The Impact of Automatic Exposure Control Technology on the In Vivo Radiation Dose in Digital Mammography: A Comparison Between Different Systems and Target/Filter Combinations
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Collection
2.2. Assessing Dose Variation Among Mammography Machines
2.3. Analysis of AEC Optimization of Acquisition Parameters Across Mammography Systems
3. Results
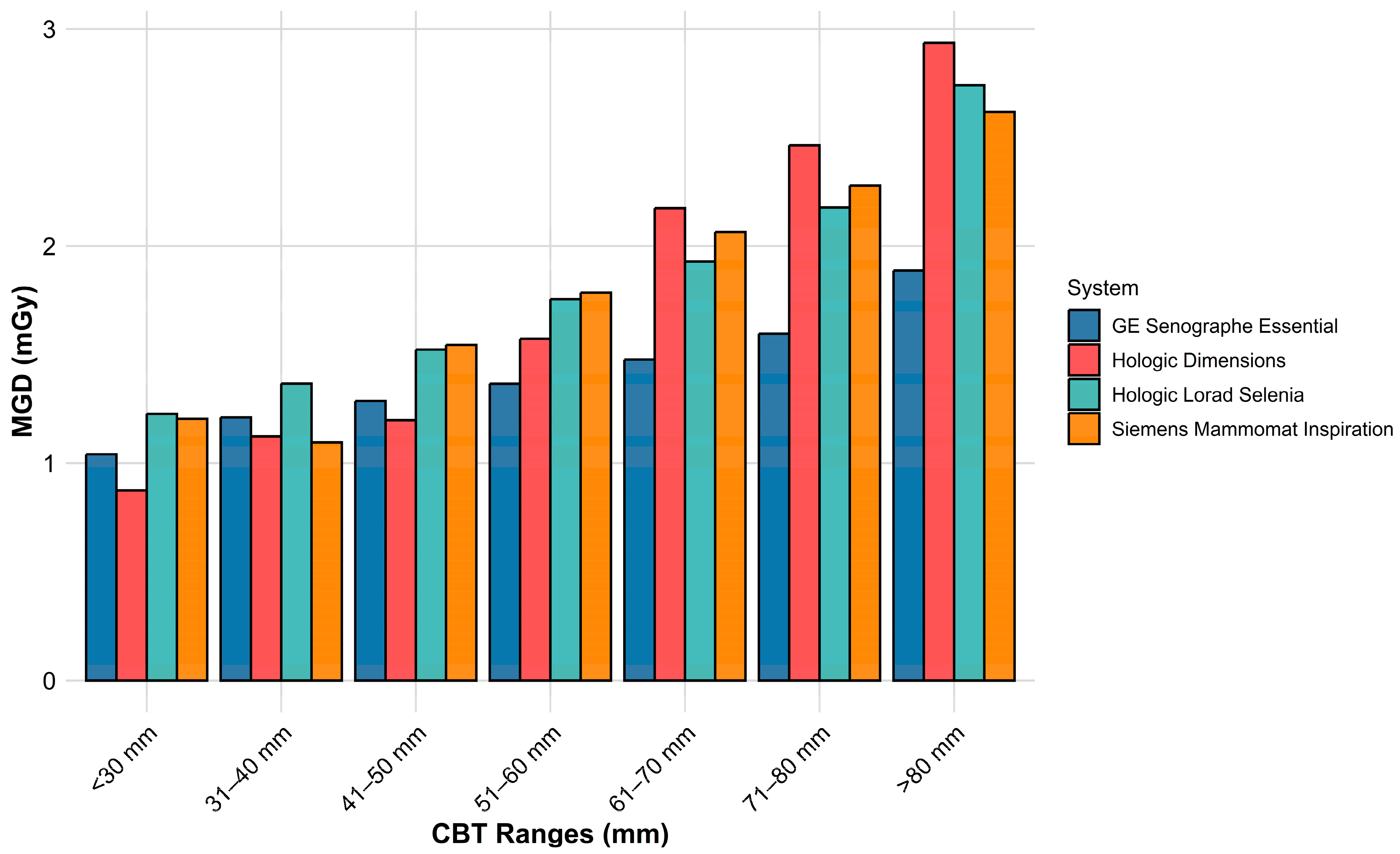
3.1. Variation in MGD Across Machines
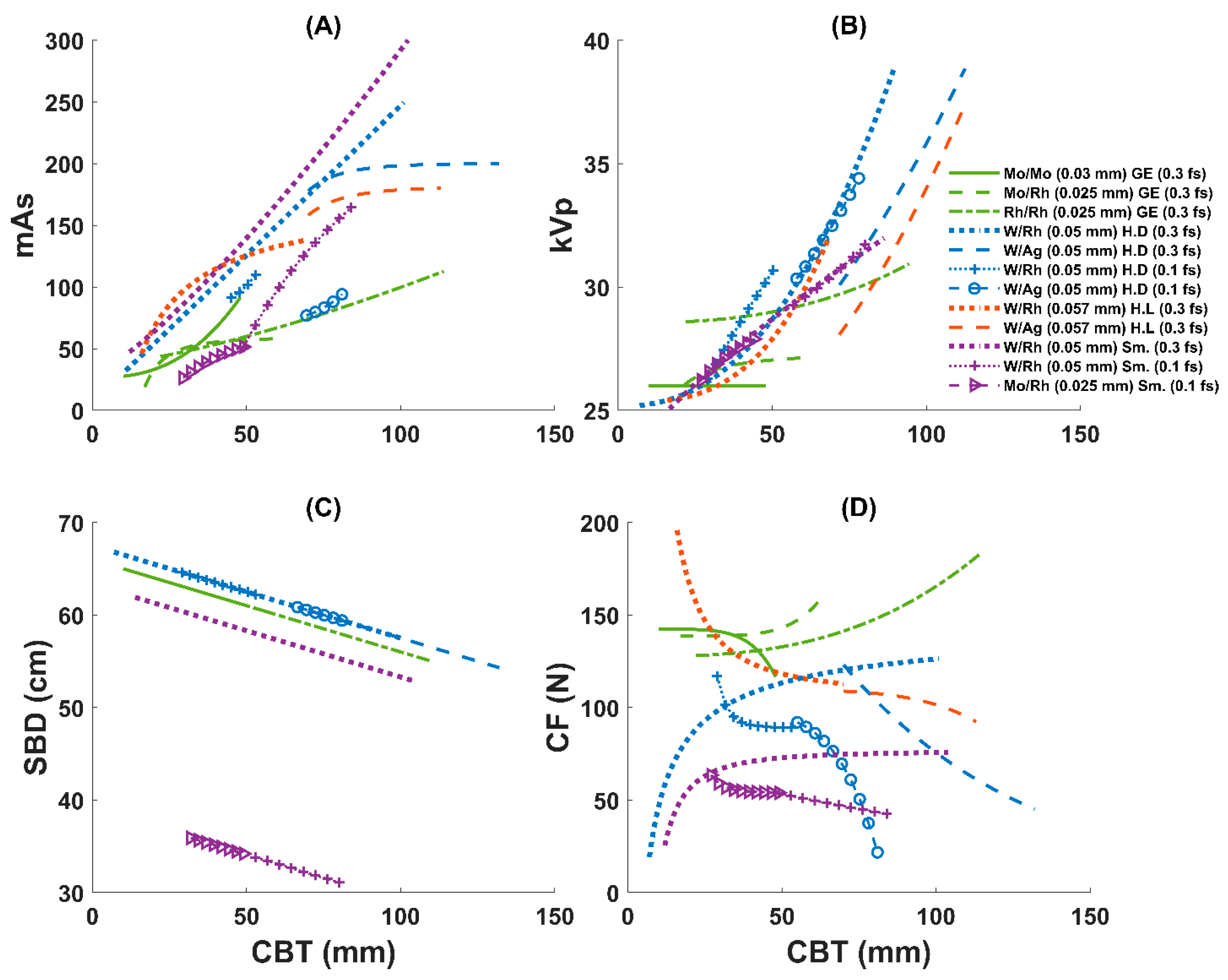
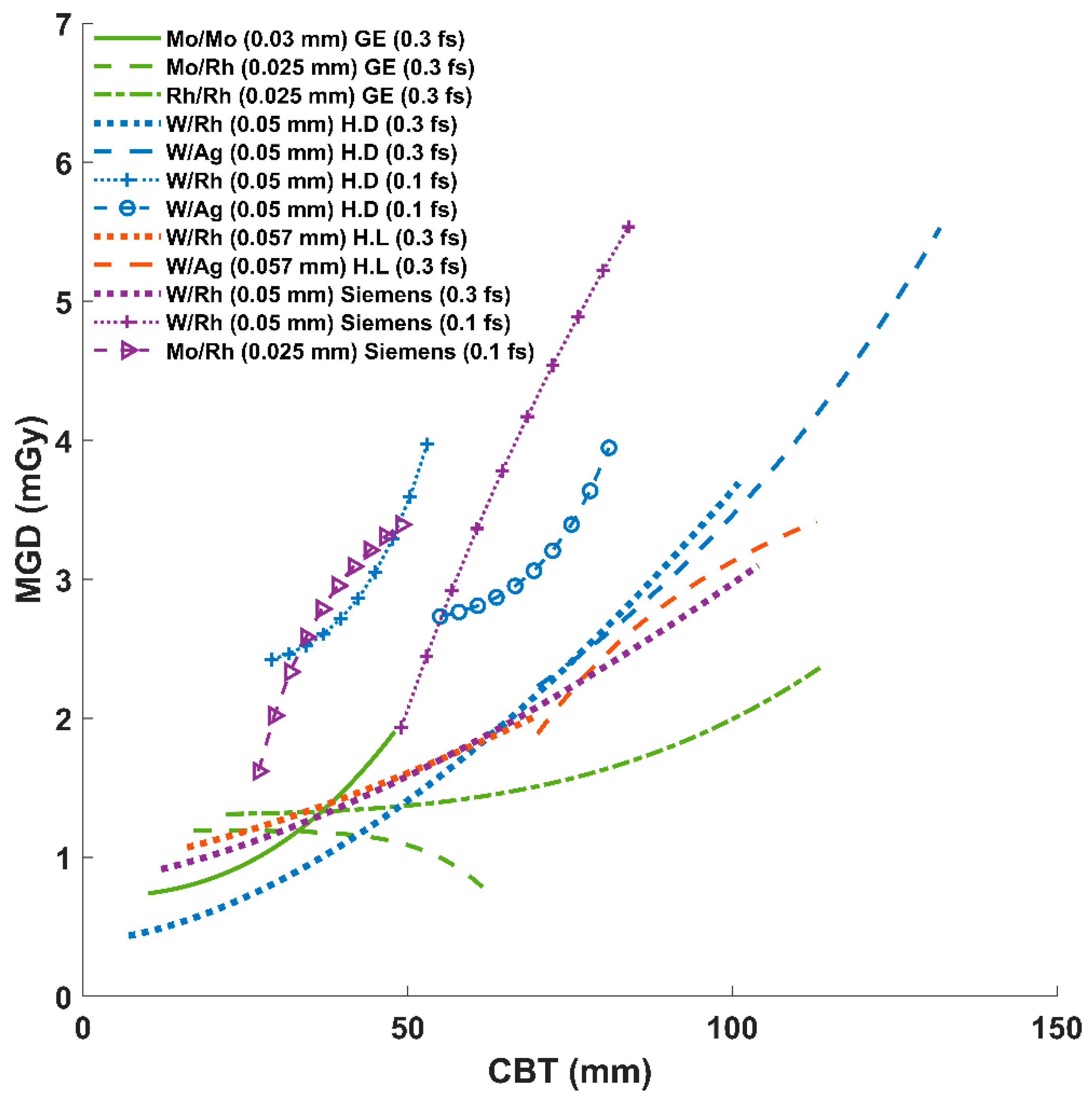
3.2. AEC Parameter Optimization Variation Across Systems
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- WHO. A Short Guide to Cancer Screening: Increase Effectiveness, Maximize Benefits and Minimize Harm; World Health Organization, Regional Office for Europe: Copenhagen, Denmark, 2022; ISBN 9289057564. [Google Scholar]
- Monticciolo, D.L.; Malak, S.F.; Friedewald, S.M.; Eby, P.R.; Newell, M.S.; Moy, L.; Destounis, S.; Leung, J.W.T.; Hendrick, R.E.; Smetherman, D. Breast cancer screening recommendations inclusive of all women at average risk: Update from the ACR and Society of Breast Imaging. J. Am. Coll. Radiol. 2021, 18, 1280–1288. [Google Scholar] [CrossRef] [PubMed]
- Bicchierai, G.; Di Naro, F.; De Benedetto, D.; Cozzi, D.; Pradella, S.; Miele, V.; Nori, J. A review of breast imaging for timely diagnosis of disease. Int. J. Environ. Res. Public Health 2021, 18, 5509. [Google Scholar] [CrossRef] [PubMed]
- Pauwels, E.K.J.; Foray, N.; Bourguignon, M.H. Breast cancer induced by X-ray mammography screening? A review based on recent understanding of low-dose radiobiology. Med. Princ. Pract. 2016, 25, 101–109. [Google Scholar] [CrossRef] [PubMed]
- Dance, D.R. Monte-Carlo calculation of conversion factors for the estimation of mean glandular breast dose. Phys. Med. Biol. 1990, 35, 1211–1219. [Google Scholar] [CrossRef] [PubMed]
- ICRP. Diagnostic reference levels in medical imaging. Ann. ICRP 2017, 46, 1–144. [Google Scholar] [CrossRef] [PubMed]
- IAEA. Patient Radiation Exposure Monitoring in Medical Imaging; International Atomic Energy Agency: Vienna, Austria, 2023; ISBN 9201493223. [Google Scholar]
- Albeshan, S.M.; Alhulail, A.A.; Almuqbil, M.M. Reporting average glandular dose and its associations with age and exposure parameters. Radiat. Phys. Chem. 2024, 223, 112004. [Google Scholar] [CrossRef]
- Alakhras, M.M.; Mello-Thoms, C.; Bourne, R.; Rickard, M.; Diffey, J.; Brennan, P.C. Radiation dose differences between digital mammography and digital breast tomosynthesis are dependent on breast thickness. In Proceedings of the Medical Imaging 2016: Physics of Medical Imaging, San Diego, CA, USA, 28 February–2 March 2016; SPIE: Bellingham, WA, USA, 2016; Volume 9783, pp. 697–703. [Google Scholar]
- Bick, U.; Diekmann, F. Digital Mammography; Springer: Berlin, Germany, 2010; ISBN 3540784500. [Google Scholar]
- Williams, M.B.; Raghunathan, P.; More, M.J.; Seibert, J.A.; Kwan, A.; Lo, J.Y.; Samei, E.; Ranger, N.T.; Fajardo, L.L.; McGruder, A. Optimization of exposure parameters in full field digital mammography. Med. Phys. 2008, 35, 2414–2423. [Google Scholar] [CrossRef] [PubMed]
- Berns, E.A.; Hendrick, R.E.; Cutter, G.R. Optimization of technique factors for a silicon diode array full-field digital mammography system and comparison to screen-film mammography with matched average glandular dose. Med. Phys. 2003, 30, 334–340. [Google Scholar] [CrossRef] [PubMed]
- Bernhardt, P.; Mertelmeier, T.; Hoheisel, M. X-ray spectrum optimization of full-field digital mammography: Simulation and phantom study. Med. Phys. 2006, 33, 4337–4349. [Google Scholar] [CrossRef] [PubMed]
- Huda, W.; Sajewicz, A.M.; Ogden, K.M.; Dance, D.R. Experimental investigation of the dose and image quality characteristics of a digital mammography imaging system. Med. Phys. 2003, 30, 442–448. [Google Scholar] [CrossRef] [PubMed]
- Dance, D.R.; Thilander, A.K.; Sandborg, M.; Skinner, C.L.; Castellano, I.A.; Carlsson, G.A. Influence of anode/filter material and tube potential on contrast, signal-to-noise ratio and average absorbed dose in mammography: A Monte Carlo study. Br. J. Radiol. 2000, 73, 1056–1067. [Google Scholar] [CrossRef] [PubMed]
- Baldelli, P.; Phelan, N.; Egan, G. Investigation of the effect of anode/filter materials on the dose and image quality of a digital mammography system based on an amorphous selenium flat panel detector. Br. J. Radiol. 2010, 83, 290–295. [Google Scholar] [CrossRef] [PubMed]
- Gholamkar, L.; Mowlavi, A.A.; Sadeghi, M.; Athari, M. Assessment of mean glandular dose in mammography system with different anode-filter combinations using MCNP code. Iran. J. Radiol. 2016, 13, e36484. [Google Scholar] [CrossRef] [PubMed]
- Kruger, R.L.; Schueler, B.A. A survey of clinical factors and patient dose in mammography. Med. Phys. 2001, 28, 1449–1454. [Google Scholar] [CrossRef] [PubMed]
- Kanaga, K.C.; Yap, H.H.; Laila, S.E.; Sulaiman, T.; Zaharah, M.; Shantini, A.A. A critical comparison of three full field digital mammography systems using figure of merit. Med. J. Malaysia 2010, 65, 119–122. [Google Scholar] [PubMed]
- Gershan, V.; Ristova, M.; Ivanovski, Z. Evaluation of the hologic selenia ffdm system with tungsten tube. Invit. Pap. 2012, 167, 167–170. [Google Scholar]
- Waade, G.G.; Moshina, N.; Sebuødegård, S.; Hogg, P.; Hofvind, S. Compression forces used in the Norwegian Breast Cancer Screening Program. Br. J. Radiol. 2017, 90, 20160770. [Google Scholar] [CrossRef] [PubMed]

| Manufacturer | Model | Number of Images | Target/Filter (Filter Thickness) | CBT [mm] | CF [N] | kVp | mAs | SBD [cm] | MGD [mGy] |
|---|---|---|---|---|---|---|---|---|---|
| Mean ± SD (Range) | |||||||||
| GE | Senographe Essential * | 168,000 | Mo/Mo (0.03 mm) [0.64%] | 31.8 ± 7.5 (10–48) | 138 ± 42 (30–200) | 26 ± 0.3 (25–27) | 51 ± 18 (5–113) | 63 ± 1 (61–65) | 1.2 ± 0.4 (0.15–2.3) |
| Mo/Rh (0.025 mm) [15.52%] | 43 ± 7 (17–63) | 141 ± 37 (30–270) | 27 ± 0.5 (26–28) | 56 ± 14 (6–230) | 62 ± 1 (60–64) | 1.1 ± 0.3 (0.16–3.7) | |||
| Rh/Rh (0.025 mm) [83.84%] | 61 ± 10 (22–114) | 138 ± 37 (20–270) | 29 ± 0.6 (27–31) | 69 ± 19 (2–335) | 60 ± 1 (55–64) | 1.5 ± 0.3 (0.12–7.8) | |||
| All combinations | 58 ± 12 (10–114) | 138 ± 37 (20–270) | 29 ± 1.1 (25–31) | 67 ± 19 (2–335) | 60 ± 1 (55–65) | 1.4 ± 0.4 (0.12–7.8) | |||
| Hologic | Selenia Dimensions | 2660 | W/Rh (0.05 mm) [f.s: 0.3; 86.6%] | 55 ± 11 (7–101) | 115 ± 46 (18–312) | 30 ± 2 (25–39) | 140 ± 53 (31–462) | 62 ± 1 (57–67) | 1.6 ± 0.7 (0.4–6.3) |
| W/Ag (0.05 mm) [f.s: 0.3; 11.4%] | 77 ± 8 (70–132) | 111 ± 48 (25.4–301) | 31 ± 2 (30–39) | 190 ± 59 (57–477) | 60 ± 1 (54–61) | 2.6 ± 0.9 (0.7–6.9) | |||
| W/Rh (0.05 mm) [f.s: 0.1; 1.1%] | 41 ± 6 (29–53) | 92 ± 33 (32–150) | 29 ± 1 (26–31) | 89 ± 16 (67–119) | 63 ± 1 (62–64) | 2.9 ± 0.6 (2–4.2) | |||
| W/Ag (0.05 mm) [f.s: 0.1; 0.9%] | 65 ± 7 (55–81) | 74 ± 38 (21–158) | 32 ± 1 (30–35) | 76 ± 21 (54–134) | 61 ± 0.7 (59–62) | 3 ± 1 (1.6–5.0) | |||
| All combinations | 57 ± 13 (7–132) | 114 ± 46 (18–312) | 30 ± 2 (25–39) | 145 ± 56 (31–477) | 62 ± 1 (54–67) | 1.8 ± 0.8 (0.4–6.9) | |||
| Hologic | Lorad Selenia * | 23,168 | W/Rh (0.057 mm) [80.2%] | 53 ± 10 (16–70) | 123 ± 55 (44–325) | 29 ± 2 (25–32) | 128 ± 39 (15–378) | NA | 1.7 ± 0.5 (0.2–5.9) |
| W/Ag (0.057 mm) [19.8%] | 77 ± 6 (70–113) | 111 ± 35 (44–320) | 29 ± 1 (28–37) | 169 ± 41 (14–404) | NA | 2.3 ± 0.6 (0.2–6.4) | |||
| All combinations | 58 ± 13 (16–113) | 121 ± 52 (44–325) | 29 ± 2 (25–39) | 136 ± 42 (14–404) | NA | 1.8 ± 0.6 (0.2–6.4) | |||
| Siemens | Mammomat Inspiration | 780 | W/Rh (0.05 mm) [f.s: 0.3; 66%] | 58 ± 15 (12–104) | 74 ± 37 (23–196) | 29 ± 1 (25–32) | 166 ± 82 (47–488) | 58 ± 1.5 (53–62) | 1.8 ± 0.8 (0.6–5.0) |
| W/Rh (0.05 mm) [f.s: 0.1; 22.5%] | 60 ± 8 (49–84) | 50 ± 20 (27–145) | 30 ± 1 (29–32) | 96 ± 37 (46–200) | 33 ± 0.8 (31–34) | 3.3 ± 1.2 (1.7–6.8) | |||
| Mo/Rh (0.025 mm) [f.s: 0.1; 11.5%] | 40 ± 6 (27–49) | 56 ± 22 (28–124) | 27 ± 1 (26–28) | 43 ± 14 (21–93) | 35 ± 0.6 (34–36) | 3.0 ± 0.9 (1.6–6.2) | |||
| All combinations | 56 ± 14 (12–104) | 67 ± 34 (23–196) | 29 ± 1 (25–32) | 136 ± 82 (21–488) | 50 ± 11 (31–62) | 2.3 ± 1.1 (0.6–6.8) | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alhulail, A.A.; Albeshan, S.M.; Alshuhri, M.S.; Alkhybari, E.M.; Almanaa, M.A.; Alahmad, H.; Alenazi, K.; Alshabibi, A.S.; Alsufayan, M.; Alsulaiman, S.A.; et al. The Impact of Automatic Exposure Control Technology on the In Vivo Radiation Dose in Digital Mammography: A Comparison Between Different Systems and Target/Filter Combinations. Diagnostics 2025, 15, 1185. https://doi.org/10.3390/diagnostics15101185
Alhulail AA, Albeshan SM, Alshuhri MS, Alkhybari EM, Almanaa MA, Alahmad H, Alenazi K, Alshabibi AS, Alsufayan M, Alsulaiman SA, et al. The Impact of Automatic Exposure Control Technology on the In Vivo Radiation Dose in Digital Mammography: A Comparison Between Different Systems and Target/Filter Combinations. Diagnostics. 2025; 15(10):1185. https://doi.org/10.3390/diagnostics15101185
Chicago/Turabian StyleAlhulail, Ahmad A., Salman M. Albeshan, Mohammed S. Alshuhri, Essam M. Alkhybari, Mansour A. Almanaa, Haitham Alahmad, Khaled Alenazi, Abdulaziz S. Alshabibi, Mohammed Alsufayan, Saleh A. Alsulaiman, and et al. 2025. "The Impact of Automatic Exposure Control Technology on the In Vivo Radiation Dose in Digital Mammography: A Comparison Between Different Systems and Target/Filter Combinations" Diagnostics 15, no. 10: 1185. https://doi.org/10.3390/diagnostics15101185
APA StyleAlhulail, A. A., Albeshan, S. M., Alshuhri, M. S., Alkhybari, E. M., Almanaa, M. A., Alahmad, H., Alenazi, K., Alshabibi, A. S., Alsufayan, M., Alsulaiman, S. A., Almuqbil, M. M., Elsharkawi, M. M., & Alghamdi, S. (2025). The Impact of Automatic Exposure Control Technology on the In Vivo Radiation Dose in Digital Mammography: A Comparison Between Different Systems and Target/Filter Combinations. Diagnostics, 15(10), 1185. https://doi.org/10.3390/diagnostics15101185






