New Therapeutic Approaches for the Treatment of Patients with Metabolic Dysfunction-Associated Steatotic Liver Disease (MASLD) and Increased Cardiovascular Risk
Abstract
1. Introduction
2. Association between Metabolic Dysfunction-Associated Steatotic Liver Disease (MASLD)/Metabolic Dysfunction-Associated Steatohepatitis (MASH) and Cardiovascular Diseases (CVD)
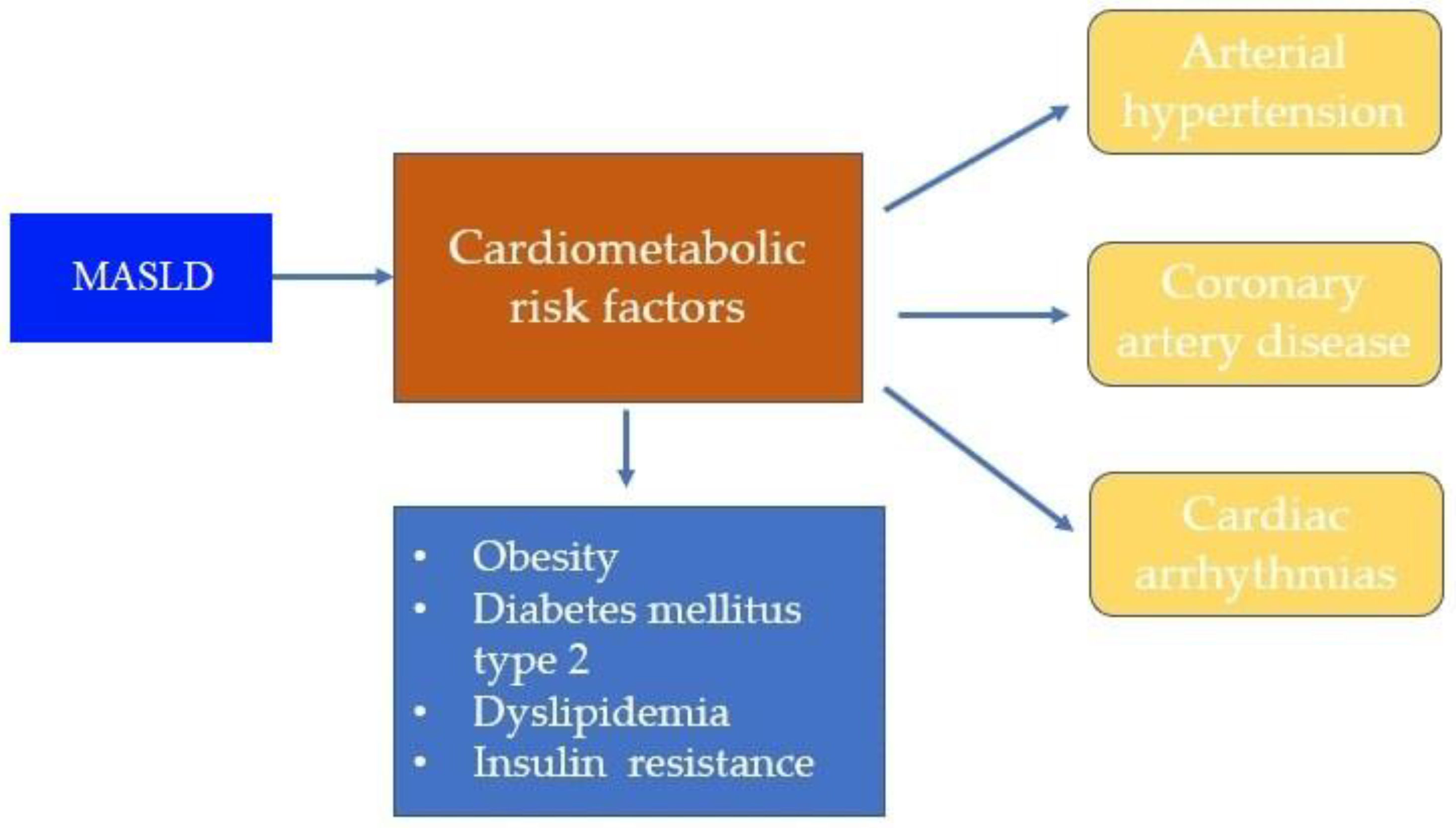
3. Common Cardiovascular Diseases (CVD) in Patients with Metabolic Dysfunction-Associated Steatotic Liver Disease (MASLD)
3.1. Arterial Hypertension
3.2. Coronary Artery Disease
3.3. Cardiac Arrhythmias
3.4. Heart Failure
4. New Therapeutic Approaches for the Treatment of Metabolic Dysfunction-Associated Steatotic Liver Disease (MASLD)/Metabolic Dysfunction-Associated Steatohepatitis (MASH) and Their Effects on Cardiovascular Risk Reduction
4.1. Farnesoid X Receptor (FXR) Agonists
4.2. Peroxisome Proliferator-Activated Receptor (PPAR) Agonists
4.3. Analogues of Fibroblast Growth Factors (FGF)
4.4. The Other Drugs Which Have Beneficial Effects
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Roeb, E.; Geier, A. Nonalcoholic steatohepatitis (NASH)—Current treatment recommendations and future developments. Z. Gastroenterol. 2019, 57, 508–517. [Google Scholar] [CrossRef]
- Wong, R.J.; Aguilar, M.; Cheung, R.; Perumpail, B.R.; Harrison, A.S.; Younossi, M.Z.; Ahmed, A. Nonalcoholic steatohepatitis is the second leading etiology of liver disease among adults awaiting liver transplantation in the United States. Gastroenterology 2015, 148, 547–555. [Google Scholar] [CrossRef]
- Branković, M.; Jovanović, I.; Dukić, M.; Radonjić, T.; Oprić, S.; Klašnja, S.; Zdravković, M. Lipotoxicity as the Leading Cause of Non-Alcoholic Steatohepatitis. Int. J. Mol. Sci. 2022, 23, 5146. [Google Scholar] [CrossRef]
- Rinella, M.E.; Lazarus, J.V.; Ratziu, V.; Francque, S.M.; Sanyal, A.J.; Kanwal, F.; Romero, D.; Abdelmalek, M.F.; Anstee, Q.M.; Arab, J.P.; et al. A multisociety Delphi consensus statement on new fatty liver disease nomenclature. Hepatology 2023, 78, 1966–1986. [Google Scholar] [CrossRef]
- Eslam, M.; Newsome, P.N.; Sarin, S.K.; Anstee, Q.M.; Targher, G.; Romero-Gomez, M.; Zelber-Sagi, S.; Wai-Sun Wong, V.; Dufour, J.F.; Schattenberg, J.M.; et al. A new definition for metabolic dysfunction-associated fatty liver disease: An international expert consensus statement. J. Hepatol. 2020, 73, 202–209. [Google Scholar] [CrossRef]
- Wang, X.; Wu, S.; Yuan, X.; Chen, S.; Fu, Q.; Sun, Y.; Lan, Y.; Hu, S.; Wang, Y.; Lu, Y.; et al. Metabolic Dysfunctionassociated Fatty Liver Disease and Mortality Among Chinese Adults: A Prospective Cohort Study. J. Clin. Endocrinol. Metab. 2022, 107, e745–e755. [Google Scholar] [CrossRef]
- Lin, S.; Huang, J.; Wang, M.; Kumar, R.; Liu, Y.; Liu, S.; Wu, Y.; Wang, X.; Zhu, Y. Comparison of MAFLD and NAFLD diagnostic criteria in real world. Liver Int. 2020, 40, 2082–2089. [Google Scholar] [CrossRef]
- Lin, Y.C.; Wu, C.C.; Ni, Y.H. New Perspectives on Genetic Prediction for Pediatric Metabolic Associated Fatty Liver Disease. Front. Pediatr. 2020, 8, 603654. [Google Scholar] [CrossRef]
- Radonjić, T.; Dukić, M.; Jovanović, I.; Zdravković, M.; Mandić, O.; Popadić, V.; Popović, M.; Nikolić, N.; Klašnja, S.; Divac, A.; et al. Aging of Liver in Its Different Diseases. Int. J. Mol. Sci. 2022, 23, 13085. [Google Scholar] [CrossRef]
- Llovet, J.M.; Willoughby, C.E.; Singal, A.G.; Greten, T.F.; Heikenwälder, M.; El-Serag, H.B.; Finn, R.S.; Friedman, S.L. Nonalcoholic steatohepatitis-related hepatocellular carcinoma: Pathogenesis and treatment. Nat. Rev. Gastroenterol. Hepatol. 2023, 20, 487–503. [Google Scholar] [CrossRef]
- Heymann, F.; Tacke, F. Immunology in the liver—From homeostasis to disease. Nat. Rev. Gastroenterol. Hepatol. 2016, 13, 88–110. [Google Scholar] [CrossRef]
- Gao, J.; Wei, B.; de Assuncao, T.M.; Liu, Z.; Hu, X.; Ibrahim, S.; Cooper, S.A.; Cao, S.; Shah, V.H.; Kostallari, E. Hepatic stellate cell autophagy inhibits extracellular vesicle release to attenuate liver fibrosis. J. Hepatol. 2020, 73, 1144–1154. [Google Scholar] [CrossRef]
- Chen, Q.T.; Zhang, Z.Y.; Huang, Q.L.; Chen, H.Z.; Hong, W.B.; Lin, T.; Zhao, W.X.; Wang, X.M.; Ju, C.Y.; Wu, L.Z.; et al. HK1 from hepatic stellate cell-derived extracellular vesicles promotes progression of hepatocellular carcinoma. Nat. Metab. 2022, 4, 1306–1321. [Google Scholar] [CrossRef]
- Gómez, M.; Vila, J.; Elosua, R.; Molina, L.; Bruguera, J.; Sala, J.; Masià, R.; Covas, M.I.; Marrugat, J.; Fitó, M. Relationship of lipid oxidation with subclinical atherosclerosis and 10-year coronary events in general population. Atherosclerosis 2014, 232, 134–140. [Google Scholar] [CrossRef]
- Sookoian, S.; Gianotti, T.F.; Rosselli, M.S.; Burgueño, A.L.; Castaño, G.O.; Pirola, C.J. Liver transcriptional profile of atherosclerosis-related genes in human nonalcoholic fatty liver disease. Atherosclerosis 2011, 218, 378–385. [Google Scholar] [CrossRef]
- Ertunc, M.E.; Hotamisligil, G.S. Lipid signaling and lipotoxicity in metaflammation: Indications for metabolic disease pathogenesis and treatment. J. Lipid Res. 2016, 57, 2099–2114. [Google Scholar] [CrossRef]
- European Association for the Study of the Liver (EASL); European Association for the Study of Diabetes (EASD); European Association for the Study of Obesity (EASO). EASL-EASD-EASO Clinical Practice Guidelines for the management of non-alcoholic fatty liver disease. Diabetologia 2016, 59, 1121–1140. [Google Scholar] [CrossRef]
- Ye, R.; Onodera, T.; Scherer, P.E. Lipotoxicity and β Cell Maintenance in Obesity and Type 2 Diabetes. J. Endocr. Soc. 2019, 3, 617–631. [Google Scholar] [CrossRef]
- Wende, A.R.; Abel, E.D. Lipotoxicity in the heart. Biochim. Biophys. Acta 2010, 1801, 311–319. [Google Scholar] [CrossRef]
- Jang, H.S.; Noh, M.R.; Kim, J.; Padanilam, B.J. Defective Mitochondrial Fatty Acid Oxidation and Lipotoxicity in Kidney Diseases. Front. Med. 2020, 7, 65. [Google Scholar] [CrossRef]
- Zhao, S.M.; Ren, L.J.; Chen, L.; Zhang, X.; Cheng, M.L.; Li, W.Z.; Zhang, Y.Y.; Gao, S.Z. Differential expression of lipid metabolism related genes in porcine muscle tissue leading to different intramuscular fat deposition. Lipids 2009, 44, 1029–1037. [Google Scholar] [CrossRef]
- Schrauwen, P.; Schrauwen-Hinderling, V.; Hoeks, J.; Hesselink, M.K. Mitochondrial dysfunction and lipotoxicity. Biochim. Biophys. Acta 2010, 1801, 266–271. [Google Scholar] [CrossRef]
- Vanni, E.; Bugianesi, E.; Kotronen, A.; De Minicis, S.; Yki-Järvinen, H.; Svegliati-Baroni, G. From the metabolic syndrome to NAFLD or vice versa? Dig. Liver Dis. 2010, 42, 320–330. [Google Scholar] [CrossRef]
- Fan, J.; Watanabe, T. Atherosclerosis: Known and unknown. Pathol. Int. 2022, 72, 151–160. [Google Scholar] [CrossRef]
- Lonardo, A.; Sookoian, S.; Pirola, C.J.; Targher, G. Non-alcoholic fatty liver disease and risk of cardiovascular disease. Metabolism 2016, 65, 1136–1150. [Google Scholar] [CrossRef]
- Lee, H.H.; Cho, Y.; Choi, Y.J.; Huh, B.W.; Lee, B.W.; Kang, E.S.; Park, S.W.; Cha, B.S.; Lee, E.J.; Lee, Y.H.; et al. Non-alcoholic steatohepatitis and progression of carotid atherosclerosis in patients with type 2 diabetes: A Korean cohort study. Cardiovasc. Diabetol. 2020, 19, 81. [Google Scholar] [CrossRef]
- Rampally, V.; Biri, S.K.; Nair, I.K.; Vadlakonda, A. Determination of association between nonalcoholic fatty liver disease and carotid artery atherosclerosis among nondiabetic individuals. J. Fam. Med. Prim. Care 2020, 9, 1182–1186. [Google Scholar] [CrossRef]
- Ridker, P.M. Testing the inflammatory hypothesis of atherothrombosis: Scientific rationale for the cardiovascular inflammation reduction trial (CIRT). J. Thromb. Haemost. 2009, 7 (Suppl. S1), 332–339. [Google Scholar] [CrossRef]
- Lechner, K.; McKenzie, A.L.; Kränkel, N.; Von Schacky, C.; Worm, N.; Nixdorff, U.; Lechner, B.; Scherr, J.; Weingärtner, O.; Krauss, R.M. High-Risk Atherosclerosis and Metabolic Phenotype: The Roles of Ectopic Adiposity, Atherogenic Dyslipidemia, and Inflammation. Metab. Syndr. Relat. Disord. 2020, 18, 176–185. [Google Scholar] [CrossRef]
- Francque, S.M.; van der Graaff, D.; Kwanten, W.J. Non-alcoholic fatty liver disease and cardiovascular risk: Pathophysiological mechanisms and implications. J. Hepatol. 2016, 65, 425–443. [Google Scholar] [CrossRef]
- Mahfood Haddad, T.; Hamdeh, S.; Kanmanthareddy, A.; Alla, V.M. Nonalcoholic fatty liver disease and the risk of clinical cardiovascular events: A systematic review and meta-analysis. Diabetes Metab. Syndr. 2017, 11 (Suppl. S1), S209–S216. [Google Scholar] [CrossRef] [PubMed]
- Wong, C.R.; Lim, J.K. The Association Between Nonalcoholic Fatty Liver Disease and Cardiovascular Disease Outcomes. Clin. Liver Dis. 2018, 12, 39–44. [Google Scholar] [CrossRef]
- Iacobellis, G. Epicardial adipose tissue in contemporary cardiology. Nat. Rev. Cardiol. 2022, 19, 593–606. [Google Scholar] [CrossRef] [PubMed]
- Donati, G.; Stagni, B.; Piscaglia, F.; Venturoli, N.; Morselli-Labate, A.M.; Rasciti, L.; Bolondi, L. Increased prevalence of fatty liver in arterial hypertensive patients with normal liver enzymes: Role of insulin resistance. Gut 2004, 53, 1020–1023. [Google Scholar] [CrossRef]
- López-Suárez, A.; Guerrero, J.M.; Elvira-González, J.; Beltrán-Robles, M.; Cañas-Hormigo, F.; Bascuñana-Quirell, A. Nonalcoholic fatty liver disease is associated with blood pressure in hypertensive and nonhypertensive individuals from the general population with normal levels of alanine aminotransferase. Eur. J. Gastroenterol. Hepatol. 2011, 23, 1011–1017. [Google Scholar] [CrossRef] [PubMed]
- Aneni, E.C.; Oni, E.T.; Martin, S.S.; Blaha, M.J.; Agatston, A.S.; Feldman, T.; Veledar, E.; Conçeicao, R.D.; Carvalho, J.A.; Santos, R.D.; et al. Blood pressure is associated with the presence and severity of nonalcoholic fatty liver disease across the spectrum of cardiometabolic risk. J. Hypertens. 2015, 33, 1207–1214. [Google Scholar] [CrossRef] [PubMed]
- Ryoo, J.H.; Suh, Y.J.; Shin, H.C.; Cho, Y.K.; Choi, J.M.; Park, S.K. Clinical association between non-alcoholic fatty liver disease and the development of hypertension. J. Gastroenterol. Hepatol. 2014, 29, 1926–1931. [Google Scholar] [CrossRef] [PubMed]
- Latea, L.; Negrea, S.; Bolboaca, S. Primary non-alcoholic fatty liver disease in hypertensive patients. Australas. Med. J. 2013, 6, 325–330. [Google Scholar] [CrossRef]
- Ren, Z.; Simons, P.I.H.G.; Wesselius, A.; Stehouwer, C.D.A.; Brouwers, M.C.G.J. Relationship between NAFLD and coronary artery disease: A Mendelian randomization study. Hepatology 2023, 77, 230–238. [Google Scholar] [CrossRef]
- Peng, H.; Wang, S.; Wang, M.; Ye, Y.; Xue, E.; Chen, X.; Wang, X.; Fan, M.; Gao, W.; Qin, X.; et al. Nonalcoholic fatty liver disease and cardiovascular diseases: A Mendelian randomization study. Metabolism 2022, 133, 155220. [Google Scholar] [CrossRef]
- Osawa, K.; Miyoshi, T.; Yamauchi, K.; Koyama, Y.; Nakamura, K.; Sato, S.; Kanazawa, S.; Ito, H. Nonalcoholic Hepatic Steatosis Is a Strong Predictor of High-Risk Coronary-Artery Plaques as Determined by Multidetector CT. PLoS ONE 2015, 10, e0131138. [Google Scholar] [CrossRef] [PubMed]
- Keskin, M.; Hayıroğlu, M.İ.; Uzun, A.O.; Güvenç, T.S.; Şahin, S.; Kozan, Ö. Effect of Nonalcoholic Fatty Liver Disease on In-Hospital and Long-Term Outcomes in Patients With ST-Segment Elevation Myocardial Infarction. Am. J. Cardiol. 2017, 120, 1720–1726. [Google Scholar] [CrossRef] [PubMed]
- Targher, G.; Mantovani, A.; Pichiri, I.; Rigolon, R.; Dauriz, M.; Zoppini, G.; Morani, G.; Vassanelli, C.; Bonora, E. Non-alcoholic fatty liver disease is associated with an increased prevalence of atrial fibrillation in hospitalized patients with type 2 diabetes. Clin. Sci. 2013, 125, 301–309. [Google Scholar] [CrossRef] [PubMed]
- Targher, G.; Valbusa, F.; Bonapace, S.; Bertolini, L.; Zenari, L.; Rodella, S.; Zoppini, G.; Mantovani, W.; Barbieri, E.; Byrne, C.D. Non-alcoholic fatty liver disease is associated with an increased incidence of atrial fibrillation in patients with type 2 diabetes. PLoS ONE 2013, 8, e57183. [Google Scholar] [CrossRef] [PubMed]
- Sinner, M.F.; Wang, N.; Fox, C.S.; Fontes, J.D.; Rienstra, M.; Magnani, J.W.; Vasan, R.S.; Calderwood, A.H.; Pencina, M.; Sullivan, L.M.; et al. Relation of circulating liver transaminase concentrations to risk of new-onset atrial fibrillation. Am. J. Cardiol. 2013, 111, 219–224. [Google Scholar] [CrossRef]
- Alonso, A.; Misialek, J.R.; Amiin, M.A.; Hoogeveen, R.C.; Chen, L.Y.; Agarwal, S.K.; Loehr, L.R.; Soliman, E.Z.; Selvin, E. Circulating levels of liver enzymes and incidence of atrial fibrillation: The Atherosclerosis Risk in Communities cohort. Heart 2014, 100, 1511–1516. [Google Scholar] [CrossRef]
- Käräjämäki, A.J.; Pätsi, O.P.; Savolainen, M.; Kesäniemi, Y.A.; Huikuri, H.; Ukkola, O. Non-Alcoholic Fatty Liver Disease as a Predictor of Atrial Fibrillation in Middle-Aged Population (OPERA Study). PLoS ONE 2015, 10, e0142937. [Google Scholar] [CrossRef]
- Lazzerini, P.E.; Capecchi, P.L.; Laghi-Pasini, F. Long QT Syndrome: An Emerging Role for Inflammation and Immunity. Front. Cardiovasc. Med. 2015, 2, 26. [Google Scholar] [CrossRef]
- Targher, G.; Valbusa, F.; Bonapace, S.; Bertolini, L.; Zenari, L.; Pichiri, I.; Mantovani, A.; Zoppini, G.; Bonora, E.; Barbieri, E.; et al. Association of nonalcoholic fatty liver disease with QTc interval in patients with type 2 diabetes. Nutr. Metab. Cardiovasc. Dis. 2014, 24, 663–669. [Google Scholar] [CrossRef]
- Hung, C.S.; Tseng, P.H.; Tu, C.H.; Chen, C.C.; Liao, W.C.; Lee, Y.C.; Chiu, H.M.; Lin, H.J.; Ho, Y.L.; Yang, W.S.; et al. Nonalcoholic Fatty Liver Disease Is Associated with QT Prolongation in the General Population. J. Am. Heart Assoc. 2015, 4, e001820. [Google Scholar] [CrossRef]
- Mantovani, A.; Rigamonti, A.; Bonapace, S.; Bolzan, B.; Pernigo, M.; Morani, G.; Franceschini, L.; Bergamini, C.; Bertolini, L.; Valbusa, F.; et al. Nonalcoholic Fatty Liver Disease Is Associated with Ventricular Arrhythmias in Patients with Type 2 Diabetes Referred for Clinically Indicated 24-Hour Holter Monitoring. Diabetes Care 2016, 39, 1416–1423. [Google Scholar] [CrossRef] [PubMed]
- Fudim, M.; Zhong, L.; Patel, K.V.; Khera, R.; Abdelmalek, M.F.; Diehl, A.M.; McGarrah, R.W.; Molinger, J.; Moylan, C.A.; Rao, V.N.; et al. Nonalcoholic Fatty Liver Disease and Risk of Heart Failure Among Medicare Beneficiaries. J. Am. Heart Assoc. 2021, 10, e021654. [Google Scholar] [CrossRef] [PubMed]
- Mantovani, A.; Petracca, G.; Csermely, A.; Beatrice, G.; Bonapace, S.; Rossi, A.; Tilg, H.; Byrne, C.D.; Targher, G. Non-alcoholic fatty liver disease and risk of new-onset heart failure: An updated meta-analysis of about 11 million individuals. Gut 2023, 72, 372–380. [Google Scholar] [CrossRef] [PubMed]
- Paternostro, R.; Trauner, M. Current treatment of non-alcoholic fatty liver disease. J. Intern. Med. 2022, 292, 190–204. [Google Scholar] [CrossRef] [PubMed]
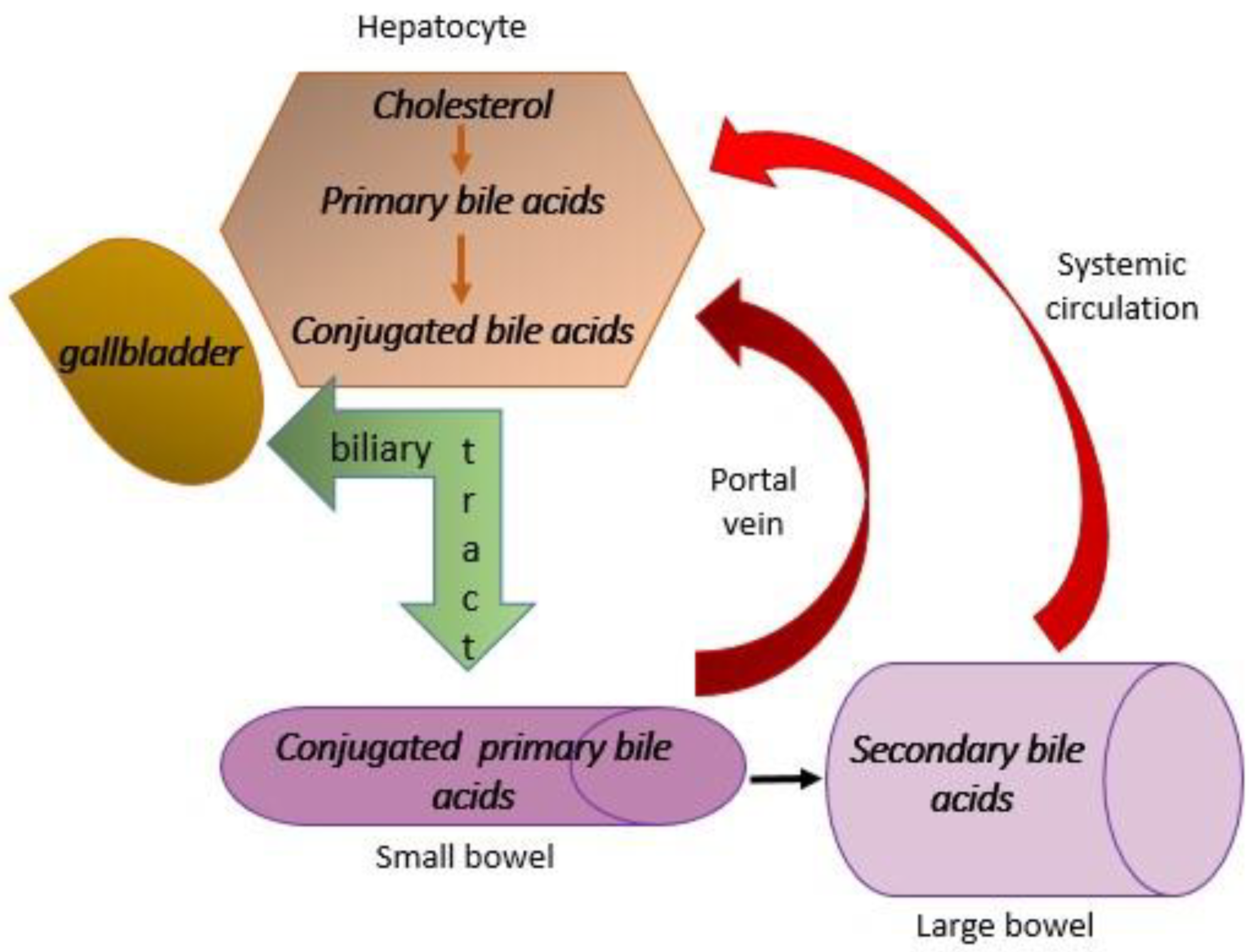
- Kumar, A.; Al-Hassi, H.O.; Steed, H.; Phipps, O.; Brookes, M.J. Bile Acids and the Microbiome: Making Sense of This Dynamic Relationship in Their Role and Management in Crohn’s Disease. Can. J. Gastroenterol. Hepatol. 2022, 2022, 8416578. [Google Scholar] [CrossRef]
- Cai, J.; Sun, L.; Gonzalez, F.J. Gut microbiota-derived bile acids in intestinal immunity, inflammation, and tumorigenesis. Cell Host Microbe 2022, 30, 289–300. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Yang, D.; Wang, X.; Asare, P.T.; Zhang, Q.; Na, L.; Shao, L. Gut Microbiota Targeted Approach in the Management of Chronic Liver Diseases. Front. Cell. Infect. Microbiol. 2022, 12, 774335. [Google Scholar] [CrossRef]
- Cowen, A.E.; Κοrman, M.G.; Hofmann, A.F.; Cass, O.W.; Coffin, S.B. Metabolism of Lithocholate in Healthy Man. II. Enterohepatic circulation. Gastroenterology 1975, 69, 67–76. [Google Scholar] [CrossRef]
- Xiang, J.; Zhang, Z.; Xie, H.; Zhang, C.; Bai, Y.; Cao, H.; Che, Q.; Guo, J.; Su, Z. Effect of different bile acids on the intestine through enterohepatic circulation based on FXR. Gut Microbes 2021, 13, 1949095. [Google Scholar] [CrossRef]
- Younossi, M.Z.; Ratziu, V.; Loomba, R.; Rinella, M.; Anstee, Q.; Goodman, Z.; Bedossa, P.; Geier, A.; Beckebaum, S.; Newsome, P.; et al. Obeticholic acid for the treatment of non-alcoholic statohepatitis: Interim analysis from multicentre, randomised, placebo-controlled phase 3 trial. Lancet 2019, 394, 21842196. [Google Scholar] [CrossRef]
- Modica, S.; Gadaleta, M.R.; Moschetta, A. Deciphering the nuclear bile acid receptor FXR paradigm. Nucl. Recept. Signal. 2010, 8, e005. [Google Scholar] [CrossRef] [PubMed]
- Jhaveri, A.M.; Kowdley, V.K. New developments in the treatment of primary biliary cholangitis—Role of obeticholic acid. Ther. Clin. Risk Manag. 2017, 13, 1053–1060. [Google Scholar] [CrossRef] [PubMed]
- Ratziu, V.; Sanyal, J.A.; Loomba, R.; Rinella, M.; Harrison, S.; Anstee, M.Q.; Goodman, Z.; Bedossa, P.; MacConell, L.; Shringarpure, R.; et al. REGENERATE: Design of a pivotal, randomised, phase 3 study evaluating the safety and efficacy of obeticholic acid in patients with fibrosis due to nonalcoholic steatohepatitis. Contemp. Clin. Trials 2019, 84, 105803. [Google Scholar] [CrossRef] [PubMed]
- Neuschwander-Tetri, A.B.; Loomba, R.; Sanyal, J.A.; Lavine, E.J.; Van Natta, L.M.; Abdelmalek, F.M.; Chalasani, N.; Dasarathz, S.; Mae Diehl, A.; Hameed, B.; et al. Farnesoid X nuclear receptor ligand obeticholic acid for non-cirrhotic, nonalcoholic steatohepatitis (FLINT): A multicentre, randomised, placebo-controlled trial. Lancet 2015, 385, 956–965. [Google Scholar] [CrossRef] [PubMed]
- Pedrosa, M.; Seyedkazemi, S.; Francque, S.; Sanyal, A.; Rinella, M.; Charlton, M.; Loomba, R.; Ratzu, V.; Kochuparampil, J.; Fischer, L.; et al. A randomized, double-blind, multicenter, phase 2b study to evaluate the safety and efficacy of a combination of tropifexor and cenicriviroc in patients with nonalcoholic steatohepatitis and liver fibrosis: Study design of the TANDEM trial. Contemp. Clin. Trials 2020, 88, 105889. [Google Scholar] [CrossRef] [PubMed]
- Gross, B.; Pawlak, M.; Lefebvre, P.; Staels, B. PPARs in obesity-induced T2DM, dyslipidaemia and NAFLD. Nat. Rev. Endocrinol. 2017, 13, 36–49. [Google Scholar] [CrossRef]
- Tiikkainen, M.; Häkkinen, A.M.; Korsheninnikova, E.; Nyman, T.; Mäkimattila, S.; Yki-Järvinen, H. Effects of rosiglitazone and metformin on liver fat content, hepatic insulin resistance, insulin clearance, and gene expression in adipose tissue in patients with type 2 diabetes. Diabetes 2004, 53, 2169–2176. [Google Scholar] [CrossRef]
- Cariou, B.; Charbonnel, B.; Thiazolidinediones, B.S. PPAR gamma agonists: Time for a reassessment. Trends Endocrinol. Metab. 2012, 23, 205–215. [Google Scholar] [CrossRef]
- Musso, G.; Cassader, M.; Paschetta, E.; Gambino, R. Thiazolidinediones and advanced liver fibrosis in nonalcoholic steatohepatitis: A meta-analysis. JAMA Intern. Med. 2017, 177, 633–640. [Google Scholar] [CrossRef]
- Albhaisi, A.M.S.; Sanyal, J.A. New drugs for NASH. Liver Int. 2021, 41, 112–118. [Google Scholar] [CrossRef]
- Jain, R.M.; Giri, R.S.; Bhoi, B.; Trivedi, C.; Rath, A.; Rathod, R.; Ranvir, R.; Kadam, S.; Patel, H.; Swain, P.; et al. Dual PPARα/γ agonist saroglitazar improves liver histopathology and biochemistry in experimental NASH models. Liver Int. 2018, 38, 1084–1094. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.D.; Caffrey, R.; Marioneaux, J.; Santhekadur, K.P.; Bhat, M.; Alonso, C.; Koduru, V.S.; Philip, B.; Jain, R.M.; Giri, R.S.; et al. The PPARα/γ agonist saroglitazar improves insulin resistance and steatohepatitis in a diet induced animal model of nonalcoholic fatty liver disease. Sci. Rep. 2020, 10, 9330. [Google Scholar] [CrossRef] [PubMed]
- Gawrieh, S.; Noureddin, M.; Loo, N.; Mohseni, R.; Awasty, V.; Cusi, K.; Kowdley, V.K.; Lai, M.; Schiff, E.; Parmar, D. Saroglitazar, a PPAR-α/γAgonist, for Treatment of NAFLD: A randomized controlled double-blind phase 2 trial. Hepatology 2021, 74, 1809–1824. [Google Scholar] [CrossRef]
- Smati, S.; Canivet, M.C.; Boursier, J.; Cariou, B. Anti-diabetic drugs and NASH: From current option to promising perspectives. Expert Opin. Investig. Drugs 2021, 30, 813–825. [Google Scholar] [CrossRef] [PubMed]
- Ratziu, V.; Harrison, A.S.; Francque, S.; Bedossa, P.; Lehert, P.; Serfaty, L.; Romero-Gomez, M.; Boursier, J.; Abdelmalek, M.; Caldwell, S.; et al. Elafibranor, an Agonist of the Peroxisome Proliferator-Activated Receptor-α and -δ, Induces Resolution of Nonalcoholic Steatohepatitis without Fibrosis Worsening. Gastroenterology 2016, 150, 1147–1159.e5. [Google Scholar] [CrossRef] [PubMed]
- Wilding, H.P.J.; Batterham, L.R.; Calanna, S.; Davies, M.; Van Gaal, F.L.; Lingvay, I.; McGowan, M.B.; Rosenstock, J.; Tran, T.D.M.; Wadden, A.T. Once-weekly semaglutide in adults with overweight or obesity. N. Engl. J. Med. 2021, 384, 989–1002. [Google Scholar] [CrossRef]
- Davies, M.; Pieber, R.T.; Hartoft-Nielsen, M.-L.; Hansen, K.H.O.; Jabbour, S.; Rosenstock, J. Effect of Oral Semaglutide Compared With Placebo and Subcutaneous Semaglutide on Glycemic Control in Patients With Type 2 Diabetes. JAMA 2017, 318, 1460–1470. [Google Scholar] [CrossRef]
- Newsome, P.; Buchholtz, K.; Cusi, K.; Linder, M.; Okanoue, T.; Ratziu, V.; Sanyal, J.A.; Sejling, A.-S.; Harrison, S. A placebo-controlled trial of subcutaneous semaglutide in nonalcoholic steatohepatitis. N. Engl. J. Med. 2021, 384, 1113–1124. [Google Scholar] [CrossRef]
- Armstrong, J.M.; Gaunt, P.; Aithal, P.G.; Barton, D.; Hull, D.; Parker, R.; Hazlehurst, J.; Gou, K.; LEAN trial team; Abouda, G.; et al. Liraglutide safety and efficacy in patients with non-alcoholic steatohepatitis (LEAN): A multicentre, double-blind, randomised, placebo-controlled phase 2 study. Lancet 2016, 387, 679–690. [Google Scholar] [CrossRef]
- Maselli, D.; Atieh, J.; Clark, M.M.; Eckert, D.; Taylor, A.; Carlson, P.; Burton, D.D.; Busciglio, I.; Scott Harmsen, W.; Vella, A.; et al. Effects of liraglutide on gastrointestinal functions and weigth in obesity: A randomized clinical and pharmacogenomic trial. Obesity 2022, 30, 1608–1620. [Google Scholar] [CrossRef]
- Lazzaroni, E.; Ben Nasr, M.; Loretelli, C.; Pastore, I.; Plebani, L.; Lunati, M.E.; Vallone, L.; Bolla, A.M.; Rossi, A.; Montefusco, L.; et al. Anti-diabetic drugs and weight loss in patients with type 2 diabetes. Pharmacol. Res. 2021, 171, 105782. [Google Scholar] [CrossRef]
- Hartman, L.M.; Sanyal, J.A.; Loomba, R.; Wilson, M.J.; Nikooienejad, A.; Bray, R.; Karanikas, A.C.; Duffin, L.K.; Robins, D.; Haupt, A.; et al. Effects on novel dual GIP and GLP-1 receptor agonist tirzepatide on biomarkers of nonalcoholic steatohepatitis in patients with type 2 diabetes. Diabetes Care 2020, 43, 1352–1355. [Google Scholar] [CrossRef] [PubMed]
- Babaknejad, N.; Nayeri, H.; Hemmati, R.; Bahrami, S.; Esmaillzadeh, A. An overview of FGF19 and FGF 21: The therapeutic role in treatment of the metabolic disorders and obesity. Horm. Metab. Res. 2018, 50, 441–452. [Google Scholar] [CrossRef] [PubMed]
- Talukdar, S.; Kharitonenkov, A. FGF19 and FGF21: In NASH we trust. Mol. Metab. 2021, 46, 101152. [Google Scholar] [CrossRef] [PubMed]
- Tillman, J.E.; Rolph, T. FGF21: An emerging therapeutic target for non-alcoholic steatohepatitis and related metabolic diseases. Front. Endocrinol. 2020, 11, 601290. [Google Scholar] [CrossRef] [PubMed]
- Sanyal, A.; Charles, D.E.; Neuschwander-Tetri, A.B.; Loomba, R.; Harrison, A.S.; Abdelmalek, F.M.; Lawitz, J.E.; Halegoua-DeMarzio, D.; Kundu, S.; Noviello, S.; et al. Pegbelfermin (BMS-986036), PEGylated fibroblast growth factor 21 analogue, in patients with non-alcoholic steatohepatitis: A randomised, double-blind, placebo-controlled, phase 2a trial. Lancet 2019, 392, 2705–2717. [Google Scholar] [CrossRef]
- Harrison, A.S.; Abdelmalek, F.M.; Neff, G.; Gunn, N.; Guy, D.C.; Alkhouri, N.; Bashir, R.M.; Freilich, B.; Kohli, A.; Khazanchi, A.; et al. Aldafermin in patients with non-alcoholic steatohepatitis (ALPINE 2/3): A randomised, double-blind, placebo-controlled, phase 2b trial. Lancet Gastroenetrol. Hepatol. 2022, 7, 603–616. [Google Scholar] [CrossRef]
- Harrison, A.S.; Rinella, E.M.; Abdelmalek, F.M.; Trotter, F.J.; Paredes, H.A.; Arnold, L.H.; Kugelmas, M.; Bashir, R.M.; Jaros, J.M.; Ling, L.; et al. NGM282 for treatment of non-alcoholic steatohepatitis: A multicentre randomised, double-blind placebo-controlled, phase 2b trial. Lancet 2018, 391, 1174–1185. [Google Scholar] [CrossRef]
- Ratziu, V.; De Guevara, L.; Safadi, R.; Poordad, F.; Fuster, F.; Flores-Figueroa, J.; Arrese, M.; Fracanzani, L.A.; Ben Bashat, D.; Lackner, K.; et al. Aramchol in patients with nonalcoholic steatohepatitis: A randomized, double-blind, placebo-controlled phase 2b trial. Nat. Med. 2021, 27, 1825–1835. [Google Scholar] [CrossRef]
- Ratziu, V.; Sanyal, A.; Harrison, A.S.; Wai-Sung Wong, V.; Francque, S.; Goodman, Z.; Aithal, P.G.; Kowdley, V.K.; Seyedkazemi, S.; Fischer, L.; et al. Cenicriviroc Treatment for Adults with Nonalcoholic Steatohepatitis and Fibrosis: Final Analysis of the Phase 2b CENTAUR Study. Hepatology 2020, 72, 892–905. [Google Scholar] [CrossRef]
- Harrison, A.S.; Wai-Sung Wong, V.; Okanoue, T.; Bzowej, N.; Vuppalanchi, R.; Younes, Z.; Kohli, A.; Sarin, S.; Caldwell, H.S.; Alkhouri, N.; et al. Selonsertib for patienets with bridging fibrosis or compensated cirrhosis due to NASH: Result from randomized phase III STELLAR trials. J. Hepatol. 2020, 73, 26–39. [Google Scholar] [CrossRef] [PubMed]
| Drugs | Mechanism of Action |
|---|---|
| Obeticholic acid | Farnesoid X receptor (FXR) agonist |
| Tropifexor | FXR agonist |
| Pioglitazone | Peroxisome proliferator-activated receptor (PPAR) γ agonist |
| Saroglitazar | PPARα/γ agonist |
| Elafibranor | PPARα/δ agonist |
| Semaglutide | Glucagon-like peptide (GLP)-1 agonist |
| Liraglutide | GLP-1 agonists |
| Tirzepatide | GLP-1 and GIP agonist |
| Pegbelfermin | Fibroblast growth factors (FGF) 21 analogue |
| Efruxifermin | FGF21 analogue |
| Aldafermin | FGF19 analogue |
| Aramchol | Inhibitor of de novo synthesis of lipids |
| Cenicriviroc | Agonist of chemokine receptors 2 and 5 |
| Selonsertib | Apoptosis signal-regulating kinase (ASK)1 inhibitor |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Branković, M.; Dukić, M.; Gmizić, T.; Popadić, V.; Nikolić, N.; Sekulić, A.; Brajković, M.; Đokić, J.; Mahmutović, E.; Lasica, R.; et al. New Therapeutic Approaches for the Treatment of Patients with Metabolic Dysfunction-Associated Steatotic Liver Disease (MASLD) and Increased Cardiovascular Risk. Diagnostics 2024, 14, 229. https://doi.org/10.3390/diagnostics14020229
Branković M, Dukić M, Gmizić T, Popadić V, Nikolić N, Sekulić A, Brajković M, Đokić J, Mahmutović E, Lasica R, et al. New Therapeutic Approaches for the Treatment of Patients with Metabolic Dysfunction-Associated Steatotic Liver Disease (MASLD) and Increased Cardiovascular Risk. Diagnostics. 2024; 14(2):229. https://doi.org/10.3390/diagnostics14020229
Chicago/Turabian StyleBranković, Marija, Marija Dukić, Tijana Gmizić, Višeslav Popadić, Novica Nikolić, Ana Sekulić, Milica Brajković, Jelena Đokić, Edvin Mahmutović, Ratko Lasica, and et al. 2024. "New Therapeutic Approaches for the Treatment of Patients with Metabolic Dysfunction-Associated Steatotic Liver Disease (MASLD) and Increased Cardiovascular Risk" Diagnostics 14, no. 2: 229. https://doi.org/10.3390/diagnostics14020229
APA StyleBranković, M., Dukić, M., Gmizić, T., Popadić, V., Nikolić, N., Sekulić, A., Brajković, M., Đokić, J., Mahmutović, E., Lasica, R., Vojnović, M., & Milovanović, T. (2024). New Therapeutic Approaches for the Treatment of Patients with Metabolic Dysfunction-Associated Steatotic Liver Disease (MASLD) and Increased Cardiovascular Risk. Diagnostics, 14(2), 229. https://doi.org/10.3390/diagnostics14020229












