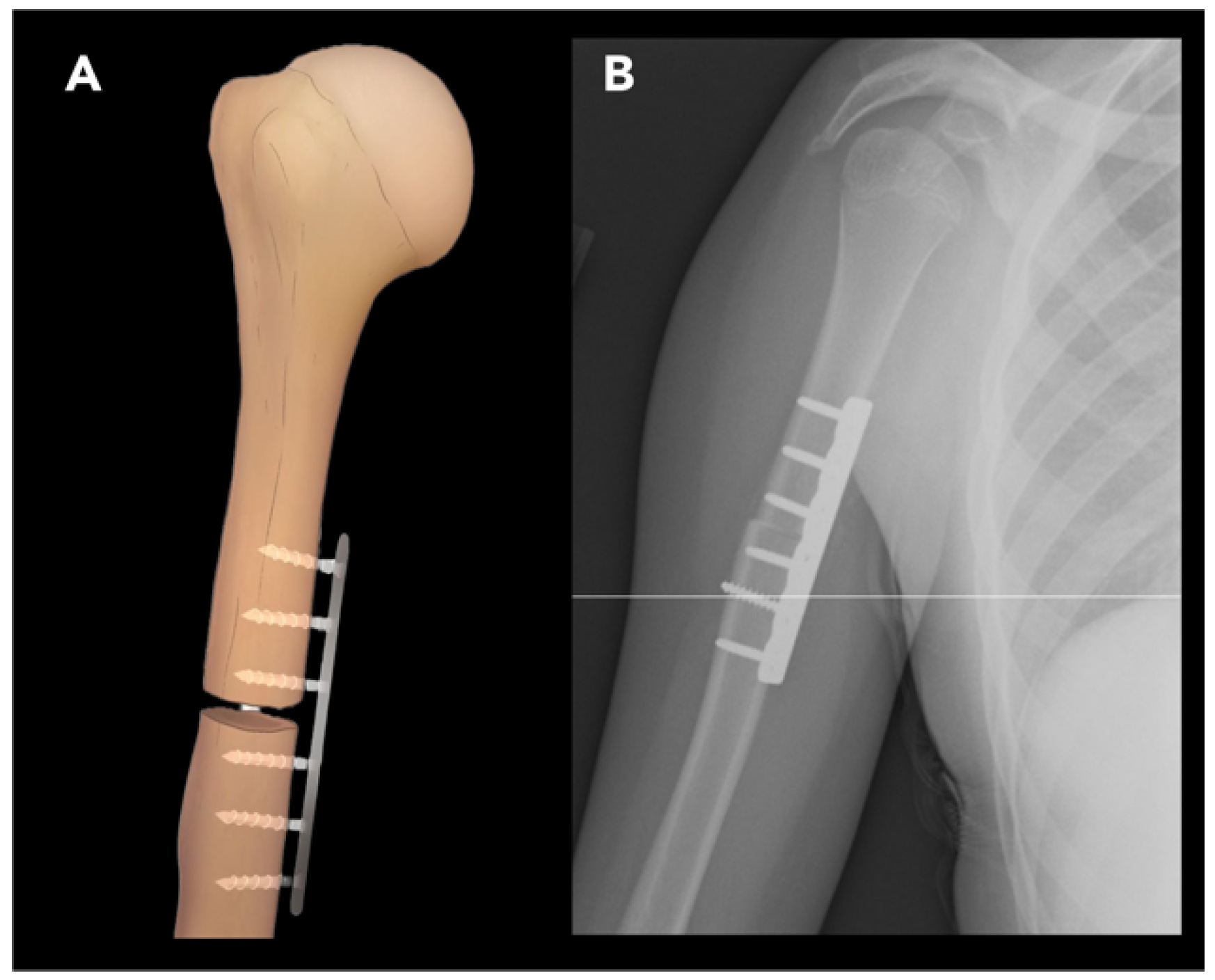
2.2.1. External Rotation Humeral Osteotomy
2.2.2. Ulnar Shortening Osteotomy
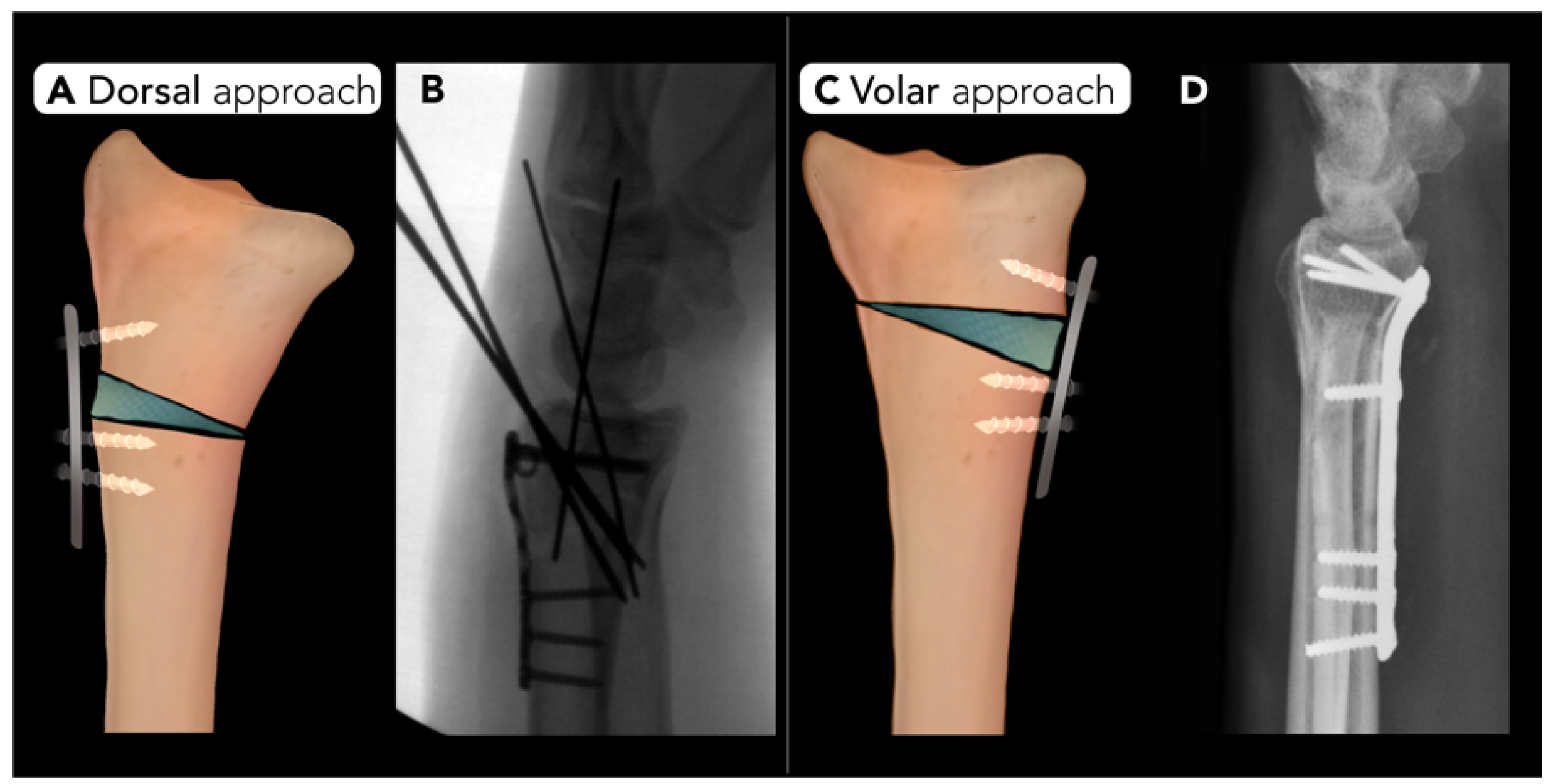
2.2.3. Distal Radius Corrective Osteotomy
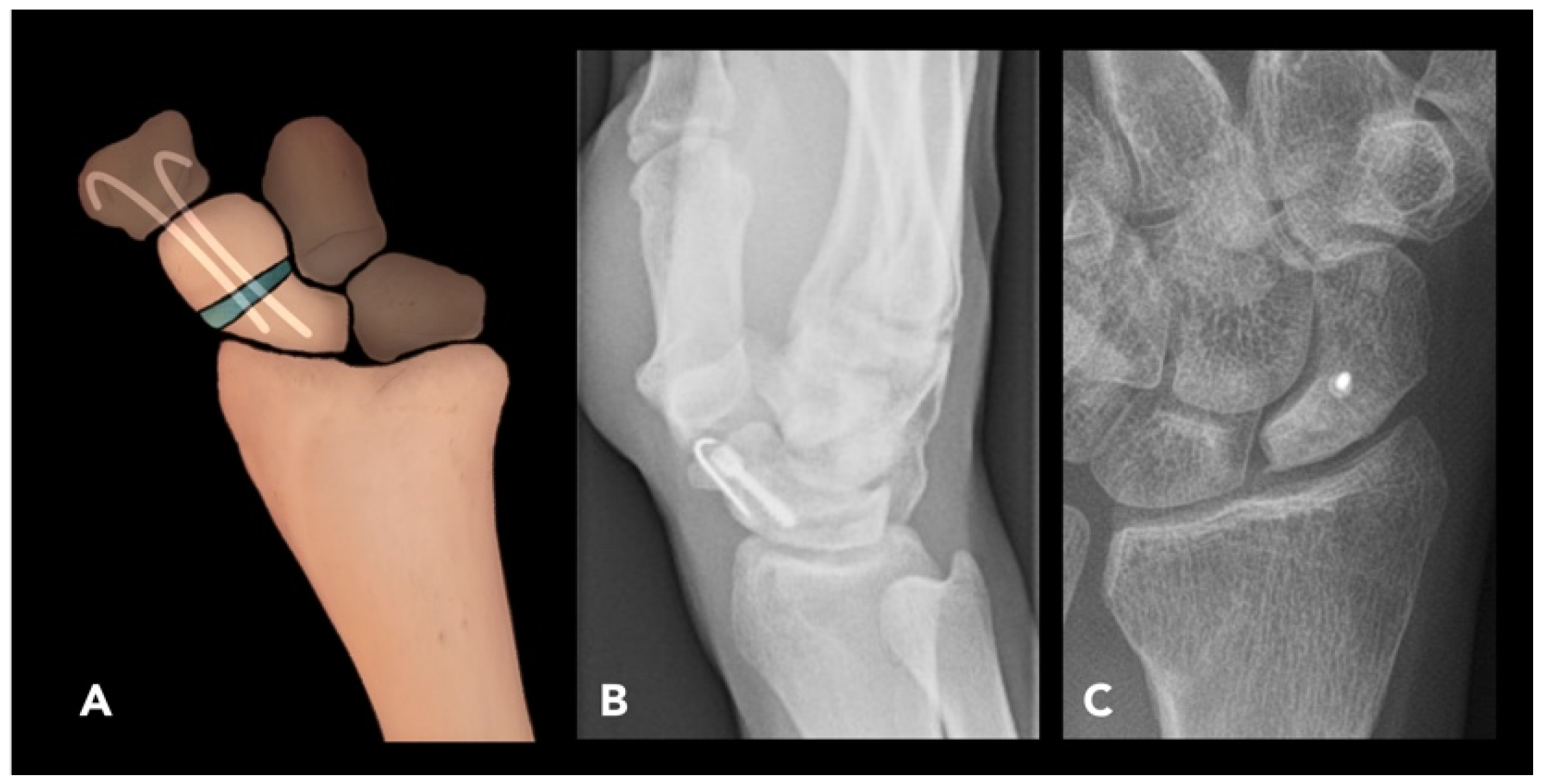
2.2.4. Scaphoid Corrective Osteotomy
2.3. Lower Extremity Osteotomies
2.3.1. High Tibial Osteotomy
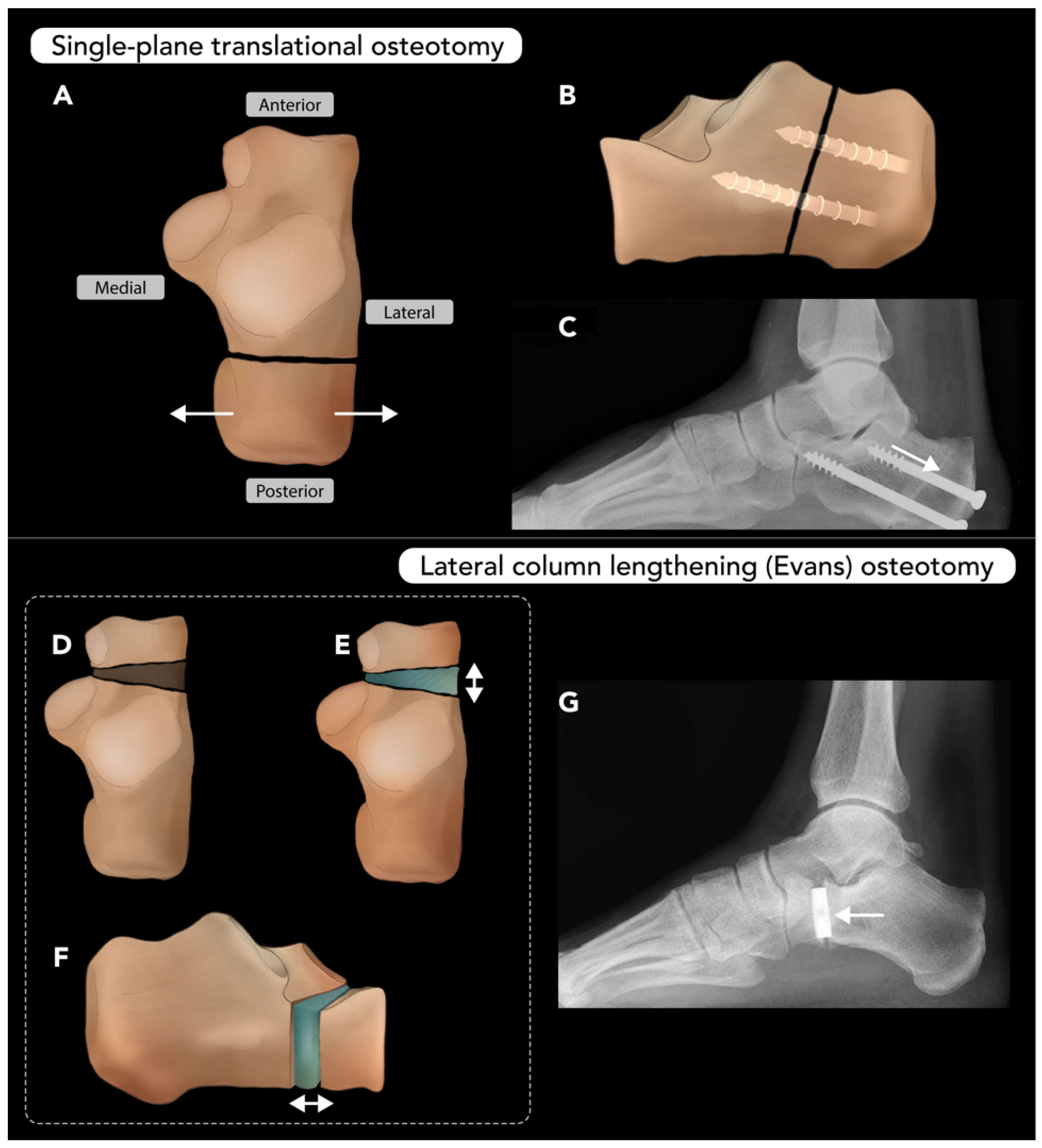
2.3.2. Talar Neck Osteotomy
2.3.3. Calcaneal Osteotomy
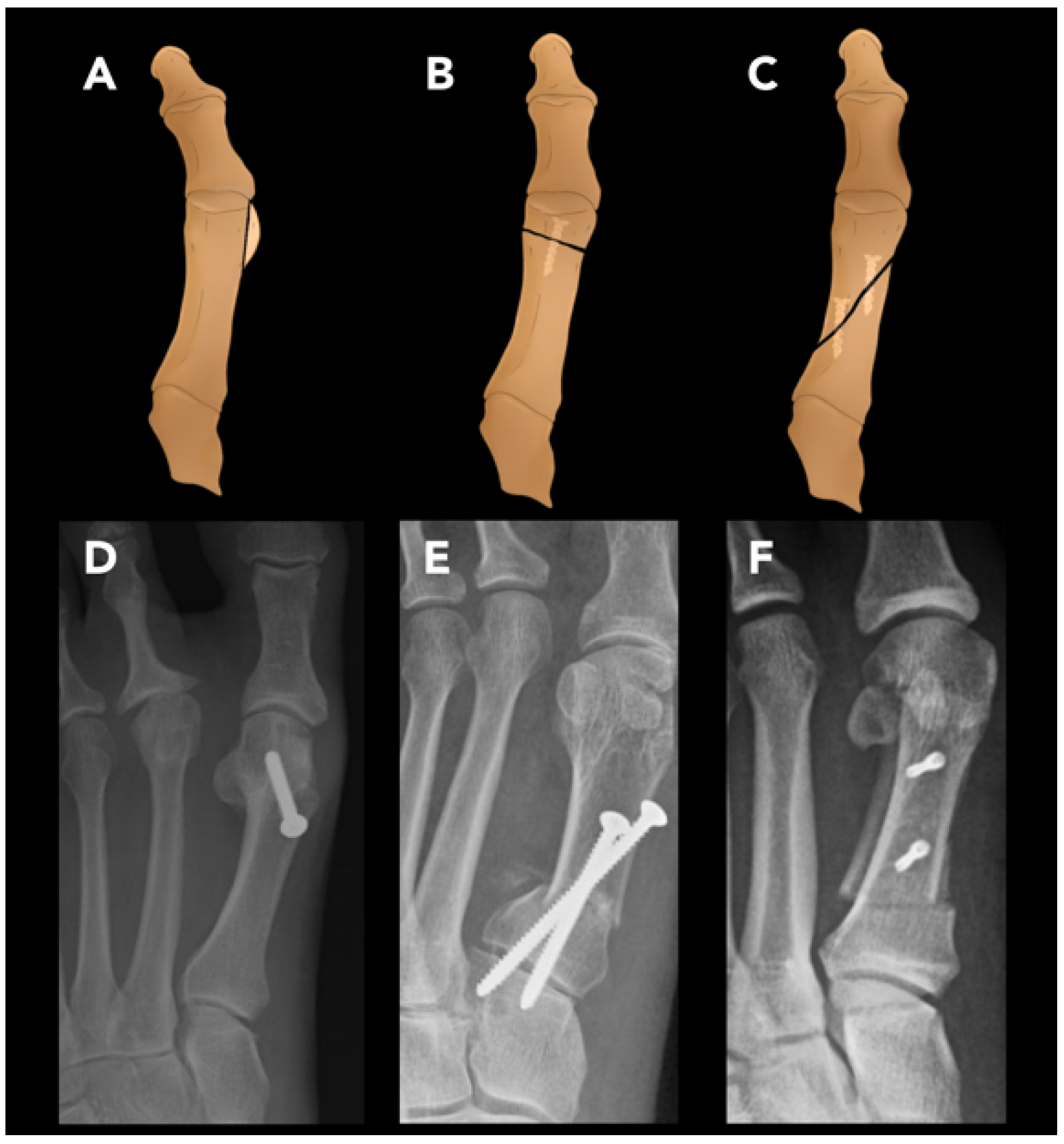
2.3.4. Hallux Valgus Osteotomy
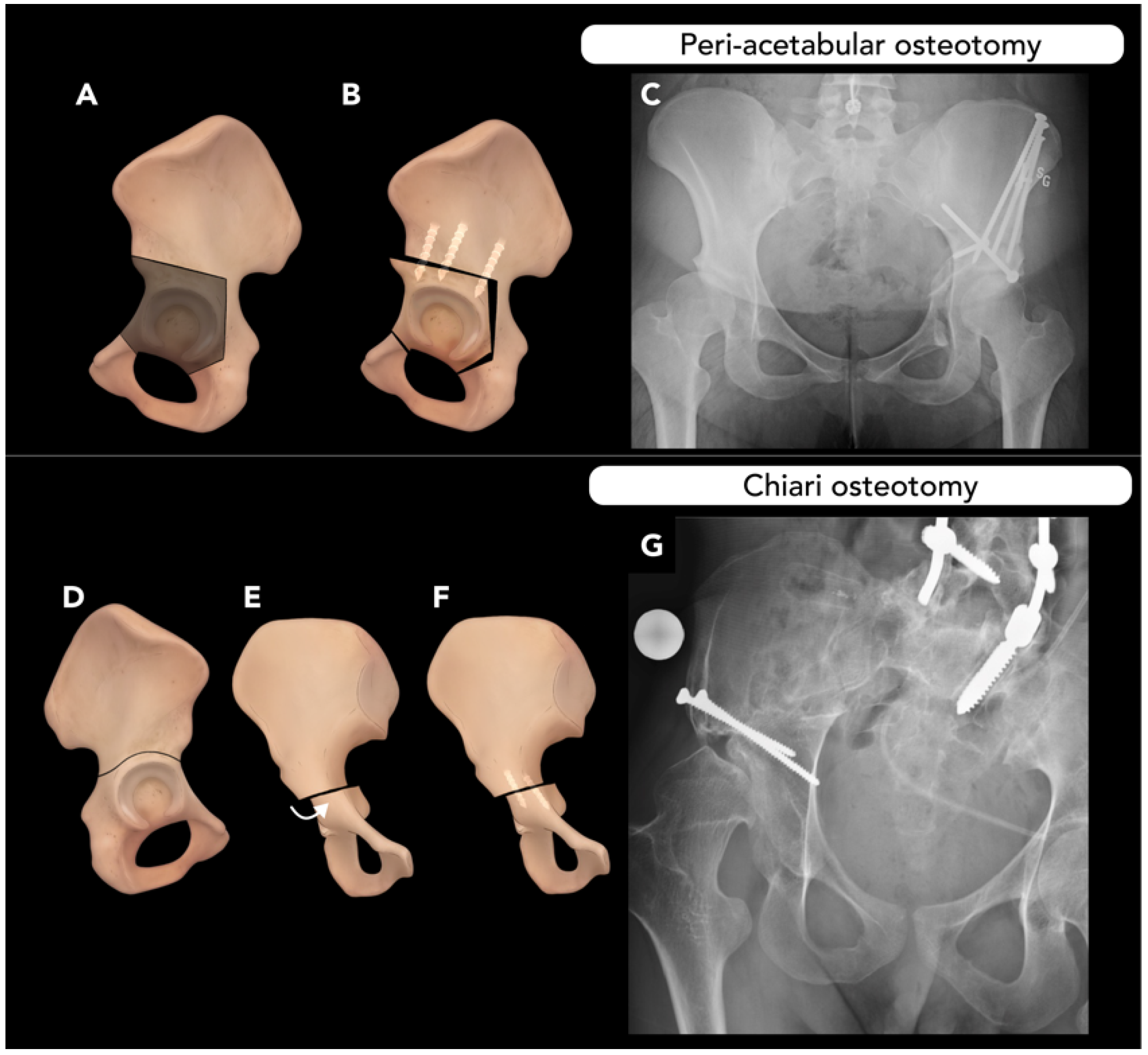
2.4. Pelvic Osteotomy
2.5. Vertebral and Rib Osteotomies
2.5.1. Thoracolumbar Vertebral Osteotomy
2.5.2. Rib Osteotomy
3. Conclusions
4. Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CORA | Center of rotation and angulation |
| ACA | Axis of correction of angulation |
| OWO | Opening-wedge osteotomy |
| CWO | Closing-wedge osteotomy |
| CT | Computed tomography |
| LNO | Lateral nasal osteotomy |
| LTL | Low-to-low |
| LTH | Low-to-high |
| RM | Reduction malarplasty |
| BSSO | Bilateral sagittal split osteotomy |
| IVRO | Intra-oral vertical ramus osteotomy |
| LO | Lingual osteotomy |
| BO | Buccal osteotomy |
| ERO | External rotation osteotomy |
| DISI | Dorsal segmental intercalated instability |
| OA | Osteoarthritis |
| HTO | High tibial osteotomy |
| ACL | Anterior cruciate ligament |
| PCL | Posterior cruciate ligament |
| TNO | Talar neck osteotomy |
| AVN | Avascular necrosis |
| CO | Calcaneal osteotomy |
| HV | Hallux valgus |
| MTP | Metatarsophalangeal |
| HVO | Hallux valgus osteotomy |
| HVA | Hallux valgus angle |
| IMA | Intermetatarsal angle |
| DDH | Developmental dysplasia of hip |
| LCA | Lateral center-edge angle |
| PAO | Peri-acetabular osteotomy |
| VO | Vertebral osteotomy |
| SVA | Sagittal vertical axis |
| PSO | Pedicle subtraction osteotomy |
| BDBO | Bone–disc–bone osteotomy |
| VCR | Vertebral column resection |
| RO | Rib osteotomy |
References
- Paley, D. Principles of Deformity Correction; Springer: Berlin/Heidelberg, Germany, 2014. [Google Scholar]
- Volpin, G.; Shtarker, H. Management of Delayed Union, Non-Union and Mal-Union of Long Bone Fractures. In European Surgical Orthopaedics and Traumatology: The EFORT Textbook; Bentley, G., Ed.; Springer Berlin Heidelberg: Berlin/Heidelberg, Germany, 2014; pp. 241–266. [Google Scholar]
- Pandey, A.; Kumar, J.A. Principles of Osteosynthesis. In Maxillofacial Trauma: A Clinical Guide; Springer: Singapore, 2021; pp. 31–59. [Google Scholar]
- Impieri, L.; Pezzi, A.; Hadad, H.; Peretti, G.M.; Mangiavini, L.; Rossi, N. Orthobiologics in delayed union and non-union of adult long bones fractures: A systematic review. Bone Rep. 2024, 21, 101760. [Google Scholar] [CrossRef] [PubMed]
- Herman, B.V.; Giffin, J.R. High tibial osteotomy in the ACL-deficient knee with medial compartment osteoarthritis. J. Orthop. Traumatol. 2016, 17, 277–285. [Google Scholar] [CrossRef] [PubMed]
- Park, H.; Kim, H.W.; Park, H.-W.; Lee, K.S. Limb angular deformity correction using Dyna-ATC: Surgical technique, calculation method, and clinical outcome. Yonsei Med. J. 2011, 52, 818–830. [Google Scholar] [CrossRef] [PubMed]
- Neiman, R. Focal dome osteotomy for the treatment of diaphyseal malunion of the lower extremity. Medicina 2022, 58, 308. [Google Scholar] [CrossRef]
- Li, Y.; Li, J.; Gan, T.; Gong, H.; Ma, X.; Yin, S.; Qin, B.; Wu, S.; Xie, H.; Zhang, H. Focal dome osteotomy combined with Ilizarov technique for treating femoral multiplanar deformity. Orthop. Surg. 2024, 16, 2230–2241. [Google Scholar] [CrossRef]
- Alaia, E.F.; Burke, C.J.; Alaia, M.J.; Strauss, E.J.; Ciavarra, G.A.; Rossi, I.; Rosenberg, Z.S. Imaging features of iBalance, a new high tibial osteotomy: What the radiologist needs to know. Skelet. Radiol. 2017, 46, 1–6. [Google Scholar] [CrossRef]
- Li, O.L.; Pritchett, S.; Giffin, J.R.; Spouge, A.R. High tibial osteotomy: An update for radiologists. Am. J. Roentgenol. 2022, 218, 701–712. [Google Scholar] [CrossRef]
- Sabharwal, S.; Kumar, A. Methods for assessing leg length discrepancy. Clin. Orthop. Relat. Res. 2008, 466, 2910–2922. [Google Scholar] [CrossRef]
- Hamad, M.N.; Livshetz, I.; Sood, A.; Patetta, M.; Gonzalez, M.H.; Amirouche, F.A. Effects of pelvic obliquity and limb position on radiographic leg length discrepancy measurement: A Sawbones model. J. Exp. Orthop. 2022, 9, 71. [Google Scholar] [CrossRef]
- Goh, G.S.; Kuiper, J.W.; El Khadrawe, T.A.; Jutte, P.C.; Erdoğan, F.; Aitelhadj, L.; Ettema, H.B.; Assi, C. Should Patients Be Weight-Bearing When Obtaining Preoperative Radiographs of the Hip and Knee? J. Arthroplast. 2025, 40, S25–S29. [Google Scholar] [CrossRef]
- Abdollahi, M.; Rashedi, E.; Kuber, P.M.; Jahangiri, S.; Kazempour, B.; Dombovy, M.; Azadeh-Fard, N. Post-Stroke Functional Changes: In-Depth Analysis of Clinical Tests and Motor-Cognitive Dual-Tasking Using Wearable Sensors. Bioengineering 2024, 11, 349. [Google Scholar] [CrossRef]
- van Houten, A.H.; Heesterbeek, P.J.; van Heerwaarden, R.J.; van Tienen, T.G.; Wymenga, A.B. Medial open wedge high tibial osteotomy: Can delayed or nonunion be predicted? Clin. Orthop. Relat. Res. 2014, 472, 1217–1223. [Google Scholar] [CrossRef] [PubMed]
- Nicholson, J.; Yapp, L.; Keating, J.; Simpson, A. Monitoring of fracture healing. Update on current and future imaging modalities to predict union. Injury 2021, 52, S29–S34. [Google Scholar] [CrossRef] [PubMed]
- Sinclair, A.G.; Scoffings, D.J. Imaging of the post-operative cranium. Radiographics 2010, 30, 461–482. [Google Scholar] [CrossRef]
- Yang, J.; Shen, M. Comparison of Craniotomy versus Decompressive Craniectomy for Acute Subdural Hematoma: A Systematic Review and Meta-Analysis. World Neurosurg. 2024, 188, e194–e206. [Google Scholar] [CrossRef]
- Ghanaatpisheh, M.; Sajjadian, A.; Daniel, R.K. Superior rhinoplasty outcomes with precise nasal osteotomy: An individualized approach for maintaining function and achieving aesthetic goals. Aesthetic Surg. J. 2015, 35, 28–39. [Google Scholar] [CrossRef]
- Hontanilla, B.; Cabello, A.; Olivas, J. A predictable approach for osteotomy in rhinoplasty: A new concept of open external osteotomy. Plast. Reconstr. Surg. Glob. Open 2016, 4, e764. [Google Scholar] [CrossRef] [PubMed]
- Mu, X. Experience in East Asian facial recontouring: Reduction malarplasty and mandibular reshaping. Arch. Facial Plast. Surg. 2010, 12, 222–229. [Google Scholar] [CrossRef]
- Choung, J.-W. Rotation technique of reduction malar plasty. J. Craniofacial Surg. 2015, 26, 238–239. [Google Scholar] [CrossRef]
- Monson, L.A. Bilateral sagittal split osteotomy. In Seminars in Plastic Surgery; Thieme Medical Publishers: Stuttgart, Germany, 2013; pp. 145–148. [Google Scholar]
- Böckmann, R.; Meyns, J.; Dik, E.; Kessler, P. The modifications of the sagittal ramus split osteotomy: A literature review. Plast. Reconstr. Surg. Glob. Open 2014, 2, e271. [Google Scholar] [CrossRef]
- Ylikontiola, L.; Moberg, K.; Huumonen, S.; Soikkonen, K.; Oikarinen, K. Comparison of three radiographic methods used to locate the mandibular canal in the buccolingual direction before bilateral sagittal split osteotomy. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontol. 2002, 93, 736–742. [Google Scholar] [CrossRef] [PubMed]
- Zeynalzadeh, F.; Shooshtari, Z.; Eshghpour, M.; Zarch, S.H.H.; Tohidi, E.; Samieirad, S. Dal Pont vs Hunsuck: Which technique can lead to a lower incidence of bad split during bilateral sagittal split osteotomy? A triple-blind randomized clinical trial. World J. Plast. Surg. 2021, 10, 25. [Google Scholar] [CrossRef]
- Rühmann, O.; Lipka, W.; Bohnsack, M. External rotation osteotomy of the humerus for treatment of external rotation deficit in palsies. Oper. Orthopädie und Traumatol. 2008, 20, 145–156. [Google Scholar] [CrossRef] [PubMed]
- Acan, A.E.; Gursan, O.; Demirkiran, N.D.; Havitcioglu, H. Late treatment of obstetrical brachial plexus palsy by humeral rotational osteotomy and lengthening with an intramedullary elongation nail. Acta Orthop. Traumatol. Turc. 2018, 52, 75–80. [Google Scholar] [CrossRef]
- Akinci, M.; Ay, S.; Kamiloglu, S.; Ercetin, O. External rotation osteotomy of the humerus for the treatment of shoulder problems secondary to obstetric brachial plexus palsy. Acta Orthop. Traumatol. Turc. 2005, 39, 328–333. [Google Scholar]
- Menashe, S.J.; Tse, R.; Nixon, J.N.; Ishak, G.E.; Thapa, M.M.; McBroom, J.A.; Iyer, R.S. Brachial plexus birth palsy: Multimodality imaging of spine and shoulder abnormalities in children. Am. J. Roentgenol. 2015, 204, W199–W206. [Google Scholar] [CrossRef]
- Sharifi, A.; Siebert, M.J.; Chhabra, A. How to measure glenoid bone stock and version and why it is important: A practical guide. Radiographics 2020, 40, 1671–1683. [Google Scholar] [CrossRef]
- Bae, D.S.; Waters, P.M. External rotation humeral osteotomy for brachial plexus birth palsy. Tech. Hand Up. Extrem. Surg. 2007, 11, 8–14. [Google Scholar] [CrossRef]
- Abdelgawad, A.A.; Pirela-Cruz, M.A. Humeral rotational osteotomy for shoulder deformity in obstetric brachial plexus palsy: Which direction should I rotate? Open Orthop. J. 2014, 8, 130. [Google Scholar] [CrossRef]
- Roulet, S.; Gubbiotti, L.; Lakhal, W.; Chaves, C.; Marteau, E.; Laulan, J.; Bacle, G. Ulna shortening osteotomy for ulnar impaction syndrome: Impact of distal radioulnar joint morphology on clinical outcome. Orthop. Traumatol. Surg. Res. 2021, 107, 102970. [Google Scholar] [CrossRef]
- Farr, S.; Kalish, L.A.; Bae, D.S.; Waters, P.M. Radiographic criteria for undergoing an ulnar shortening osteotomy in Madelung deformity: A long-term experience from a single institution. J. Pediatr. Orthop. 2016, 36, 310–315. [Google Scholar] [CrossRef] [PubMed]
- Delclaux, S.; Pham, T.T.; Bonnevialle, N.; Aprédoaei, C.; Rongières, M.; Bonnevialle, P.; Mansat, P. Distal radius fracture malunion: Importance of managing injuries of the distal radio-ulnar joint. Orthop. Traumatol. Surg. Res. 2016, 102, 327–332. [Google Scholar] [CrossRef] [PubMed]
- Evans, B.T.; Jupiter, J.B. Best approaches in distal radius fracture malunions. Curr. Rev. Musculoskelet. Med. 2019, 12, 198–203. [Google Scholar] [CrossRef] [PubMed]
- de Muinck Keizer, R.; Lechner, K.; Mulders, M.; Schep, N.; Eygendaal, D.; Goslings, J. Three-dimensional virtual planning of corrective osteotomies of distal radius malunions: A systematic review and meta-analysis. Strateg. Trauma Limb Reconstr. 2017, 12, 77–89. [Google Scholar]
- Peterson, B.; Gajendran, V.; Szabo, R.M. Corrective osteotomy for deformity of the distal radius using a volar locking plate. Hand 2008, 3, 61–68. [Google Scholar] [CrossRef]
- Haines, S.C.; Bott, A. Current Concepts: Corrective Osteotomy for Extra-Articular Deformity Following a Distal Radius Fracture. Cureus 2023, 15, e47019. [Google Scholar] [CrossRef]
- Trumble, T.E.; Gilbert, M.; Murray, L.W.; Smith, J.; McCallister, W.V. Displaced Scaphoid Fractures Treated with Open Reductio and Internal Fixation with a Cannulated Screw. JBJS 2000, 82, 633. [Google Scholar] [CrossRef]
- Meaike, J.J.; Meaike, J.D.; Collins, M.S.; Bishop, A.T.; Shin, A.Y. Utility of preoperative MRI for assessing proximal fragment vascularity in scaphoid nonunion. Bone Jt. J. 2023, 105, 657–662. [Google Scholar] [CrossRef]
- Kawamura, K.; Chung, K.C. Treatment of scaphoid fractures and nonunions. J. Hand Surg. 2008, 33, 988–997. [Google Scholar] [CrossRef]
- Bervian, M.R.; Ribak, S.; Livani, B. Scaphoid fracture nonunion: Correlation of radiographic imaging, proximal fragment histologic viability evaluation, and estimation of viability at surgery: Diagnosis of scaphoid pseudarthrosis. Int. Orthop. 2015, 39, 67–72. [Google Scholar] [CrossRef]
- Shomal Zadeh, F.; Azhideh, A.; Mantilla, J.G.; Kosaraju, V.; Venugopal, N.; Gaskin, C.M.; Pooyan, A.; Alipour, E.; Chalian, M. Imaging features of intraosseous schwannoma: A case series and review of the literature. Diagnostics 2023, 13, 1610. [Google Scholar] [CrossRef]
- El-Karef, E.A. Corrective osteotomy for symptomatic scaphoid malunion. Injury 2005, 36, 1440–1448. [Google Scholar] [CrossRef] [PubMed]
- Buijze, G.; Bulstra, A.; Ho, P. Management of complications of scaphoid fracture fixation. In Management of Complications in Common Hand and Wrist Procedures: FESSH Instructional Course Book; Thieme Medical Pub: Stuttgart, Germany, 2021; Volume 1, pp. 89–104. [Google Scholar]
- Babatunde, O.M.; Danoff, J.R.; Patrick, D.A., Jr.; Lee, J.H.; Kazam, J.K.; Macaulay, W. The combination of the tunnel view and weight-bearing anteroposterior radiographs improves the detection of knee arthritis. Arthritis 2016, 2016, 9786924. [Google Scholar] [CrossRef]
- Brown, G.A.; Amendola, A. Radiographic evaluation and preoperative planning for high tibial osteotomies. Oper. Tech. Sports Med. 2012, 20, 93–102. [Google Scholar] [CrossRef]
- Chahla, J.; Dean, C.S.; Mitchell, J.J.; Moatshe, G.; Cruz, R.S.; LaPrade, R.F. Medial opening wedge proximal tibial osteotomy. Arthrosc. Tech. 2016, 5, e919–e928. [Google Scholar] [CrossRef]
- Luís, N.M.; Varatojo, R. Radiological assessment of lower limb alignment. EFORT Open Rev. 2021, 6, 487–494. [Google Scholar] [CrossRef] [PubMed]
- Wolcott, M.; Traub, S.; Efird, C. High tibial osteotomies in the young active patient. Int. Orthop. 2010, 34, 161–166. [Google Scholar] [CrossRef]
- Wang, J.-W.; Hsu, C.-C. Distal femoral varus osteotomy for osteoarthritis of the knee. JBJS 2005, 87, 127–133. [Google Scholar] [CrossRef]
- Kunze, K.N.; Beletsky, A.; Hannon, C.P.; LaPrade, R.F.; Yanke, A.B.; Cole, B.J.; Forsythe, B.; Chahla, J. Return to work and sport after proximal tibial osteotomy and the effects of opening versus closing wedge techniques on adverse outcomes: A systematic review and meta-analysis. Am. J. Sports Med. 2020, 48, 2295–2304. [Google Scholar] [CrossRef]
- Cotter, E.J.; Gowd, A.K.; Bohl, D.D.; Getgood, A.; Cole, B.J.; Frank, R.M. Medical comorbidities and functional dependent living are independent risk factors for short-term complications following osteotomy procedures about the knee. Cartilage 2020, 11, 423–430. [Google Scholar] [CrossRef]
- Vena, G.; D’Adamio, S.; Amendola, A. Complications of osteotomies about the knee. Sports Med. Arthrosc. Rev. 2013, 21, 113–120. [Google Scholar] [CrossRef] [PubMed]
- Rossi, R.; Bonasia, D.E.; Amendola, A. The role of high tibial osteotomy in the varus knee. JAAOS—J. Am. Acad. Orthop. Surg. 2011, 19, 590–599. [Google Scholar] [CrossRef]
- Rosso, F.; Margheritini, F. Distal femoral osteotomy. Curr. Rev. Musculoskelet. Med. 2014, 7, 302–311. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-W.; Xin, Y.-Z.; Ji, J.-H.; Panchal, K.; Kwon, O.-S.; Yang, S.-J. Stress analysis of the tibial plateau according to the difference of blade path entry in opening wedge high tibial osteotomy. J. Mech. Sci. Technol. 2015, 29, 1175–1179. [Google Scholar] [CrossRef]
- Ruzbarsky, J.J.; Dare, D.M.; Marx, R.G. Closing verses opening wedge high tibial osteotomy: An evidence-based review. HSS J. 2015, 11, 291–293. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Lin, J.; Jin, Z.; Cai, X.; Gao, W. Comparison of clinical and radiological outcomes between opening-wedge and closing-wedge high tibial osteotomy: A comprehensive meta-analysis. PLoS ONE 2017, 12, e0171700. [Google Scholar] [CrossRef]
- Takeuchi, R.; Ishikawa, H.; Kumagai, K.; Yamaguchi, Y.; Chiba, N.; Akamatsu, Y.; Saito, T. Fractures around the lateral cortical hinge after a medial opening-wedge high tibial osteotomy: A new classification of lateral hinge fracture. Arthrosc. J. Arthrosc. Relat. Surg. 2012, 28, 85–94. [Google Scholar] [CrossRef]
- Daniels, T.R.; Smith, J.W.; Ross, T.I. Varus malalignment of the talar neck. Its effect on the position of the foot and on subtalar motion. JBJS 1996, 78, 1559–1567. [Google Scholar] [CrossRef]
- Vallier, H.A.; Nork, S.E.; Benirschke, S.K.; Sangeorzan, B.J. Surgical treatment of talar body fractures. JBJS 2003, 85, 1716–1724. [Google Scholar] [CrossRef]
- Azhideh, A.; Pouramini, A.; Haseli, S.; Abbaspour, E.; Karande, G.; Kafi, F.; Chalian, M. Radiological assessment of extremity bone involvement in Erdheim-Chester disease: A systematic review of case reports. Skelet. Radiol. 2024, 1–15. [Google Scholar] [CrossRef]
- Zwipp, H.; Rammelt, S. Posttraumatic deformity correction at the foot. Zentralblatt Fur. Chir. 2003, 128, 218–226. [Google Scholar] [CrossRef] [PubMed]
- Rammelt, S.; Zwipp, H. Talar neck and body fractures. Injury 2009, 40, 120–135. [Google Scholar] [CrossRef]
- Whitaker, C.; Turvey, B.; Illical, E.M. Current concepts in talar neck fracture management. Curr. Rev. Musculoskelet. Med. 2018, 11, 456–474. [Google Scholar] [CrossRef]
- Meyr, A.J.; Sansosti, L.E.; Ali, S. A pictorial review of reconstructive foot and ankle surgery: Evaluation and intervention of the flatfoot deformity. J. Radiol. Case Rep. 2017, 11, 26. [Google Scholar] [CrossRef] [PubMed]
- Soltanolkotabi, M.; Mallory, C.; Allen, H.; Chan, B.Y.; Mills, M.K.; Leake, R.L. Postoperative Findings of Common Foot and Ankle Surgeries: An Imaging Review. Diagnostics 2022, 12, 1090. [Google Scholar] [CrossRef]
- Tennant, J.N.; Carmont, M.; Phisitkul, P. Calcaneus osteotomy. Curr. Rev. Musculoskelet. Med. 2014, 7, 271–276. [Google Scholar] [CrossRef]
- Saltzman, C.L.; El-Khoury, G.Y. The hindfoot alignment view. Foot Ankle Int. 1995, 16, 572–576. [Google Scholar] [CrossRef] [PubMed]
- Greenfield, S.; Cohen, B. Calcaneal osteotomies: Pearls and pitfalls. Foot Ankle Clin. 2017, 22, 563–571. [Google Scholar] [CrossRef]
- Zirngibl, B.; Grifka, J.; Baier, C.; Götz, J. Hallux valgus. Der Orthopäde 2017, 46, 283–296. [Google Scholar] [CrossRef]
- Heineman, N.; Liu, G.; Pacicco, T.; Dessouky, R.; Wukich, D.K.; Chhabra, A. Clinical and imaging assessment and treatment of hallux valgus. Acta Radiol. 2020, 61, 56–66. [Google Scholar] [CrossRef]
- Salet, E.; Legghe, B.; Barouk, P.; Stigliz, Y.; Dallaudiere, B.; Lintingre, P.-F.; Pesquer, L. Imaging of the post-operative hallux valgus: What do radiologists need to know? Skelet. Radiol. 2023, 52, 1629–1637. [Google Scholar] [CrossRef]
- Phillips, M.; Krackow, K. High tibial osteotomy and distal femoral osteotomy for valgus or varus deformity around the knee. Instr. Course Lect. 1998, 47, 429–436. [Google Scholar] [PubMed]
- Venkatadass, K.; Prasad, V.D.; Al Ahmadi, N.M.M.; Rajasekaran, S. Pelvic osteotomies in hip dysplasia: Why, when and how? EFORT Open Rev. 2022, 7, 153–163. [Google Scholar] [CrossRef]
- Starr, V.; Ha, B.Y. Imaging update on developmental dysplasia of the hip with the role of MRI. Am. J. Roentgenol. 2014, 203, 1324–1335. [Google Scholar] [CrossRef] [PubMed]
- Mills, M.K.; Strickland, C.D.; Jesse, M.K.; Lowry, P.A.; Mei-Dan, O.; Flug, J.A. Postoperative imaging in the setting of hip preservation surgery. Radiographics 2016, 36, 1746–1758. [Google Scholar] [CrossRef]
- Beck, M.; Lash, N.J.; Ganz, R. Surgical Technique: Periacetabular Osteotomy. In Hip Arthroscopy and Hip Joint Preservation Surgery; Springer: Berlin/Heidelberg, Germany, 2022; pp. 663–682. [Google Scholar]
- Hsu, J.-Y.; Lee, C.-C.; Lin, S.-C.; Wang, T.-M.; Kuo, K.N.; Wu, K.-W. Radiographic outcomes of ganz versus modified triple osteotomies in femoral head medialization and coverage in acetabular dysplasia. J. Clin. Med. 2022, 11, 1924. [Google Scholar] [CrossRef] [PubMed]
- Shibata, K.R.; Matsuda, S.; Safran, M.R. Open treatment of dysplasia—Other than PAO: Does it have to be a PAO? J. Hip Preserv. Surg. 2017, 4, 131–144. [Google Scholar] [CrossRef]
- Vrgoč, G.; Bulat, S.; Vuletić, F. Role of Chiari osteotomy in treating degenerative hip arthritis: A review. J. Clin. Orthop. Trauma 2022, 24, 101687. [Google Scholar] [CrossRef]
- Weber, M.-A.; Egermann, M.; Thierjung, H.; Kloth, J. Modern radiological postoperative diagnostics of the hip joint in children and adults. In RöFo-Fortschritte Auf Dem Gebiet der Röntgenstrahlen und der bildgebenden Verfahren; Georg Thieme Verlag KG: Stuttgart, Germany, 2015; pp. 525–542. [Google Scholar]
- Fabry, G.; Macewen, G.D.; Shands, A., Jr. Torsion of the femur: A follow-up study in normal and abnormal conditions. JBJS 1973, 55, 1726–1738. [Google Scholar] [CrossRef]
- Nelitz, M.; Dreyhaupt, J.; Williams, S.R.M.; Dornacher, D. Combined supracondylar femoral derotation osteotomy and patellofemoral ligament reconstruction for recurrent patellar dislocation and severe femoral anteversion syndrome: Surgical technique and clinical outcome. Int. Orthop. 2015, 39, 2355–2362. [Google Scholar] [CrossRef]
- Rippstein, J. Determination of the antetorsion of the femur neck by means of two x-ray pictures. Z. Fur. Orthop. und Ihre Grenzgeb. 1955, 86, 345–360. [Google Scholar]
- Nelitz, M. Femoral derotational osteotomies. Curr. Rev. Musculoskelet. Med. 2018, 11, 272–279. [Google Scholar] [CrossRef] [PubMed]
- Schwab, F.; Blondel, B.; Chay, E.; Demakakos, J.; Lenke, L.; Tropiano, P.; Ames, C.; Smith, J.S.; Shaffrey, C.I.; Glassman, S. The comprehensive anatomical spinal osteotomy classification. Neurosurgery 2015, 76, S33–S41. [Google Scholar] [CrossRef] [PubMed]
- Schwab, F.J.; Blondel, B.; Bess, S.; Hostin, R.; Shaffrey, C.I.; Smith, J.S.; Boachie-Adjei, O.; Burton, D.C.; Akbarnia, B.A.; Mundis, G.M. Radiographical spinopelvic parameters and disability in the setting of adult spinal deformity: A prospective multicenter analysis. Spine 2013, 38, E803–E812. [Google Scholar] [CrossRef]
- Kose, K.C.; Bozduman, O.; Yenigul, A.E.; Igrek, S. Spinal osteotomies: Indications, limits and pitfalls. EFORT Open Rev. 2017, 2, 73–82. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, T.; Kainth, D.; Marette, S.; Polly, D. Alphabet Soup: Sagittal Balance Correction Osteotomies of the Spine—What Radiologists Should Know. Am. J. Neuroradiol. 2018, 39, 606–611. [Google Scholar] [CrossRef] [PubMed]
- Bridwell, K.H. Decision making regarding Smith-Petersen vs. pedicle subtraction osteotomy vs. vertebral column resection for spinal deformity. Spine 2006, 31, S171–S178. [Google Scholar] [CrossRef]
- Corona-Cedillo, R.; Saavedra-Navarrete, M.-T.; Espinoza-Garcia, J.-J.; Mendoza-Aguilar, A.-N.; Ternovoy, S.K.; Roldan-Valadez, E. Imaging assessment of the postoperative spine: An updated pictorial review of selected complications. BioMed Res. Int. 2021, 2021, 9940001. [Google Scholar] [CrossRef]
- Dorward, I.G.; Lenke, L.G. Osteotomies in the posterior-only treatment of complex adult spinal deformity: A comparative review. Neurosurg. Focus 2010, 28, E4. [Google Scholar] [CrossRef]
- Deslauriers, J.; Mehran, R.J. Posterolateral thoracotomy. Oper. Tech. Thorac. Cardiovasc. Surg. 2003, 8, 51–57. [Google Scholar] [CrossRef]
- Thongtrangan, I.; Le, H.N.; Park, J.; Kim, D.H. Thoracoabdominal approach. In Surgical Anatomy & Techniques to the Spine; Elsevier: Amsterdam, The Netherlands, 2006; pp. 89–100. [Google Scholar]
- Hachenberg, T.; Loop, T. Postthoracotomy complications. In Cohen’s Comprehensive Thoracic Anesthesia; Elsevier: Amsterdam, The Netherlands, 2022; pp. 376–391. [Google Scholar]







Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Azhideh, A.; Hosseini, N.; Haseli, S.; Park, C.; Venugopal, N.; Abadi, A.; Masroori, Z.; Chen, E.; Miller, Z.; Camacho, D.; et al. Osteotomies: Indications, Imaging Appearance, Surgical Techniques, and Complications. Diagnostics 2025, 15, 1184. https://doi.org/10.3390/diagnostics15101184
Azhideh A, Hosseini N, Haseli S, Park C, Venugopal N, Abadi A, Masroori Z, Chen E, Miller Z, Camacho D, et al. Osteotomies: Indications, Imaging Appearance, Surgical Techniques, and Complications. Diagnostics. 2025; 15(10):1184. https://doi.org/10.3390/diagnostics15101184
Chicago/Turabian StyleAzhideh, Arash, Nastaran Hosseini, Sara Haseli, Chankue Park, Nitin Venugopal, Ali Abadi, Zahra Masroori, Eric Chen, Zachary Miller, David Camacho, and et al. 2025. "Osteotomies: Indications, Imaging Appearance, Surgical Techniques, and Complications" Diagnostics 15, no. 10: 1184. https://doi.org/10.3390/diagnostics15101184
APA StyleAzhideh, A., Hosseini, N., Haseli, S., Park, C., Venugopal, N., Abadi, A., Masroori, Z., Chen, E., Miller, Z., Camacho, D., & Chalian, M. (2025). Osteotomies: Indications, Imaging Appearance, Surgical Techniques, and Complications. Diagnostics, 15(10), 1184. https://doi.org/10.3390/diagnostics15101184





