Unraveling Chronic Cardiovascular and Kidney Disorder through the Butterfly Effect
Abstract
1. Cardiorenal Syndrome (CRS): A Heterogenous Entity
1.1. Initial Paradigm: A Bidirectional Interaction between Heart and Kidney Dysfunction
1.2. New Paradigm: A Continuum Process That Promotes the Dysfunction of Both Organs
1.3. Other Classifications
1.3.1. Pliquett Classification
1.3.2. Cardiovascular–Kidney–Metabolic (CKM) Disease: A New Larger Entity

1.3.3. CCKD: Chronic Cardiovascular and Kidney Disorder
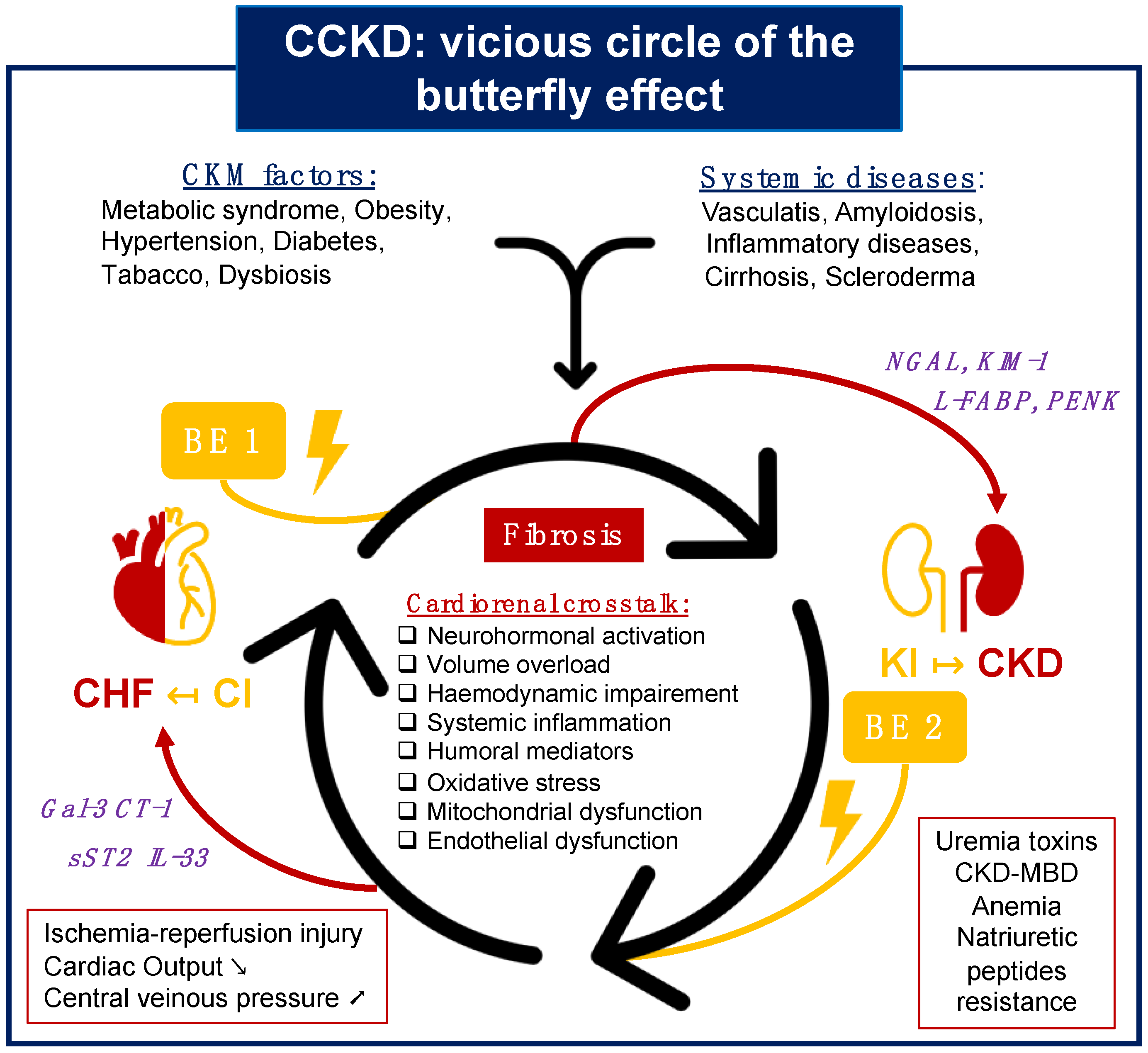
2. From Epidemiology to a Vicious Cycle of Cardiorenal Interaction
2.1. Heart and Kidney Disease: A Perilous Connection
2.2. Focus on Cardiorenal Syndrome Type 1
2.2.1. Definition and Peculiarity
2.2.2. Myocardial Infarction Associated with AKI
Epidemiology and Prognosis
MI Early Phase and Controversial Contrast-Induced Nephropathy
Prognostic Impact Amidst Diverse AKI Definitions
2.3. CCKD Definition: The Vicious Cycle of Cardiorenal Interaction
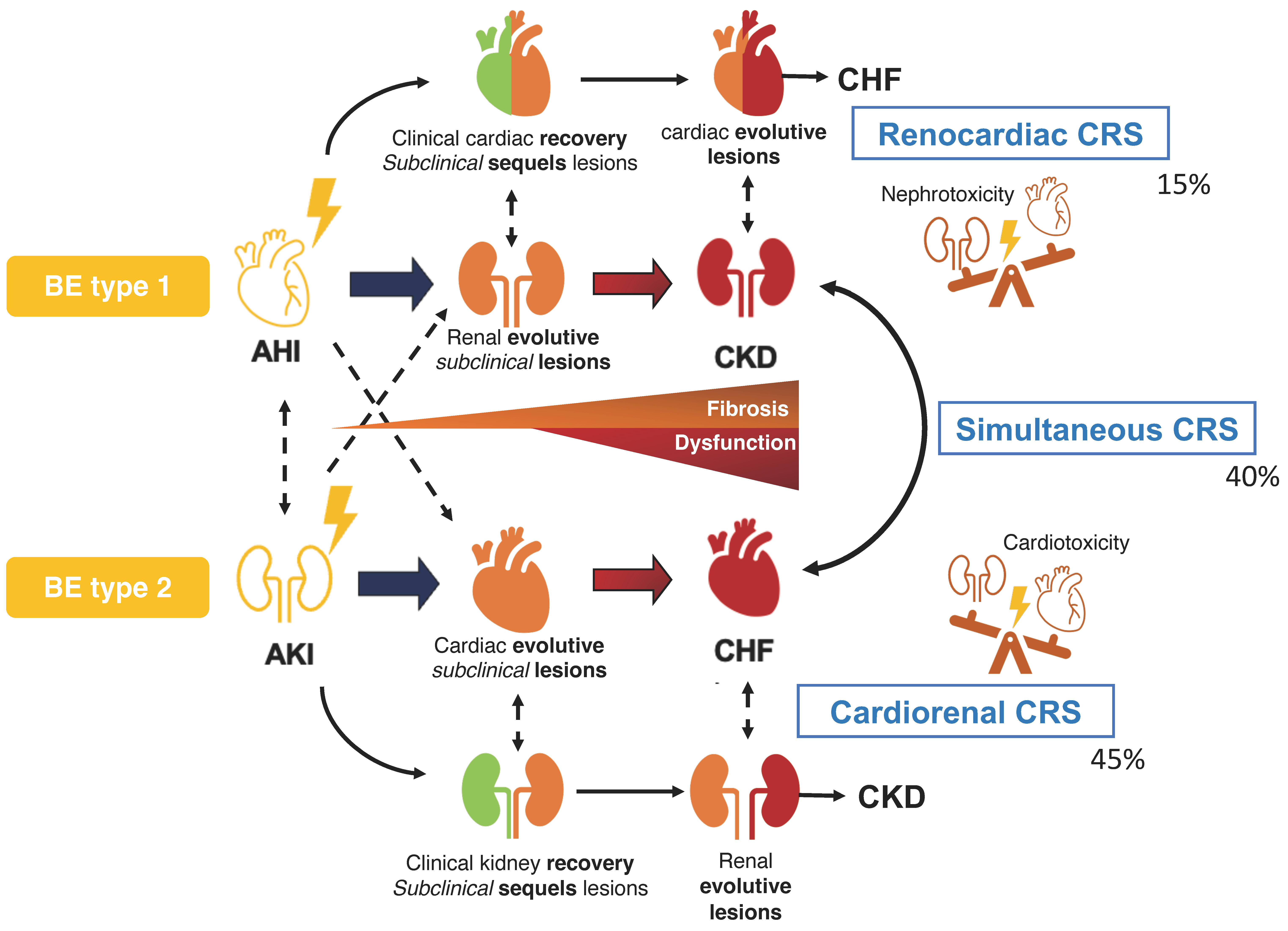
3. Cardiorenal Butterfly Effect
3.1. Definition of the Concept
- ■
- The initially affected organ, particularly if it does not fully recover or if there are cardio/nephrotoxic conditions involved.
- ■
- The other remote organ, especially if the initially affected organ recovers but there are predominant toxic factors impacting the other organ.
- ■
- Both organs simultaneously, if the initial insult is severe enough to trigger a rapid and harmful cycle of mutual dysfunction.
- ■
- It is not required, as CKD or HF can evolve into CRS without an acute insult. However, it is important to acknowledge that even a reversible, acute renal or cardiac insult could have underappreciated long-term consequences.
- ■
- It is not sufficient on its own, depending instead on the presence of metabolic and systemic risk factors. These factors play a crucial role in initiating cardiorenal crosstalk and, subsequently, cardiorenal fibrogenesis.
3.2. Cardiorenal Butterfly Effect Type 1
3.2.1. Description
3.2.2. Clinical Evidence Supporting the Concept
3.2.3. Biomarkers
3.2.4. Preclinical Evidence Supporting the Concept
3.3. Cardiorenal Butterfly Effect Type 2
3.3.1. Description
3.3.2. Clinical Evidence Supporting the Concept
3.3.3. Preclinical Evidence Supporting of Concept
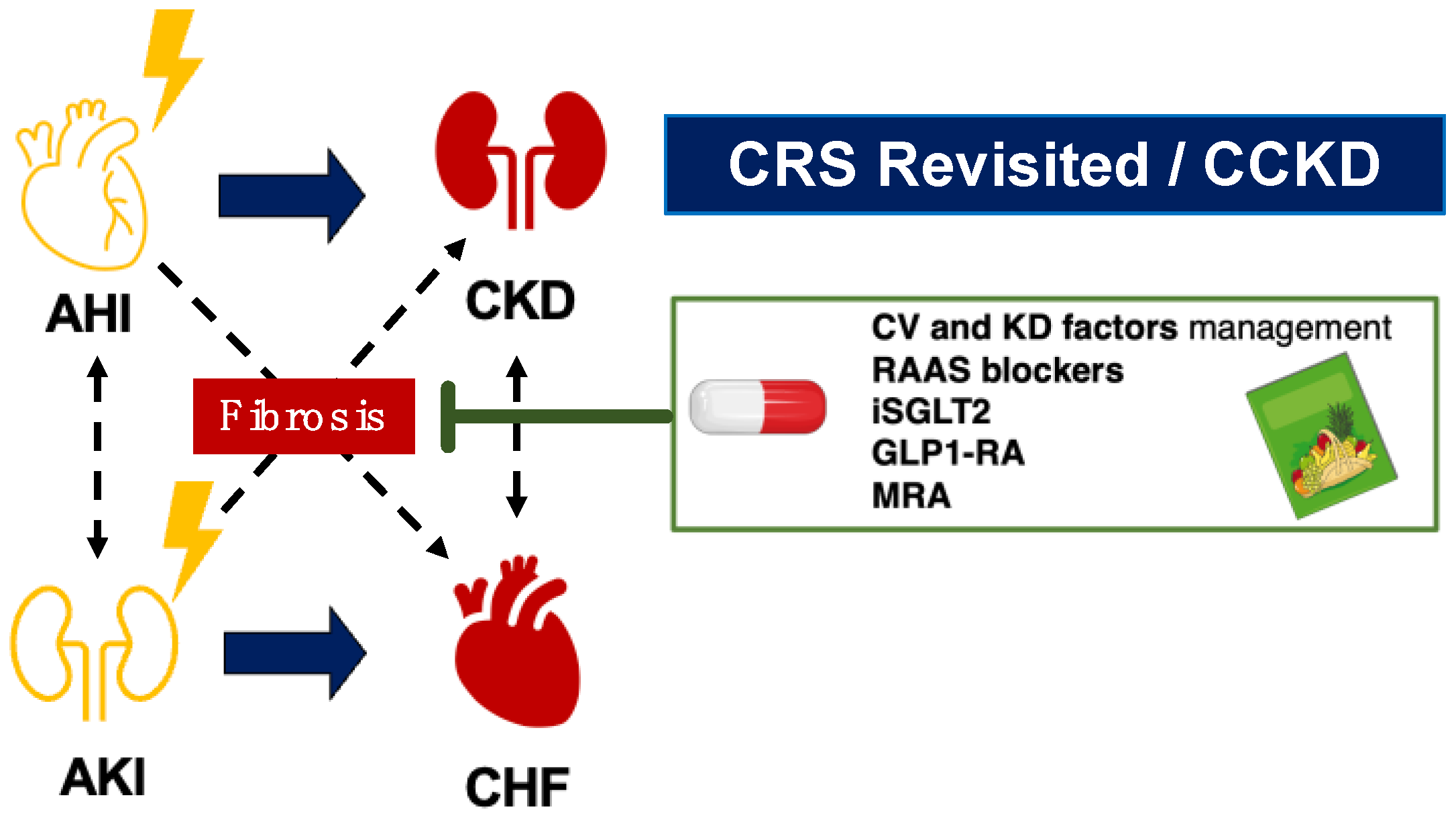
4. Therapeutic Approach for Alleviating Butterfly Effect
4.1. Potential Therapies in Cardiorenal Medicine
4.2. Addressing the Butterfly Effect in Current Cardiorenal Trials
5. Limitations and Challenges with the Cardiorenal Butterfly Effect Approach
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Townsend, N.; Wilson, L.; Bhatnagar, P.; Wickramasinghe, K.; Rayner, M.; Nichols, M. Cardiovascular disease in Europe: Epidemiological update 2016. Eur. Heart J. 2016, 37, 3232–3245. [Google Scholar] [CrossRef]
- Roger, V.L. Epidemiology of Heart Failure: A Contemporary Perspective. Circ. Res. 2021, 128, 1421–1434. [Google Scholar] [CrossRef]
- Savarese, G.; Becher, P.M.; Lund, L.H.; Seferovic, P.; Rosano, G.M.C.; Coats, A.J.S. Global burden of heart failure: A comprehensive and updated review of epidemiology. Cardiovasc. Res. 2023, 118, 3272–3287. [Google Scholar] [CrossRef] [PubMed]
- Bikbov, B.; Purcell, C.A.; Levey, A.S.; Smith, M.; Abdoli, A.; Abebe, M.; Adebayo, O.M.; Afarideh, M.; Agarwal, S.K.; Agudelo-Botero, M.; et al. Global, regional, and national burden of chronic kidney disease, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2020, 395, 709–733. [Google Scholar] [CrossRef] [PubMed]
- Liyanage, T.; Ninomiya, T.; Jha, V.; Neal, B.; Patrice, H.M.; Okpechi, I.; Zhao, M.-H.; Lv, J.; Garg, A.X.; Knight, J.; et al. Worldwide access to treatment for end-stage kidney disease: A systematic review. Lancet 2015, 385, 1975–1982. [Google Scholar] [CrossRef]
- Bongartz, L.G.; Cramer, M.J.M.; Braam, B. The cardiorenal connection. Hypertens. Dallas Tex 1979 2004, 43, e14. [Google Scholar]
- Ronco, C.; Haapio, M.; House, A.A.; Anavekar, N.; Bellomo, R. Cardiorenal Syndrome. J. Am. Coll. Cardiol. 2008, 52, 1527–1539. [Google Scholar] [CrossRef] [PubMed]
- Ronco, C.; McCullough, P.; Anker, S.D.; Anand, I.; Aspromonte, N.; Bagshaw, S.M.; Bellomo, R.; Berl, T.; Bobek, I.; Cruz, D.N.; et al. Cardio-renal syndromes: Report from the consensus conference of the Acute Dialysis Quality Initiative. Eur. Heart J. 2010, 31, 703–711. [Google Scholar] [CrossRef]
- Zannad, F.; Rossignol, P. Cardiorenal Syndrome Revisited. Circulation 2018, 138, 929–944. [Google Scholar] [CrossRef] [PubMed]
- Pliquett, R.U. Cardiorenal Syndrome: An Updated Classification Based on Clinical Hallmarks. J. Clin. Med. 2022, 11, 2896. [Google Scholar] [CrossRef]
- Farkas, J.D.; Long, B.; Koyfman, A.; Menson, K. BRASH Syndrome: Bradycardia, Renal Failure, AV Blockade, Shock, and Hyperkalemia. J. Emerg. Med. 2020, 59, 216–223. [Google Scholar] [CrossRef] [PubMed]
- Ronco, C.; Kaushik, M.; Valle, R.; Aspromonte, N.; Peacock, W.F. Diagnosis and Management of Fluid Overload in Heart Failure and Cardio-Renal Syndrome: The “5B” Approach. Semin. Nephrol. 2012, 32, 129–141. [Google Scholar] [CrossRef] [PubMed]
- Taramasso, M.; Benfari, G.; van der Bijl, P.; Alessandrini, H.; Attinger-Toller, A.; Biasco, L.; Lurz, P.; Braun, D.; Brochet, E.; Connelly, K.A.; et al. Transcatheter Versus Medical Treatment of Patients with Symptomatic Severe Tricuspid Regurgitation. J. Am. Coll. Cardiol. 2019, 74, 2998–3008. [Google Scholar] [CrossRef]
- Mack, M.J.; Lindenfeld, J.; Abraham, W.T.; Kar, S.; Lim, D.S.; Mishell, J.M.; Whisenant, B.K.; Grayburn, P.A.; Rinaldi, M.J.; Kapadia, S.R.; et al. 3-Year Outcomes of Transcatheter Mitral Valve Repair in Patients with Heart Failure. J. Am. Coll. Cardiol. 2021, 77, 1029–1040. [Google Scholar] [CrossRef] [PubMed]
- Pliquett, R.U.; Radler, D.; Tamm, A.; Greinert, D.; Greinert, R.; Girndt, M. Oliguric acute kidney injury as a main symptom of bradycardia and arteriosclerosis resolved by pacemaker implantation: A case report. J. Med. Case Rep. 2014, 8, 289. [Google Scholar] [CrossRef]
- NCD Risk Factor Collaboration (NCD-RisC). Worldwide trends in body-mass index, underweight, overweight, and obesity from 1975 to 2016: A pooled analysis of 2416 population-based measurement studies in 128·9 million children, adolescents, and adults. Lancet Lond. Engl. 2017, 390, 2627–2642. [Google Scholar] [CrossRef]
- Ndumele, C.E.; Neeland, I.J.; Tuttle, K.R.; Chow, S.L.; Mathew, R.O.; Khan, S.S.; Coresh, J.; Baker-Smith, C.M.; Carnethon, M.R.; Després, J.-P.; et al. A Synopsis of the Evidence for the Science and Clinical Management of Cardiovascular-Kidney-Metabolic (CKM) Syndrome: A Scientific Statement from the American Heart Association. Circulation 2023, 148, 1636–1664. [Google Scholar] [CrossRef]
- Sebastian, S.A.; Padda, I.; Johal, G. Cardiovascular-Kidney-Metabolic (CKM) syndrome: A state-of-the-art review. Curr. Probl. Cardiol. 2023, 49, 102344. [Google Scholar] [CrossRef]
- Zoccali, C.; Mallamaci, F.; Halimi, J.M.; Rossignol, P.; Sarafidis, P.; De Caterina, R.; Giugliano, R.; Zannad, F. From Cardiorenal Syndrome to Chronic Cardiovascular and Kidney Disorder: A Conceptual Transition. Clin. J. Am. Soc. Nephrol. 2024. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhu, H.; Hou, X.; Wang, Z.; Zou, F.; Qian, Z.; Wei, Y.; Wang, X.; Zhang, L.; Li, X.; et al. Randomized Trial of Left Bundle Branch vs Biventricular Pacing for Cardiac Resynchronization Therapy. J. Am. Coll. Cardiol. 2022, 80, 1205–1216. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Rha, S.W.; Choi, B.G.; Cho, J.H.; Park, S.H.; Lee, J.B.; Kim, Y.H.; Park, S.M.; Choi, J.W.; Park, J.Y.; et al. Immediate versus staged complete revascularization in patients with ST-segment elevation myocardial infarction and multivessel coronary artery disease: Results from a prematurely discontinued randomized multicenter trial. Am. Heart J. 2023, 259, 58–67. [Google Scholar] [CrossRef]
- Ledwidge, M.; Dodd, J.D.; Ryan, F.; Sweeney, C.; McDonald, K.; Fox, R.; Shorten, E.; Zhou, S.; Watson, C.; Gallagher, J.; et al. Effect of Sacubitril/Valsartan vs Valsartan on Left Atrial Volume in Patients with Pre-Heart Failure with Preserved Ejection Fraction: The PARABLE Randomized Clinical Trial. JAMA Cardiol. 2023, 8, 366–375. [Google Scholar] [CrossRef]
- Patel, R.B.; Ter Maaten, J.M.; Ferreira, J.P.; McCausland, F.R.; Shah, S.J.; Rossignol, P.; Solomon, S.D.; Vaduganathan, M.; Packer, M.; Thompson, A.; et al. Challenges of Cardio-Kidney Composite Outcomes in Large-Scale Clinical Trials. Circulation 2021, 143, 949–958. [Google Scholar] [CrossRef]
- Webster, A.C.; Nagler, E.V.; Morton, R.L.; Masson, P. Chronic Kidney Disease. Lancet Lond. Engl. 2017, 389, 1238–1252. [Google Scholar] [CrossRef]
- Cheung, A.K.; Sarnak, M.J.; Yan, G.; Berkoben, M.; Heyka, R.; Kaufman, A.; Lewis, J.; Rocco, M.; Toto, R.; Windus, D.; et al. Cardiac diseases in maintenance hemodialysis patients: Results of the HEMO Study. Kidney Int. 2004, 65, 2380–2389. [Google Scholar] [CrossRef]
- Mitsnefes, M.M.; Betoko, A.; Schneider, M.F.; Salusky, I.B.; Wolf, M.S.; Jüppner, H.; Warady, B.A.; Furth, S.L.; Portale, A.A. FGF23 and Left Ventricular Hypertrophy in Children with CKD. Clin. J. Am. Soc. Nephrol. 2018, 13, 45. [Google Scholar] [CrossRef]
- Paoletti, E.; Bellino, D.; Cassottana, P.; Rolla, D.; Cannella, G. Left Ventricular Hypertrophy in Nondiabetic Predialysis CKD. Am. J. Kidney Dis. 2005, 46, 320–327. [Google Scholar] [CrossRef] [PubMed]
- Foley, R.N.; Parfrey, P.S.; Harnett, J.D.; Kent, G.M.; Martin, C.J.; Murray, D.C.; Barre, P.E. Clinical and echocardiographic disease in patients starting end-stage renal disease therapy. Kidney Int. 1995, 47, 186–192. [Google Scholar] [CrossRef] [PubMed]
- London, G.M.; Pannier, B.; Guerin, A.P.; Blacher, J.; Marchais, S.J.; Darne, B.; Metivier, F.; Adda, H.; Safar, M.E. Alterations of left ventricular hypertrophy in and survival of patients receiving hemodialysis: Follow-up of an interventional study. J. Am. Soc. Nephrol. JASN 2001, 12, 2759–2767. [Google Scholar] [CrossRef] [PubMed]
- Blecker, S.; Matsushita, K.; Köttgen, A.; Loehr, L.R.; Bertoni, A.G.; Boulware, L.E.; Coresh, J. High-Normal Albuminuria and Risk of Heart Failure in the Community. Am. J. Kidney Dis. 2011, 58, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.D.; Schulze, P.C. Cardiac Metabolism in Heart Failure and Implications for Uremic Cardiomyopathy. Circ. Res. 2023, 132, 1034–1049. [Google Scholar] [CrossRef]
- Hiraiwa, H.; Kasugai, D.; Okumura, T.; Murohara, T. Implications of uremic cardiomyopathy for the practicing clinician: An educational review. Heart Fail. Rev. 2023, 28, 1129–1139. [Google Scholar] [CrossRef]
- Alhaj, E.; Alhaj, N.; Rahman, I.; Niazi, T.O.; Berkowitz, R.; Klapholz, M. Uremic cardiomyopathy: An underdiagnosed disease. Congest. Heart Fail. Greenwich Conn. 2013, 19, E40–E45. [Google Scholar] [CrossRef]
- Wali, R.K.; Wang, G.S.; Gottlieb, S.S.; Bellumkonda, L.; Hansalia, R.; Ramos, E.; Drachenberg, C.; Papadimitriou, J.; Brisco, M.A.; Blahut, S.; et al. Effect of kidney transplantation on left ventricular systolic dysfunction and congestive heart failure in patients with end-stage renal disease. J. Am. Coll. Cardiol. 2005, 45, 1051–1060. [Google Scholar] [CrossRef]
- Tuegel, C.; Bansal, N. Heart failure in patients with kidney disease. Heart 2017, 103, 1848–1853. [Google Scholar] [CrossRef]
- Schefold, J.C.; Filippatos, G.; Hasenfuss, G.; Anker, S.D.; von Haehling, S. Heart failure and kidney dysfunction: Epidemiology, mechanisms and management. Nat. Rev. Nephrol. 2016, 12, 610–623. [Google Scholar] [CrossRef]
- Rahman, M.; Xie, D.; Feldman, H.I.; Go, A.S.; He, J.; Kusek, J.W.; Lash, J.; Miller, E.R.; Ojo, A.; Pan, Q.; et al. Association between Chronic Kidney Disease Progression and Cardiovascular Disease: Results from the CRIC Study. Am. J. Nephrol. 2014, 40, 399–407. [Google Scholar] [CrossRef] [PubMed]
- Shlipak, M.G.; Smith, G.L.; Rathore, S.S.; Massie, B.M.; Krumholz, H.M. Renal function, digoxin therapy, and heart failure outcomes: Evidence from the digoxin intervention group trial. J. Am. Soc. Nephrol. JASN 2004, 15, 2195–2203. [Google Scholar] [CrossRef] [PubMed]
- Uduman, J. Epidemiology of Cardiorenal Syndrome. Adv. Chronic. Kidney Dis. 2018, 25, 391–399. [Google Scholar] [CrossRef]
- Odutayo, A.; Wong, C.X.; Farkouh, M.; Altman, D.G.; Hopewell, S.; Emdin, C.A.; Hunn, B.H. AKI and Long-Term Risk for Cardiovascular Events and Mortality. J. Am. Soc. Nephrol. 2017, 28, 377. [Google Scholar] [CrossRef] [PubMed]
- Ronco, C.; Cicoira, M.; McCullough, P.A. Cardiorenal syndrome type 1: Pathophysiological crosstalk leading to combined heart and kidney dysfunction in the setting of acutely decompensated heart failure. J. Am. Coll. Cardiol. 2012, 60, 1031–1042. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Bellomo, R. Cardiac surgery-associated acute kidney injury: Risk factors, pathophysiology and treatment. Nat. Rev. Nephrol. 2017, 13, 697–711. [Google Scholar] [CrossRef]
- Shaw, A. Models of preventable disease: Contrast-induced nephropathy and cardiac surgery-associated acute kidney injury. Contrib. Nephrol. 2011, 174, 156–162. [Google Scholar] [PubMed]
- Holzmann, M.; Jernberg, T.; Szummer, K.; Sartipy, U. Long-term Cardiovascular Outcomes in Patients with Chronic Kidney Disease Undergoing Coronary Artery Bypass Graft Surgery for Acute Coronary Syndromes. J. Am. Heart Assoc. 2014, 3, e000707. [Google Scholar] [CrossRef] [PubMed]
- Warren, J.; Mehran, R.; Baber, U.; Xu, K.; Giacoppo, D.; Gersh, B.J.; Guagliumi, G.; Witzenbichler, B.; Ohman, E.M.; Pocock, S.J.; et al. Incidence and impact of acute kidney injury in patients with acute coronary syndromes treated with coronary artery bypass grafting: Insights from the Harmonizing Outcomes with Revascularization and Stents in Acute Myocardial Infarction (HORIZONS-AMI) and Acute Catheterization and Urgent Intervention Triage Strategy (ACUITY) trials. Am. Heart J. 2016, 171, 40–47. [Google Scholar]
- Heywood, J.T.; Fonarow, G.C.; Costanzo, M.R.; Mathur, V.S.; Wigneswaran, J.R.; Wynne, J. High prevalence of renal dysfunction and its impact on outcome in 118,465 patients hospitalized with acute decompensated heart failure: A report from the ADHERE database. J. Card. Fail. 2007, 13, 422–430. [Google Scholar] [CrossRef] [PubMed]
- Ghionzoli, N.; Sciaccaluga, C.; Mandoli, G.E.; Vergaro, G.; Gentile, F.; D’Ascenzi, F.; Mondillo, S.; Emdin, M.; Valente, S. Cardiogenic shock and acute kidney injury: The rule rather than the exception. Heart Fail. Rev. 2021, 26, 487–496. [Google Scholar] [CrossRef] [PubMed]
- Lauridsen, M.D.; Gammelager, H.; Schmidt, M.; Rasmussen, T.B.; Shaw, R.E.; Bøtker, H.E.; Sørensen, H.T.; Christiansen, C.F. Acute kidney injury treated with renal replacement therapy and 5-year mortality after myocardial infarction-related cardiogenic shock: A nationwide population-based cohort study. Crit. Care Lond. Engl. 2015, 19, 452. [Google Scholar] [CrossRef]
- Zweck, E.; Thayer, K.L.; Helgestad, O.K.L.; Kanwar, M.; Ayouty, M.; Garan, A.R.; Hernandez-Montfort, J.; Mahr, C.; Wencker, D.; Sinha, S.S.; et al. Phenotyping Cardiogenic Shock. J. Am. Heart Assoc. 2021, 10, e020085. [Google Scholar] [CrossRef]
- Zweck, E.; Kanwar, M.; Li, S.; Sinha, S.S.; Garan, A.R.; Hernandez-Montfort, J.; Zhang, Y.; Li, B.; Baca, P.; Dieng, F.; et al. Clinical Course of Patients in Cardiogenic Shock Stratified by Phenotype. JACC Heart Fail. 2023, 11, 1304–1315. [Google Scholar] [CrossRef]
- Goldberg, A.; Hammerman, H.; Petcherski, S.; Zdorovyak, A.; Yalonetsky, S.; Kapeliovich, M.; Agmon, Y.; Markiewicz, W.; Aronson, D. Inhospital and 1-year mortality of patients who develop worsening renal function following acute ST-elevation myocardial infarction. Am. Heart J. 2005, 150, 330–337. [Google Scholar] [CrossRef]
- Marbach, J.A.; Wells, G.; Santo, P.D.; So, D.; Chong, A.Y.; Russo, J.; Labinaz, M.; Dick, A.; Froeschl, M.; Glover, C.; et al. Acute kidney injury after radial or femoral artery access in ST-segment elevation myocardial infarction: AKI-SAFARI. Am. Heart J. 2021, 234, 12–22. [Google Scholar] [CrossRef]
- Owan, T.E.; Hodge, D.O.; Herges, R.M.; Jacobsen, S.J.; Roger, V.L.; Redfield, M.M. Secular Trends in Renal Dysfunction and Outcomes in Hospitalized Heart Failure Patients. J. Card. Fail. 2006, 12, 257–262. [Google Scholar] [CrossRef]
- Landi, A.; Branca, M.; Leonardi, S.; Frigoli, E.; Vranckx, P.; Tebaldi, M.; Varbella, F.; Calabró, P.; Esposito, G.; Sardella, G.; et al. Transient vs In-Hospital Persistent Acute Kidney Injury in Patients with Acute Coronary Syndrome. JACC Cardiovasc. Interv. 2023, 16, 193–205. [Google Scholar] [CrossRef] [PubMed]
- Chawla, L.S.; Amdur, R.L.; Shaw, A.D.; Faselis, C.; Palant, C.E.; Kimmel, P.L. Association between AKI and long-term renal and cardiovascular outcomes in United States veterans. Clin. J. Am. Soc. Nephrol. CJASN 2014, 9, 448–456. [Google Scholar] [CrossRef] [PubMed]
- Kosaki, R.; Wakabayashi, K.; Sato, S.; Tanaka, H.; Ogura, K.; Oishi, Y.; Arai, K.; Nomura, K.; Sakai, K.; Sekimoto, T.; et al. Onset time and prognostic value of acute kidney injury in patients with acute myocardial infarction. IJC Heart Vasc. 2021, 35, 100826. [Google Scholar] [CrossRef]
- Zahler, D.; Rozenfeld, K.L.; Merdler, I.; Peri, Y.; Shacham, Y. Contrast Volume to Glomerular Filtration Ratio and Acute Kidney Injury among ST-Segment Elevation Myocardial Infarction Patients Treated with Primary Percutaneous Coronary Intervention. Cardiorenal. Med. 2020, 10, 108–115. [Google Scholar] [CrossRef] [PubMed]
- Marenzi, G.; Lauri, G.; Assanelli, E.; Campodonico, J.; De Metrio, M.; Marana, I.; Grazi, M.; Veglia, F.; Bartorelli, A.L. Contrast-induced nephropathy in patients undergoing primary angioplasty for acute myocardial infarction. J. Am. Coll. Cardiol. 2004, 44, 1780–1785. [Google Scholar] [CrossRef] [PubMed]
- Van Linden, A.; Kempfert, J.; Rastan, A.J.; Holzhey, D.; Blumenstein, J.; Schuler, G.; Mohr, F.W.; Walther, T. Risk of acute kidney injury after minimally invasive transapical aortic valve implantation in 270 patients. Eur. J. Cardio-Thorac. Surg. Off. J. Eur. Assoc. Cardio-Thorac. Surg. 2011, 39, 835–842; discussion 842–843. [Google Scholar] [CrossRef]
- Lazaros, G.; Tsiachris, D.; Tousoulis, D.; Patialiakas, A.; Dimitriadis, K.; Roussos, D.; Vergopoulos, E.; Tsioufis, C.; Vlachopoulos, C.; Stefanadis, C. In-hospital worsening renal function is an independent predictor of one-year mortality in patients with acute myocardial infarction. Int. J. Cardiol. 2012, 155, 97–101. [Google Scholar] [CrossRef] [PubMed]
- McDonald, J.S.; McDonald, R.J.; Comin, J.; Williamson, E.E.; Katzberg, R.W.; Murad, M.H.; Kallmes, D.F. Frequency of acute kidney injury following intravenous contrast medium administration: A systematic review and meta-analysis. Radiology 2013, 267, 119–128. [Google Scholar] [CrossRef] [PubMed]
- Chevalier, B.; Neylon, A. Acute Kidney Injury after “Zero Contrast” Tricuspid Edge-to-Edge Repair: More than a Procedural Complication?∗. JACC Cardiovasc. Interv. 2022, 15, 1946–1947. [Google Scholar] [CrossRef] [PubMed]
- Mullens, W.; Damman, K.; Testani, J.M.; Martens, P.; Mueller, C.; Lassus, J.; Tang, W.H.W.; Skouri, H.; Verbrugge, F.H.; Orso, F.; et al. Evaluation of kidney function throughout the heart failure trajectory—A position statement from the Heart Failure Association of the European Society of Cardiology. Eur. J. Heart Fail. 2020, 22, 584–603. [Google Scholar] [CrossRef] [PubMed]
- Metra, M.; Davison, B.; Bettari, L.; Sun, H.; Edwards, C.; Lazzarini, V.; Piovanelli, B.; Carubelli, V.; Bugatti, S.; Lombardi, C.; et al. Is worsening renal function an ominous prognostic sign in patients with acute heart failure? The role of congestion and its interaction with renal function. Circ. Heart Fail. 2012, 5, 54–62. [Google Scholar] [CrossRef]
- Shirakabe, A.; Hata, N.; Kobayashi, N.; Okazaki, H.; Matsushita, M.; Shibata, Y.; Uchiyama, S.; Sawatani, T.; Asai, K.; Shimizu, W. Worsening renal failure in patients with acute heart failure: The importance of cardiac biomarkers. ESC Heart Fail. 2019, 6, 416–427. [Google Scholar] [CrossRef]
- Gottlieb, S.S.; Abraham, W.; Butler, J.; Forman, D.E.; Loh, E.; Massie, B.M.; O’Connor, C.M.; Rich, M.W.; Stevenson, L.W.; Young, J.; et al. The prognostic importance of different definitions of worsening renal function in congestive heart failure. J. Card. Fail. 2002, 8, 136–141. [Google Scholar] [CrossRef]
- Newsome, B.B. Long-term Risk of Mortality and End-Stage Renal Disease among the Elderly after Small Increases in Serum Creatinine Level during Hospitalization for Acute Myocardial Infarction. Arch. Intern. Med. 2008, 168, 609. [Google Scholar] [CrossRef]
- Anzai, A.; Anzai, T.; Naito, K.; Kaneko, H.; Mano, Y.; Jo, Y.; Nagatomo, Y.; Maekawa, Y.; Kawamura, A.; Yoshikawa, T.; et al. Prognostic Significance of Acute Kidney Injury after Reperfused ST-Elevation Myocardial Infarction: Synergistic Acceleration of Renal Dysfunction and Left Ventricular Remodeling. J. Card. Fail. 2010, 16, 381–389. [Google Scholar] [CrossRef]
- Vallon, V.; Verma, S. Effects of SGLT2 Inhibitors on Kidney and Cardiovascular Function. Annu. Rev. Physiol. 2021, 83, 503–528. [Google Scholar] [CrossRef] [PubMed]
- Brown, E.; Heerspink, H.J.L.; Cuthbertson, D.J.; Wilding, J.P.H. SGLT2 inhibitors and GLP-1 receptor agonists: Established and emerging indications. Lancet 2021, 398, 262–276. [Google Scholar] [CrossRef] [PubMed]
- Zannad, F.; Ferreira, J.P.; Pocock, S.J.; Zeller, C.; Anker, S.D.; Butler, J.; Filippatos, G.; Hauske, S.J.; Brueckmann, M.; Pfarr, E.; et al. Cardiac and Kidney Benefits of Empagliflozin in Heart Failure across the Spectrum of Kidney Function: Insights from EMPEROR-Reduced. Circulation 2021, 143, 310–321. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.G.; Kim, D.; Lee, J.J.; Lee, H.J.; Moon, R.K.; Lee, Y.J.; Lee, S.-J.; Lee, O.-H.; Kim, C.; Oh, J.; et al. Dapagliflozin attenuates diabetes-induced diastolic dysfunction and cardiac fibrosis by regulating SGK1 signaling. BMC Med. 2022, 20, 309. [Google Scholar] [CrossRef]
- Feng, L.; Chen, Y.; Li, N.; Yang, X.; Zhou, L.; Li, H.; Wang, T.; Xie, M.; Liu, H. Dapagliflozin delays renal fibrosis in diabetic kidney disease by inhibiting YAP/TAZ activation. Life Sci. 2023, 322, 121671. [Google Scholar] [CrossRef] [PubMed]
- Tian, J.; Zhang, M.; Suo, M.; Liu, D.; Wang, X.; Liu, M.; Pan, J.; Jin, T.; An, F. Dapagliflozin alleviates cardiac fibrosis through suppressing EndMT and fibroblast activation via AMPKα/TGF-β/Smad signalling in type 2 diabetic rats. J. Cell. Mol. Med. 2021, 25, 7642–7659. [Google Scholar] [CrossRef]
- Tokmakova, M.P.; Skali, H.; Kenchaiah, S.; Braunwald, E.; Rouleau, J.L.; Packer, M.; Chertow, G.M.; Moyé, L.A.; Pfeffer, M.A.; Solomon, S.D. Chronic Kidney Disease, Cardiovascular Risk, and Response to Angiotensin-Converting Enzyme Inhibition after Myocardial Infarction: The Survival and Ventricular Enlargement (SAVE) Study. Circulation 2004, 110, 3667–3673. [Google Scholar] [CrossRef] [PubMed]
- AlQudah, M.; Hale, T.M.; Czubryt, M.P. Targeting the renin-angiotensin-aldosterone system in fibrosis. Matrix Biol. 2020, 91–92, 92–108. [Google Scholar] [CrossRef]
- Murphy, D.P.; Wolfson, J.; Reule, S.; Johansen, K.L.; Ishani, A.; Drawz, P.E. Renin–Angiotensin–Aldosterone System Blockade after AKI with or without Recovery among US Veterans with Diabetic Kidney Disease. J. Am. Soc. Nephrol. 2023, 34, 1721. [Google Scholar] [CrossRef]
- Xie, X.; Liu, Y.; Perkovic, V.; Li, X.; Ninomiya, T.; Hou, W.; Zhao, N.; Liu, L.; Lv, J.; Zhang, H.; et al. Renin-Angiotensin System Inhibitors and Kidney and Cardiovascular Outcomes in Patients with CKD: A Bayesian Network Meta-analysis of Randomized Clinical Trials. Am. J. Kidney Dis. 2016, 67, 728–741. [Google Scholar] [CrossRef]
- Srivastava, P.K.; Claggett, B.L.; Solomon, S.D.; McMurray, J.J.V.; Packer, M.; Zile, M.R.; Desai, A.S.; Rouleau, J.L.; Swedberg, K.; Fonarow, G.C. Estimated 5-Year Number Needed to Treat to Prevent Cardiovascular Death or Heart Failure Hospitalization with Angiotensin Receptor-Neprilysin Inhibition vs Standard Therapy for Patients with Heart Failure with Reduced Ejection Fraction: An Analysis of Data from the PARADIGM-HF Trial. JAMA Cardiol. 2018, 3, 1226–1231. [Google Scholar]
- Tsukamoto, S.; Uehara, T.; Azushima, K.; Wakui, H.; Tamura, K. Updates for Cardio-Kidney Protective Effects by Angiotensin Receptor-Neprilysin Inhibitor: Requirement for Additional Evidence of Kidney Protection. J. Am. Heart Assoc. 2023, 12, e029565. [Google Scholar] [CrossRef]
- Agarwal, R.; Kolkhof, P.; Bakris, G.; Bauersachs, J.; Haller, H.; Wada, T.; Zannad, F. Steroidal and non-steroidal mineralocorticoid receptor antagonists in cardiorenal medicine. Eur. Heart J. 2021, 42, 152–161. [Google Scholar] [CrossRef] [PubMed]
- Bakris, G.L.; Agarwal, R.; Anker, S.D.; Pitt, B.; Ruilope, L.M.; Rossing, P.; Kolkhof, P.; Nowack, C.; Schloemer, P.; Joseph, A.; et al. Effect of Finerenone on Chronic Kidney Disease Outcomes in Type 2 Diabetes. N. Engl. J. Med. 2020, 383, 2219–2229. [Google Scholar] [CrossRef] [PubMed]
- Pitt, B.; Filippatos, G.; Agarwal, R.; Anker, S.D.; Bakris, G.L.; Rossing, P.; Joseph, A.; Kolkhof, P.; Nowack, C.; Schloemer, P.; et al. Cardiovascular Events with Finerenone in Kidney Disease and Type 2 Diabetes. N. Engl. J. Med. 2021, 385, 2252–2263. [Google Scholar] [CrossRef] [PubMed]
- Seksaria, S.; Dutta, B.J.; Kaur, M.; Gupta, G.D.; Bodakhe, S.H.; Singh, A. Role of GLP-1 receptor agonist in diabetic cardio-renal disorder: Recent updates of clinical and pre-clinical evidence. Curr. Diabetes Rev. 2024, 20, 44–57. [Google Scholar] [CrossRef] [PubMed]
- Nauck, M.A.; D’Alessio, D.A. Tirzepatide, a dual GIP/GLP-1 receptor co-agonist for the treatment of type 2 diabetes with unmatched effectiveness regrading glycaemic control and body weight reduction. Cardiovasc. Diabetol. 2022, 21, 169. [Google Scholar] [CrossRef]
- Halimi, J.M.; de Fréminville, J.B.; Gatault, P.; Bisson, A.; Gueguen, J.; Goin, N.; Sautenet, B.; Maisons, V.; Herbert, J.; Angoulvant, D.; et al. Long-term impact of cardiorenal syndromes on major outcomes based on their chronology: A comprehensive French nationwide cohort study. Nephrol. Dial. Transpl. Off. Publ. Eur. Dial. Transpl. Assoc. Eur. Ren. Assoc. 2022, 37, 2386–2397. [Google Scholar] [CrossRef]
- Maisons, V.; Halimi, J.M.; Fauchier, G.; de Fréminville, J.B.; Goin, N.; Gueguen, J.; Gatault, P.; Sautenet, B.; Angoulvant, D.; Herbert, J.; et al. Type 2 diabetes and cardiorenal syndromes. A nationwide French hospital cohort study. Diabetes Metab. 2023, 49, 101441. [Google Scholar] [CrossRef]
- Charytan, D.M.; Solomon, S.D.; Ivanovich, P.; Remuzzi, G.; Cooper, M.E.; McGill, J.B.; Parving, H.-H.; Parfrey, P.; Singh, A.K.; Burdmann, E.A.; et al. ESRD after Heart Failure, Myocardial Infarction, or Stroke in Type 2 Diabetic Patients with CKD. Am. J. Kidney Dis. Off. J. Natl. Kidney Found. 2017, 70, 522–531. [Google Scholar] [CrossRef]
- Sud, M.; Tangri, N.; Pintilie, M.; Levey, A.S.; Naimark, D.M.J. ESRD and death after heart failure in CKD. J. Am. Soc. Nephrol. JASN 2015, 26, 715–722. [Google Scholar] [CrossRef]
- Sud, M.; Tangri, N.; Pintilie, M.; Levey, A.S.; Naimark, D. Risk of end-stage renal disease and death after cardiovascular events in chronic kidney disease. Circulation 2014, 130, 458–465. [Google Scholar] [CrossRef]
- Ishigami, J.; Trevisan, M.; Lund, L.H.; Jernberg, T.; Coresh, J.; Matsushita, K.; Carrero, J.-J. Acceleration of kidney function decline after incident hospitalization with cardiovascular disease: The Stockholm CREAtinine Measurements (SCREAM) project. Eur. J. Heart Fail. 2020, 22, 1790–1799. [Google Scholar] [CrossRef] [PubMed]
- Ishigami, J.; Cowan, L.T.; Demmer, R.T.; Grams, M.E.; Lutsey, P.L.; Carrero, J.J.; Coresh, J.; Matsushita, K. Incident Hospitalization with Major Cardiovascular Diseases and Subsequent Risk of ESKD: Implications for Cardiorenal Syndrome. J. Am. Soc. Nephrol. JASN 2020, 31, 405–414. [Google Scholar] [CrossRef] [PubMed]
- Mark, P.B.; Carrero, J.J.; Matsushita, K.; Sang, Y.; Ballew, S.H.; Grams, M.E.; Coresh, J.; Surapaneni, A.; Brunskill, N.J.; Chalmers, J.; et al. Major cardiovascular events and subsequent risk of kidney failure with replacement therapy: A CKD Prognosis Consortium study. Eur. Heart J. 2023, 44, 1157–1166. [Google Scholar] [CrossRef] [PubMed]
- Helánová, K.; Pařenica, J.; Dlouhý, V.; Pávková Goldbergová, M.; Cermáková, Z.; Gottwaldová, J.; Spinar, J. The importance of NGAL and cystatin C biomarkers in cardiovascular diseases. Vnitr. Lek. 2012, 58, 286–290. [Google Scholar] [PubMed]
- McCoy, I.E.; Hsu, J.Y.; Bonventre, J.V.; Parikh, C.R.; Go, A.S.; Liu, K.D.; Ricardo, A.C.; Srivastava, A.; Cohen, D.L.; He, J.; et al. Acute Kidney Injury Associates with Long-Term Increases in Plasma TNFR1, TNFR2, and KIM-1: Findings from the CRIC Study. J. Am. Soc. Nephrol. 2022, 33, 1173. [Google Scholar] [CrossRef] [PubMed]
- Cuesta, C.; Fuentes-Calvo, I.; Sancho-Martinez, S.M.; Valentijn, F.A.; Düwel, A.; Hidalgo-Thomas, O.A.; Agüeros-Blanco, C.; Benito-Hernández, A.; Ramos-Barron, M.A.; Gómez-Alamillo, C.; et al. Urinary KIM-1 Correlates with the Subclinical Sequelae of Tubular Damage Persisting after the Apparent Functional Recovery from Intrinsic Acute Kidney Injury. Biomedicines 2022, 10, 1106. [Google Scholar] [CrossRef] [PubMed]
- Lekawanvijit, S.; Kompa, A.R.; Zhang, Y.; Wang, B.H.; Kelly, D.J.; Krum, H. Myocardial infarction impairs renal function, induces renal interstitial fibrosis, and increases renal KIM-1 expression: Implications for cardiorenal syndrome. Am. J. Physiol.-Heart Circ. Physiol. 2012, 302, H1884–H1893. [Google Scholar] [CrossRef] [PubMed]
- Karmakova, T.A.; Sergeeva, N.S.; Kanukoev, K.Y.; Alekseev, B.Y.; Kaprin, A.D. Kidney Injury Molecule 1 (KIM-1): A Multifunctional Glycoprotein and Biological Marker (Review). Sovrem. Tekhnologii V Meditsine 2021, 13, 64–78. [Google Scholar] [CrossRef]
- Mori, Y.; Ajay, A.K.; Chang, J.H.; Mou, S.; Zhao, H.; Kishi, S.; Li, J.; Brooks, C.R.; Xiao, S.; Woo, H.-M.; et al. KIM-1 mediates fatty acid uptake by renal tubular cells to promote progressive diabetic kidney disease. Cell Metab. 2021, 33, 1042–1061.e7. [Google Scholar] [CrossRef]
- Al-Bataineh, M.M.; Kinlough, C.L.; Mi, Z.; Jackson, E.K.; Mutchler, S.M.; Emlet, D.R.; Kellum, J.A.; Hughey, R.P. KIM-1-mediated anti-inflammatory activity is preserved by MUC1 induction in the proximal tubule during ischemia-reperfusion injury. Am. J. Physiol. Renal. Physiol. 2021, 321, F135–F148. [Google Scholar] [CrossRef]
- Palazzuoli, A.; Ruocco, G.; Beltrami, M.; Franci, B.; Pellegrini, M.; Lucani, B.; Nuti, R.; Ronco, C. Admission plasma neutrophil gelatinase associated lipocalin (NGAL) predicts worsening renal function during hospitalization and post discharge outcome in patients with acute heart failure. Acute Card. Care 2014, 16, 93–101. [Google Scholar] [CrossRef]
- Schröder, S.K.; Gasterich, N.; Weiskirchen, S.; Weiskirchen, R. Lipocalin 2 receptors: Facts, fictions, and myths. Front. Immunol. 2023, 14, 1229885. [Google Scholar] [CrossRef] [PubMed]
- Marakala, V. Neutrophil gelatinase-associated lipocalin (NGAL) in kidney injury—A systematic review. Clin. Chim. Acta. 2022, 536, 135–141. [Google Scholar] [CrossRef] [PubMed]
- Alvelos, M.; Pimentel, R.; Pinho, E.; Gomes, A.; Lourenço, P.; Teles, M.J.; Almeida, P.; Guimarães, J.T.; Bettencourt, P. Neutrophil gelatinase-associated lipocalin in the diagnosis of type 1 cardio-renal syndrome in the general ward. Clin. J. Am. Soc. Nephrol. CJASN 2011, 6, 476–481. [Google Scholar] [CrossRef]
- Kaufmann, M.; Schlossbauer, M.; Hubauer, U.; Stadler, S.; Fischer, M.; Wallner, S.; Hupf, J.; Zimmermann, M.; Orso, E.; Zeman, F.; et al. N-acety-b-D-glucosaminidase: A potential biomarker for early detection of acute kidney injury in acute chest pain. Nephrol. Carlton. Vic. 2020, 25, 135–143. [Google Scholar] [CrossRef]
- Jungbauer, C.G.; Birner, C.; Jung, B.; Buchner, S.; Lubnow, M.; von Bary, C.; Endemann, D.; Banas, B.; Mack, M.; Böger, C.A.; et al. Kidney injury molecule-1 and N-acetyl-β-D-glucosaminidase in chronic heart failure: Possible biomarkers of cardiorenal syndrome. Eur. J. Heart Fail. 2011, 13, 1104–1110. [Google Scholar] [CrossRef]
- Zhao, H.L.; Hu, H.J.; Zhao, X.J.; Chi, W.W.; Liu, D.M.; Wang, Q.; Cui, W. Urine N-terminal pro-B-type natriuretic peptide and plasma proenkephalin are promising biomarkers for early diagnosis of cardiorenal syndrome type 1 in acute decompensated heart failure: A prospective, double-center, observational study in real-world. Ren. Fail. 2022, 44, 1486–1497. [Google Scholar] [CrossRef]
- Ng, L.L.; Squire, I.B.; Jones, D.J.L.; Cao, T.H.; Chan, D.C.S.; Sandhu, J.K.; Quinn, P.A.; Davies, J.E.; Struck, J.; Hartmann, O.; et al. Proenkephalin, Renal Dysfunction, and Prognosis in Patients with Acute Heart Failure: A GREAT Network Study. J. Am. Coll. Cardiol. 2017, 69, 56–69. [Google Scholar] [CrossRef] [PubMed]
- Sunayama, T.; Yatsu, S.; Matsue, Y.; Dotare, T.; Maeda, D.; Ishiwata, S.; Nakamura, Y.; Suda, S.; Kato, T.; Hiki, M.; et al. Urinary liver-type fatty acid-binding protein as a prognostic marker in patients with acute heart failure. ESC Heart Fail. 2022, 9, 442–449. [Google Scholar] [CrossRef]
- van Dokkum, R.P.E.; Eijkelkamp, W.B.A.; Kluppel, A.C.A.; Henning, R.H.; van Goor, H.; Citgez, M.; Windt, W.A.K.M.; van Veldhuisen, D.J.; de Graeff, P.A.; de Zeeuw, D. Myocardial infarction enhances progressive renal damage in an experimental model for cardio-renal interaction. J. Am. Soc. Nephrol. JASN 2004, 15, 3103–3110. [Google Scholar] [CrossRef]
- Cho, E.; Kim, M.; Ko, Y.S.; Lee, H.Y.; Song, M.; Kim, M.G.; Cho, W.-Y.; Jo, S.-K. Role of inflammation in the pathogenesis of cardiorenal syndrome in a rat myocardial infarction model. Nephrol. Dial. Transpl. Off. Publ. Eur. Dial. Transpl. Assoc. Eur. Ren. Assoc. 2013, 28, 2766–2778. [Google Scholar] [CrossRef] [PubMed]
- Ruparelia, N.; Digby, J.E.; Jefferson, A.; Medway, D.J.; Neubauer, S.; Lygate, C.A.; Choudhury, R.P. Myocardial infarction causes inflammation and leukocyte recruitment at remote sites in the myocardium and in the renal glomerulus. Inflamm. Res. 2013, 62, 515–525. [Google Scholar] [CrossRef] [PubMed]
- Werner, R.A.; Hess, A.; Koenig, T.; Diekmann, J.; Derlin, T.; Melk, A.; Thackeray, J.T.; Bauersachs, J.; Bengel, F.M. Molecular imaging of inflammation crosstalk along the cardio-renal axis following acute myocardial infarction. Theranostics 2021, 11, 7984–7994. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Wang, X.; Wang, W.; Muniyappa, H.; Deshmukh, A.; Hu, C.; Das, K.; Mehta, J.L. Abrogation of lectin-like oxidized LDL receptor-1 attenuates acute myocardial ischemia-induced renal dysfunction by modulating systemic and local inflammation. Kidney Int. 2012, 82, 436–444. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Li, Q.; Yan, H.; Huang, J.; Wang, Z. Apela improves cardiac and renal function in mice with acute myocardial infarction. J. Cell. Mol. Med. 2020, 24, 10382–10390. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Wang, L.; Wang, W.; Cheng, C.; Zhang, Y.; Zhou, Y.; Wang, C.; Miao, X.; Wang, J.; Wang, C.; et al. ELABELA and an ELABELA Fragment Protect against AKI. J. Am. Soc. Nephrol. JASN 2017, 28, 2694–2707. [Google Scholar] [CrossRef] [PubMed]
- Parr, S.K.; Siew, E.D. Delayed Consequences of Acute Kidney Injury. Adv. Chronic. Kidney Dis 2016, 23, 186–194. [Google Scholar] [CrossRef] [PubMed]
- Vijayan, A.; Abdel-Rahman, E.M.; Liu, K.D.; Goldstein, S.L.; Agarwal, A.; Okusa, M.D.; Cerda, J. Recovery after Critical Illness and Acute Kidney Injury. Clin. J. Am. Soc. Nephrol. CJASN 2021, 16, 1601–1609. [Google Scholar] [CrossRef]
- Hansen, M.K.; Gammelager, H.; Jacobsen, C.J.; Hjortdal, V.E.; Layton, J.B.; Rasmussen, B.S.; Andreasen, J.J.; Johnsen, S.P.; Christiansen, C.F. Acute Kidney Injury and Long-term Risk of Cardiovascular Events after Cardiac Surgery: A Population-Based Cohort Study. J. Cardiothorac. Vasc. Anesth. 2015, 29, 617–625. [Google Scholar] [CrossRef]
- Lu, J.Y.; Boparai, M.S.; Shi, C.; Henninger, E.M.; Rangareddy, M.; Veeraraghavan, S.; Mirhaji, P.; Fisher, M.C.; Duong, T.Q. Long-term outcomes of COVID-19 survivors with hospital AKI: Association with time to recovery from AKI. Nephrol. Dial. Transpl. Off. Publ. Eur. Dial. Transpl. Assoc. Eur. Ren. Assoc. 2023, 38, 2160–2169. [Google Scholar] [CrossRef]
- Benotmane, I.; Perrin, P.; Vargas, G.G.; Bassand, X.; Keller, N.; Lavaux, T.; Ohana, M.; Bedo, D.; Baldacini, C.; Sagnard, M.; et al. Biomarkers of Cytokine Release Syndrome Predict Disease Severity and Mortality From COVID-19 in Kidney Transplant Recipients. Transplantation 2021, 105, 158–169. [Google Scholar] [CrossRef]
- Menez, S.; Coca, S.G.; Moledina, D.G.; Wen, Y.; Chan, L.; Thiessen-Philbrook, H.; Obeid, W.; Garibaldi, B.T.; Azeloglu, E.U.; Ugwuowo, U.; et al. Evaluation of Plasma Biomarkers to Predict Major Adverse Kidney Events in Hospitalized Patients with COVID-19. Am. J. Kidney Dis. 2023, 82, 322–332.e1. [Google Scholar] [CrossRef] [PubMed]
- Safranow, K.; Dziedziejko, V.; Rzeuski, R.; Czyzycka, E.; Wojtarowicz, A.; Bińczak-Kuleta, A.; Jakubowska, K.; Olszewska, M.; Ciechanowicz, A.; Kornacewicz-Jach, Z.; et al. Plasma concentrations of TNF-alpha and its soluble receptors sTNFR1 and sTNFR2 in patients with coronary artery disease. Tissue Antigens 2009, 74, 386–392. [Google Scholar] [CrossRef]
- Bae, E.; Cha, R.H.; Kim, Y.C.; An, J.N.; Kim, D.K.; Yoo, K.D.; Lee, S.M.; Kim, M.-H.; Park, J.T.; Kang, S.-W.; et al. Circulating TNF receptors predict cardiovascular disease in patients with chronic kidney disease. Medicine 2017, 96, e6666. [Google Scholar] [CrossRef]
- Bhatraju, P.K.; Zelnick, L.R.; Shlipak, M.; Katz, R.; Kestenbaum, B. Association of Soluble TNFR-1 Concentrations with Long-Term Decline in Kidney Function: The Multi-Ethnic Study of Atherosclerosis. J. Am. Soc. Nephrol. 2018, 29, 2713. [Google Scholar] [CrossRef] [PubMed]
- Schulman, I.H.; Chan, K.; Der, J.S.; Wilkins, K.J.; Corns, H.L.; Sayer, B.; Ngo, D.A.; Eggers, P.; Norton, J.; Shah, N.; et al. Readmission and Mortality after Hospitalization with Acute Kidney Injury. Am. J. Kidney Dis. Off. J. Natl. Kidney Found. 2023, 82, 63–74.e1. [Google Scholar] [CrossRef] [PubMed]
- Isaak, A.; Pomareda, I.; Mesropyan, N.; Kravchenko, D.; Endler, C.; Bischoff, L.; Pieper, C.C.; Kuetting, D.; Attenberger, U.; Zimmer, S.; et al. Cardiovascular Magnetic Resonance in Survivors of Critical Illness: Cardiac Abnormalities Are Associated with Acute Kidney Injury. J. Am. Heart Assoc. 2023, 12, e029492. [Google Scholar] [CrossRef]
- Martin, F.L.; McKie, P.M.; Cataliotti, A.; Sangaralingham, S.J.; Korinek, J.; Huntley, B.K.; Oehler, E.A.; Harders, G.E.; Ichiki, T.; Mangiafico, S.; et al. Experimental mild renal insufficiency mediates early cardiac apoptosis, fibrosis, and diastolic dysfunction: A kidney-heart connection. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2012, 302, R292–R299. [Google Scholar] [CrossRef]
- Song, Y.; Yu, Q.; Zhang, J.; Huang, W.; Liu, Y.; Pei, H.; Liu, J.; Sun, L.; Yang, L.; Li, C.; et al. Increased myocardial ischemia-reperfusion injury in renal failure involves cardiac adiponectin signal deficiency. Am. J. Physiol. Endocrinol. Metab. 2014, 306, E1055–E1064. [Google Scholar] [CrossRef]
- Dépret, F.; Prud’homme, M.; Legrand, M. A Role of Remote Organs Effect in Acute Kidney Injury Outcome. Nephron 2017, 137, 273–276. [Google Scholar] [CrossRef]
- Kaesler, N.; Cheng, M.; Nagai, J.; O’Sullivan, J.; Peisker, F.; Bindels, E.M.J.; Babler, A.; Moellmann, J.; Droste, P.; Franciosa, G.; et al. Mapping cardiac remodeling in chronic kidney disease. Sci. Adv. 2023, 9, eadj4846. [Google Scholar] [CrossRef]
- Kelly, K.J. Distant effects of experimental renal ischemia/reperfusion injury. J. Am. Soc. Nephrol. JASN 2003, 14, 1549–1558. [Google Scholar] [CrossRef] [PubMed]
- Sumida, M.; Doi, K.; Ogasawara, E.; Yamashita, T.; Hamasaki, Y.; Kariya, T.; Takimoto, E.; Yahagi, N.; Nangaku, M.; Noiri, E. Regulation of Mitochondrial Dynamics by Dynamin-Related Protein-1 in Acute Cardiorenal Syndrome. J. Am. Soc. Nephrol. 2015, 26, 2378–2387. [Google Scholar] [CrossRef] [PubMed]
- Fox, B.M.; Gil, H.W.; Kirkbride-Romeo, L.; Bagchi, R.A.; Wennersten, S.A.; Haefner, K.R.; Skrypnyk, N.I.; Brown, C.N.; Soranno, D.E.; Gist, K.M.; et al. Metabolomics assessment reveals oxidative stress and altered energy production in the heart after ischemic acute kidney injury in mice. Kidney Int. 2019, 95, 590–610. [Google Scholar] [CrossRef] [PubMed]
- Prud’homme, M.; Coutrot, M.; Michel, T.; Boutin, L.; Genest, M.; Poirier, F.; Launay, J.-M.; Kane, B.; Kinugasa, S.; Prakoura, N.; et al. Acute Kidney Injury Induces Remote Cardiac Damage and Dysfunction through the Galectin-3 Pathway. JACC Basic Transl. Sci. 2019, 4, 717–732. [Google Scholar] [CrossRef] [PubMed]
- Alam, M.L.; Katz, R.; Bellovich, K.A.; Bhat, Z.Y.; Brosius, F.C.; de Boer, I.H.; Gadegbeku, C.A.; Gipson, D.S.; Hawkins, J.J.; Himmelfarb, J.; et al. Soluble ST2 and Galectin-3 and Progression of CKD. Kidney Int. Rep. 2019, 4, 103–111. [Google Scholar] [CrossRef]
- Patel, D.M.; Thiessen-Philbrook, H.; Brown, J.R.; McArthur, E.; Moledina, D.G.; Mansour, S.G.; Shlipak, M.G.; Koyner, J.L.; Kavsak, P.; Whitlock, R.P.; et al. Association of plasma-soluble ST2 and galectin-3 with cardiovascular events and mortality following cardiac surgery. Am. Heart J. 2020, 220, 253–263. [Google Scholar] [CrossRef] [PubMed]
- Bayes-Genis, A.; de Antonio, M.; Vila, J.; Peñafiel, J.; Galán, A.; Barallat, J.; Zamora, E.; Urrutia, A.; Lupón, J. Head-to-head comparison of 2 myocardial fibrosis biomarkers for long-term heart failure risk stratification: ST2 versus galectin-3. J. Am. Coll. Cardiol. 2014, 63, 158–166. [Google Scholar] [CrossRef] [PubMed]
- Rao, V.S.; Ivey-Miranda, J.B.; Cox, Z.L.; Moreno-Villagomez, J.; Testani, J.M. Association of Urine Galectin-3 with Cardiorenal Outcomes in Patients with Heart Failure. J. Card. Fail. 2023, 30, 340–346. [Google Scholar] [CrossRef]
- Ozyildirim, S.; Dogan, O.; Barman, H.A.; Tanyolaç, S.; Atıcı, A.; Enar, R.; Doğan, S.M. Galectin-3 as a Biomarker to Predict Cardiorenal Syndrome in Patients with Acute Heart Failure. Acta Cardiol. Sin. 2023, 39, 862–870. [Google Scholar]
- Martínez-Martínez, E.; Brugnolaro, C.; Ibarrola, J.; Ravassa, S.; Buonafine, M.; López, B.; Fernández-Celis, A.; Querejeta, R.; Santamaria, E.; Fernández-Irigoyen, J.; et al. CT-1 (Cardiotrophin-1)-Gal-3 (Galectin-3) Axis in Cardiac Fibrosis and Inflammation. Hypertens Dallas Tex 1979 2019, 73, 602–611. [Google Scholar] [CrossRef]
- Hogas, S.; Bilha, S.C.; Branisteanu, D.; Hogas, M.; Gaipov, A.; Kanbay, M.; Covic, A. Potential novel biomarkers of cardiovascular dysfunction and disease: Cardiotrophin-1, adipokines and galectin-3. Arch. Med. Sci. AMS 2017, 13, 897–913. [Google Scholar] [CrossRef]
- López-Andrés, N.; Rousseau, A.; Akhtar, R.; Calvier, L.; Iñigo, C.; Labat, C.; Zhao, X.; Cruickshank, K.; Díez, J.; Zannad, F.; et al. Cardiotrophin 1 is involved in cardiac, vascular, and renal fibrosis and dysfunction. Hypertens Dallas Tex 1979 2012, 60, 563–573. [Google Scholar] [CrossRef]
- Madsen, K. Renoprotective effects of cardiotrophin-1 in a mouse model of chronic kidney disease. Acta Physiol. Oxf. Engl. 2019, 226, e13274. [Google Scholar] [CrossRef]
- Perretta-Tejedor, N.; Muñoz-Félix, J.M.; Düwel, A.; Quiros-Luis, Y.; Fernández-Martín, J.L.; Morales, A.I.; López-Hernández, F.J.; Lopez-Novoa, J.M.; Martínez-Salgado, C. Cardiotrophin-1 opposes renal fibrosis in mice: Potential prevention of chronic kidney disease. Acta Physiol. Oxf. Engl. 2019, 226, e13247. [Google Scholar] [CrossRef] [PubMed]
- Morita, H.; Nakae, S.; Saito, H.; Matsumoto, K. IL-33 in clinical practice: Size matters? J. Allergy Clin. Immunol. 2017, 140, 381–383. [Google Scholar] [CrossRef]
- Lefrançais, E.; Roga, S.; Gautier, V.; Gonzalez-de-Peredo, A.; Monsarrat, B.; Girard, J.P.; Cayrol, C. IL-33 is processed into mature bioactive forms by neutrophil elastase and cathepsin G. Proc. Natl. Acad. Sci. USA 2012, 109, 1673–1678. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.Y.; Yang, J.L.; Wu, Y.H.; Li, L.C.; Li, R.F.; Chang, Y.T.; Dai, L.-H.; Wang, W.-C.; Chang, Y.-J. IL-33/ST2 axis mediates hyperplasia of intrarenal urothelium in obstructive renal injury. Exp. Mol. Med. 2018, 50, 1–11. [Google Scholar] [CrossRef]
- Ferhat, M.; Robin, A.; Giraud, S.; Sena, S.; Goujon, J.M.; Touchard, G.; Hauet, T.; Girard, J.-P.; Gombert, J.-M.; Herbelin, A.; et al. Endogenous IL-33 Contributes to Kidney Ischemia-Reperfusion Injury as an Alarmin. J. Am. Soc. Nephrol. JASN 2018, 29, 1272–1288. [Google Scholar] [CrossRef] [PubMed]
- Akcay, A.; Nguyen, Q.; He, Z.; Turkmen, K.; Won Lee, D.; Hernando, A.A.; Altmann, C.; Toker, A.; Pacic, A.; Ljubanovic, D.G.; et al. IL-33 exacerbates acute kidney injury. J. Am. Soc. Nephrol. JASN 2011, 22, 2057–2067. [Google Scholar] [CrossRef] [PubMed]
- Sanada, S.; Hakuno, D.; Higgins, L.J.; Schreiter, E.R.; McKenzie, A.N.J.; Lee, R.T. IL-33 and ST2 comprise a critical biomechanically induced and cardioprotective signaling system. J. Clin. Investig. 2007, 117, 1538–1549. [Google Scholar] [CrossRef]
- Chen, W.Y.; Hong, J.; Gannon, J.; Kakkar, R.; Lee, R.T. Myocardial pressure overload induces systemic inflammation through endothelial cell IL-33. Proc. Natl. Acad. Sci. USA 2015, 112, 7249–7254. [Google Scholar] [CrossRef]
- Ghali, R.; Habeichi, N.J.; Kaplan, A.; Tannous, C.; Abidi, E.; Bekdash, A.; Lee, R.T. IL-33 induces type-2-cytokine phenotype but exacerbates cardiac remodeling post-myocardial infarction with eosinophil recruitment, worsened systolic dysfunction, and ventricular wall rupture. Clin. Sci. Lond. Engl. 1979 2020, 134, 1191–1218. [Google Scholar] [CrossRef]
- Florens, N.; Kasam, R.K.; Rudman-Melnick, V.; Lin, S.C.; Prasad, V.; Molkentin, J.D. Interleukin-33 Mediates Cardiomyopathy after Acute Kidney Injury by Signaling to Cardiomyocytes. Circulation 2023, 147, 746–758. [Google Scholar] [CrossRef]
- Bartunek, J.; Delrue, L.; Van Durme, F.; Muller, O.; Casselman, F.; De Wiest, B.; Croes, R.; Verstreken, S.; Goethals, M.; de Raedt, H.; et al. Nonmyocardial production of ST2 protein in human hypertrophy and failure is related to diastolic load. J. Am. Coll. Cardiol. 2008, 52, 2166–2174. [Google Scholar] [CrossRef]
- Weir, R.A.P.; Miller, A.M.; Murphy, G.E.J.; Clements, S.; Steedman, T.; Connell, J.M.C.; McInnes, I.B.; Dargie, H.J.; Mcmurray, J.J. Serum soluble ST2: A potential novel mediator in left ventricular and infarct remodeling after acute myocardial infarction. J. Am. Coll. Cardiol. 2010, 55, 243–250. [Google Scholar] [CrossRef]
- Ghali, R.; Altara, R.; Louch, W.E.; Cataliotti, A.; Mallat, Z.; Kaplan, A.; Zouein, F.A.; Booz, G.W. IL-33 (Interleukin 33)/sST2 Axis in Hypertension and Heart Failure. Hypertens Dallas Tex 1979 2018, 72, 818–828. [Google Scholar] [CrossRef] [PubMed]
- Coronado, M.J.; Bruno, K.A.; Blauwet, L.A.; Tschöpe, C.; Cunningham, M.W.; Pankuweit, S.; van Linthout, S.; Jeon, E.-S.; McNamara, D.M.; Krejčí, J.; et al. Elevated Sera sST2 Is Associated with Heart Failure in Men ≤ 50 Years Old with Myocarditis. J. Am. Heart Assoc. 2019, 8, e008968. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; He, M.; Li, H.; Chen, Y.; Nie, X.; Cai, Y.; Xie, R.; Li, L.; Chen, P.; Sun, Y.; et al. Soluble ST2 Is a Sensitive and Specific Biomarker for Fulminant Myocarditis. J. Am. Heart Assoc. 2022, 11, e024417. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Martínez, E.; Miana, M.; Jurado-López, R.; Rousseau, E.; Rossignol, P.; Zannad, F.; Cachofeiro, V.; López-Andrés, N. A role for soluble ST2 in vascular remodeling associated with obesity in rats. PLoS ONE 2013, 8, e79176. [Google Scholar] [CrossRef] [PubMed]
- Te Riet, L.; van Esch, J.H.M.; Roks, A.J.M.; van den Meiracker, A.H.; Danser, A.H.J. Hypertension: Renin-angiotensin-aldosterone system alterations. Circ. Res. 2015, 116, 960–975. [Google Scholar] [CrossRef]
- Lambers Heerspink, H.J.; de Borst, M.H.; Bakker, S.J.L.; Navis, G.J. Improving the efficacy of RAAS blockade in patients with chronic kidney disease. Nat. Rev. Nephrol. 2013, 9, 112–121. [Google Scholar] [CrossRef]
- Koszegi, S.; Molnar, A.; Lenart, L.; Hodrea, J.; Balogh, D.B.; Lakat, T.; Szkibinszkij, E.; Hosszu, A.; Sparding, N.; Genovese, F.; et al. RAAS inhibitors directly reduce diabetes-induced renal fibrosis via growth factor inhibition. J. Physiol. 2019, 597, 193–209. [Google Scholar] [CrossRef]
- Okamoto, K.; Fujii, H.; Watanabe, K.; Goto, S.; Kono, K.; Nishi, S. Changes of FGF23 and the Renin-Angiotensin-System in Male Mouse Models of Chronic Kidney Disease and Cardiac Hypertrophy. J. Endocr. Soc. 2022, 6, bvab187. [Google Scholar] [CrossRef] [PubMed]
- Desai, A.S.; Webb, D.J.; Taubel, J.; Casey, S.; Cheng, Y.; Robbie, G.J.; Foster, D.; Huang, S.A.; Rhyee, S.; Sweetser, M.T.; et al. Zilebesiran, an RNA Interference Therapeutic Agent for Hypertension. N. Engl. J. Med. 2023, 389, 228–238. [Google Scholar] [CrossRef] [PubMed]
- Chang, D.; Xu, T.T.; Zhang, S.J.; Cai, Y.; Min, S.D.; Zhao, Z.; Lu, C.-Q.; Wang, Y.-C.; Ju, S. Telmisartan ameliorates cardiac fibrosis and diastolic function in cardiorenal heart failure with preserved ejection fraction. Exp. Biol. Med. Maywood NJ 2021, 246, 2511–2521. [Google Scholar] [CrossRef] [PubMed]
- Tibi, S.; Zeynalvand, G.; Mohsin, H. Role of the Renin Angiotensin Aldosterone System in the Pathogenesis of Sepsis-Induced Acute Kidney Injury: A Systematic Review. J. Clin. Med. 2023, 12, 4566. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.Y.; Tsai, I.J.; Pan, H.C.; Liao, H.W.; Neyra, J.A.; Wu, V.C.; Chueh, J.S. The Impact of Angiotensin-Converting Enzyme Inhibitors or Angiotensin II Receptor Blockers on Clinical Outcomes of Acute Kidney Disease Patients: A Systematic Review and Meta-Analysis. Front. Pharmacol. 2021, 12, 665250. [Google Scholar] [CrossRef]
- Urbanek, K.; Cappetta, D.; Bellocchio, G.; Coppola, M.A.; Imbrici, P.; Telesca, M.; Donniacuo, M.; Riemma, M.A.; Mele, E.; Cianflone, E.; et al. Dapagliflozin protects the kidney in a non-diabetic model of cardiorenal syndrome. Pharmacol. Res. 2023, 188, 106659. [Google Scholar] [CrossRef]
- Voors, A.A.; Damman, K.; Teerlink, J.R.; Angermann, C.E.; Collins, S.P.; Kosiborod, M.; Biegus, J.; Ferreira, J.P.; Nassif, M.E.; Psotka, M.A.; et al. Renal effects of empagliflozin in patients hospitalized for acute heart failure: From the EMPULSE trial. Eur. J. Heart Fail. 2022, 24, 1844–1852. [Google Scholar] [CrossRef]
- Kluger, A.Y.; Tecson, K.M.; Barbin, C.M.; Lee, A.Y.; Lerma, E.V.; Rosol, Z.P.; Rangaswami, J.; Lepor, N.E.; Cobble, M.E.; McCullough, P.A. Cardiorenal Outcomes in the CANVAS, DECLARE-TIMI 58, and EMPA-REG OUTCOME Trials: A Systematic Review. Rev. Cardiovasc. Med. 2018, 19, 41–49. [Google Scholar] [PubMed]
- Fitchett, D.; Inzucchi, S.E.; Cannon, C.P.; McGuire, D.K.; Scirica, B.M.; Johansen, O.E.; Sambevski, S.; Kaspers, S.; Pfarr, E.; George, J.T.; et al. Empagliflozin Reduced Mortality and Hospitalization for Heart Failure across the Spectrum of Cardiovascular Risk in the EMPA-REG OUTCOME Trial. Circulation 2019, 139, 1384–1395. [Google Scholar] [CrossRef] [PubMed]
- Fisman, E.Z.; Tenenbaum, A. The dual glucose-dependent insulinotropic polypeptide (GIP) and glucagon-like peptide-1 (GLP-1) receptor agonist tirzepatide: A novel cardiometabolic therapeutic prospect. Cardiovasc. Diabetol. 2021, 20, 225. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.H.; Hwang, I.C.; Choi, H.M.; Ahn, C.H.; Yoon, Y.E.; Cho, G.Y. Differential cardiovascular and renal benefits of SGLT2 inhibitors and GLP1 receptor agonists in patients with type 2 diabetes mellitus. Int. J. Cardiol. 2022, 364, 104–111. [Google Scholar] [CrossRef] [PubMed]
- Alicic, R.Z.; Neumiller, J.J. Incretin Therapies for Patients with Type 2 Diabetes and Chronic Kidney Disease. J. Clin. Med. 2023, 13, 201. [Google Scholar] [CrossRef]
- Sattar, N.; McGuire, D.K.; Pavo, I.; Weerakkody, G.J.; Nishiyama, H.; Wiese, R.J.; Zoungas, S. Tirzepatide cardiovascular event risk assessment: A pre-specified meta-analysis. Nat. Med. 2022, 28, 591–598. [Google Scholar] [CrossRef]
- Rossing, P.; Anker, S.D.; Filippatos, G.; Pitt, B.; Ruilope, L.M.; Birkenfeld, A.L.; McGill, J.B.; Rosas, S.E.; Joseph, A.; Gebel, M.; et al. Finerenone in Patients with Chronic Kidney Disease and Type 2 Diabetes by Sodium-Glucose Cotransporter 2 Inhibitor Treatment: The FIDELITY Analysis. Diabetes Care 2022, 45, 2991–2998. [Google Scholar] [CrossRef]
- Han, Y.; Xian, Y.; Gao, X.; Qiang, P.; Hao, J.; Yang, F.; Shimosawa, T.; Chang, Y.; Xu, Q. Eplerenone inhibits the macrophage-to-myofibroblast transition in rats with UUO-induced type 4 cardiorenal syndrome through the MR/CTGF pathway. Int. Immunopharmacol. 2022, 113 Pt A, 109396. [Google Scholar] [CrossRef]
- Chang, Y.; Ben, Y.; Li, H.; Xiong, Y.; Chen, G.; Hao, J.; Ma, X.; Gao, X.; Qiang, P.; Shimosawa, T.; et al. Eplerenone Prevents Cardiac Fibrosis by Inhibiting Angiogenesis in Unilateral Urinary Obstruction Rats. J. Renin-Angiotensin-Aldosterone Syst. JRAAS 2022, 2022, 1283729. [Google Scholar] [CrossRef] [PubMed]
- Verma, S.; Pandey, A.; Pandey, A.K.; Butler, J.; Lee, J.S.; Teoh, H.; Mazer, C.D.; Kosiborod, M.N.; Cosentino, F.; Anker, S.D.; et al. Aldosterone and Aldosterone Synthase Inhibitors in Cardiorenal Disease. Am. J. Physiol. Heart Circ. Physiol. 2023. [Google Scholar] [CrossRef]
- Freeman, M.W.; Halvorsen, Y.D.; Marshall, W.; Pater, M.; Isaacsohn, J.; Pearce, C.; Murphy, B.; Alp, N.; Srivastava, A.; Bhatt, D.L.; et al. Phase 2 Trial of Baxdrostat for Treatment-Resistant Hypertension. N. Engl. J. Med. 2023, 388, 395–405. [Google Scholar] [CrossRef]
- von Lewinski, D.; Kolesnik, E.; Tripolt, N.J.; Pferschy, P.N.; Benedikt, M.; Wallner, M.; Alber, H.; Berger, R.; Lichtenauer, M.; Saely, C.H.; et al. Empagliflozin in acute myocardial infarction: The EMMY trial. Eur. Heart J. 2022, 43, 4421–4432. [Google Scholar] [CrossRef]
- James, S.; Erlinge, D.; Storey, R.F.; McGuire, D.K.; de Belder, M.; Björkgren, I.; Johansson, P.A.; Langkilde, A.M.; Ridderstråle, W.; Rizi, E.P.; et al. Rationale and design of the DAPA-MI trial: Dapagliflozin in patients without diabetes mellitus with acute myocardial infarction. Am. Heart J. 2023, 266, 188–197. [Google Scholar] [CrossRef]
- Tardif, J.C.; Kouz, S.; Waters, D.D.; Bertrand, O.F.; Diaz, R.; Maggioni, A.P.; Pinto, F.J.; Ibrahim, R.; Gamra, H.; Kiwan, G.S.; et al. Efficacy and Safety of Low-Dose Colchicine after Myocardial Infarction. N. Engl. J. Med. 2019, 381, 2497–2505. [Google Scholar] [CrossRef] [PubMed]
- Ridker, P.M.; Everett, B.M.; Thuren, T.; MacFadyen, J.G.; Chang, W.H.; Ballantyne, C.; Fonseca, F.; Nicolau, J.; Koenig, W.; Anker, S.D.; et al. Antiinflammatory Therapy with Canakinumab for Atherosclerotic Disease. N. Engl. J. Med. 2017, 377, 1119–1131. [Google Scholar] [CrossRef] [PubMed]
- Velazquez, E.J.; Morrow, D.A.; DeVore, A.D.; Duffy, C.I.; Ambrosy, A.P.; McCague, K.; Rocha, R.; Braunwald, E.; PIONEER-HF Investigators. Angiotensin-Neprilysin Inhibition in Acute Decompensated Heart Failure. N. Engl. J. Med. 2019, 380, 539–548. [Google Scholar] [CrossRef] [PubMed]
- Gayat, E.; Hollinger, A.; Cariou, A.; Deye, N.; Vieillard-Baron, A.; Jaber, S.; Chousterman, B.G.; Lu, Q.; Laterre, P.F.; Monnet, X.; et al. Impact of angiotensin-converting enzyme inhibitors or receptor blockers on post-ICU discharge outcome in patients with acute kidney injury. Intensive Care Med. 2018, 44, 598–605. [Google Scholar] [CrossRef]
- Legrand, M.; Rossignol, P. Cardiovascular Consequences of Acute Kidney Injury. N. Engl. J. Med. 2020, 382, 2238–2247. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bedo, D.; Beaudrey, T.; Florens, N. Unraveling Chronic Cardiovascular and Kidney Disorder through the Butterfly Effect. Diagnostics 2024, 14, 463. https://doi.org/10.3390/diagnostics14050463
Bedo D, Beaudrey T, Florens N. Unraveling Chronic Cardiovascular and Kidney Disorder through the Butterfly Effect. Diagnostics. 2024; 14(5):463. https://doi.org/10.3390/diagnostics14050463
Chicago/Turabian StyleBedo, Dimitri, Thomas Beaudrey, and Nans Florens. 2024. "Unraveling Chronic Cardiovascular and Kidney Disorder through the Butterfly Effect" Diagnostics 14, no. 5: 463. https://doi.org/10.3390/diagnostics14050463
APA StyleBedo, D., Beaudrey, T., & Florens, N. (2024). Unraveling Chronic Cardiovascular and Kidney Disorder through the Butterfly Effect. Diagnostics, 14(5), 463. https://doi.org/10.3390/diagnostics14050463





