Post-COVID-19 Vaccination Myocarditis: A Histopathologic Study on a Monocentric Series of Six Cases
Abstract
1. Introduction
2. Materials and Methods
Case Identification and Clinical Data
3. Results
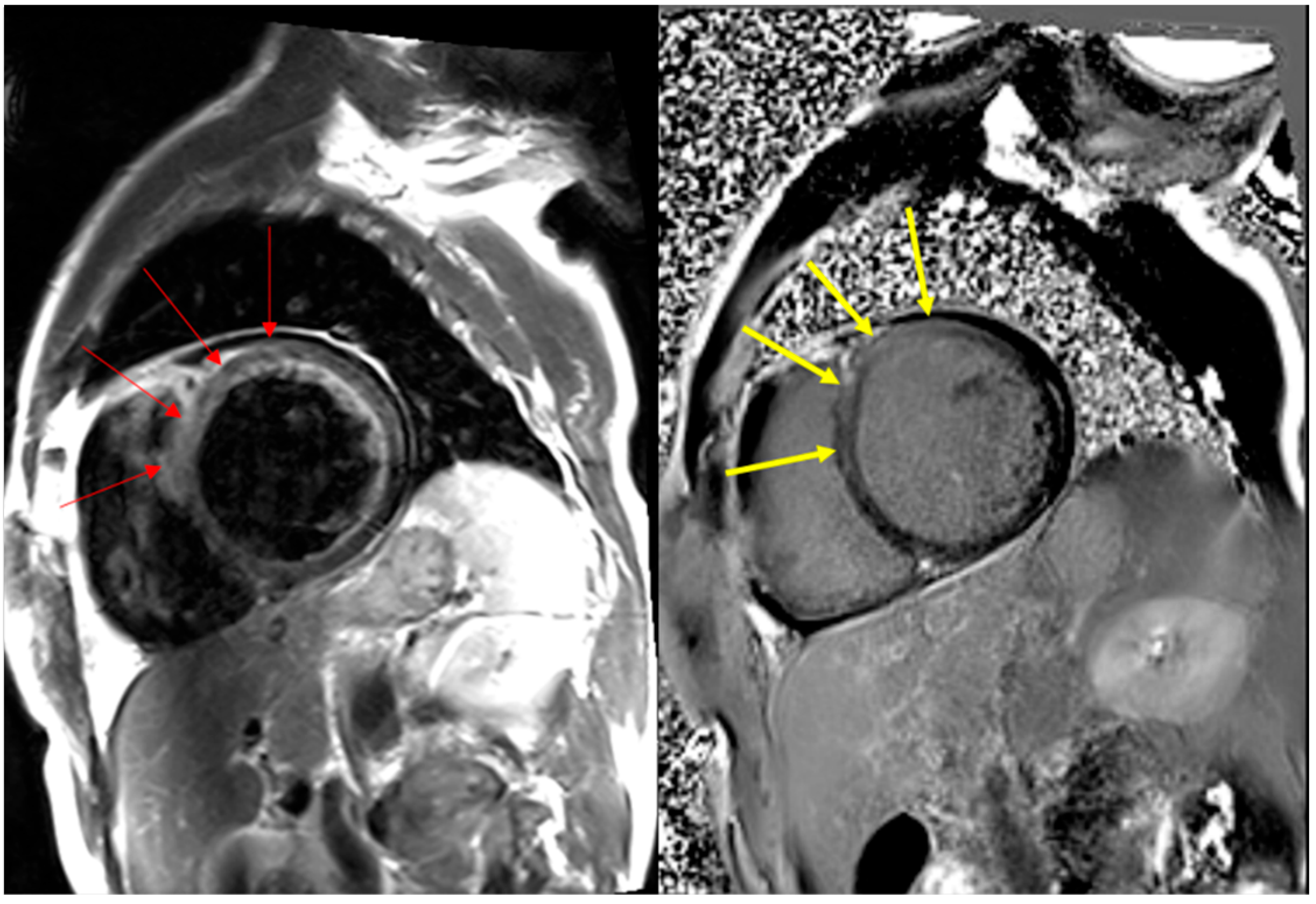
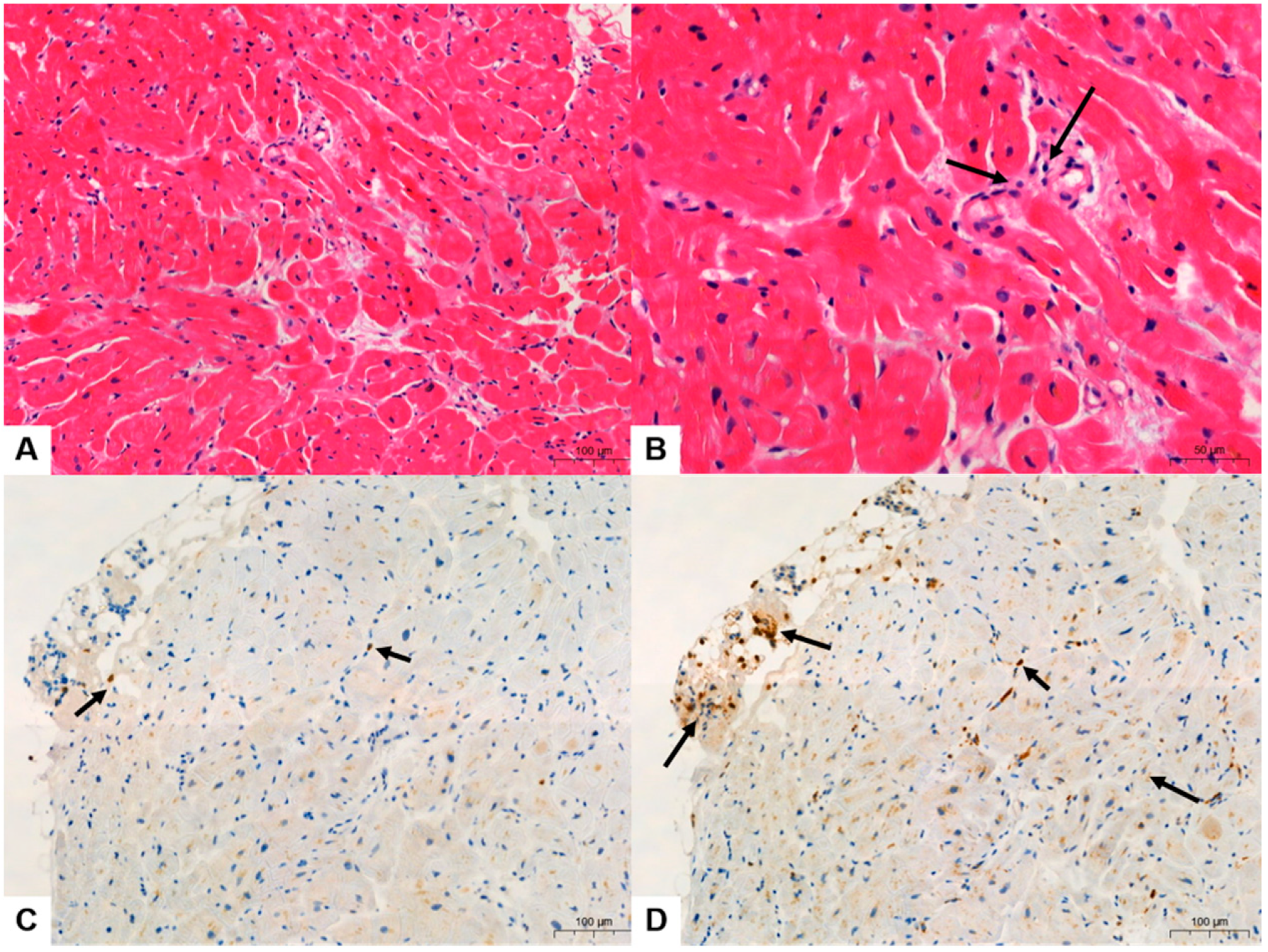
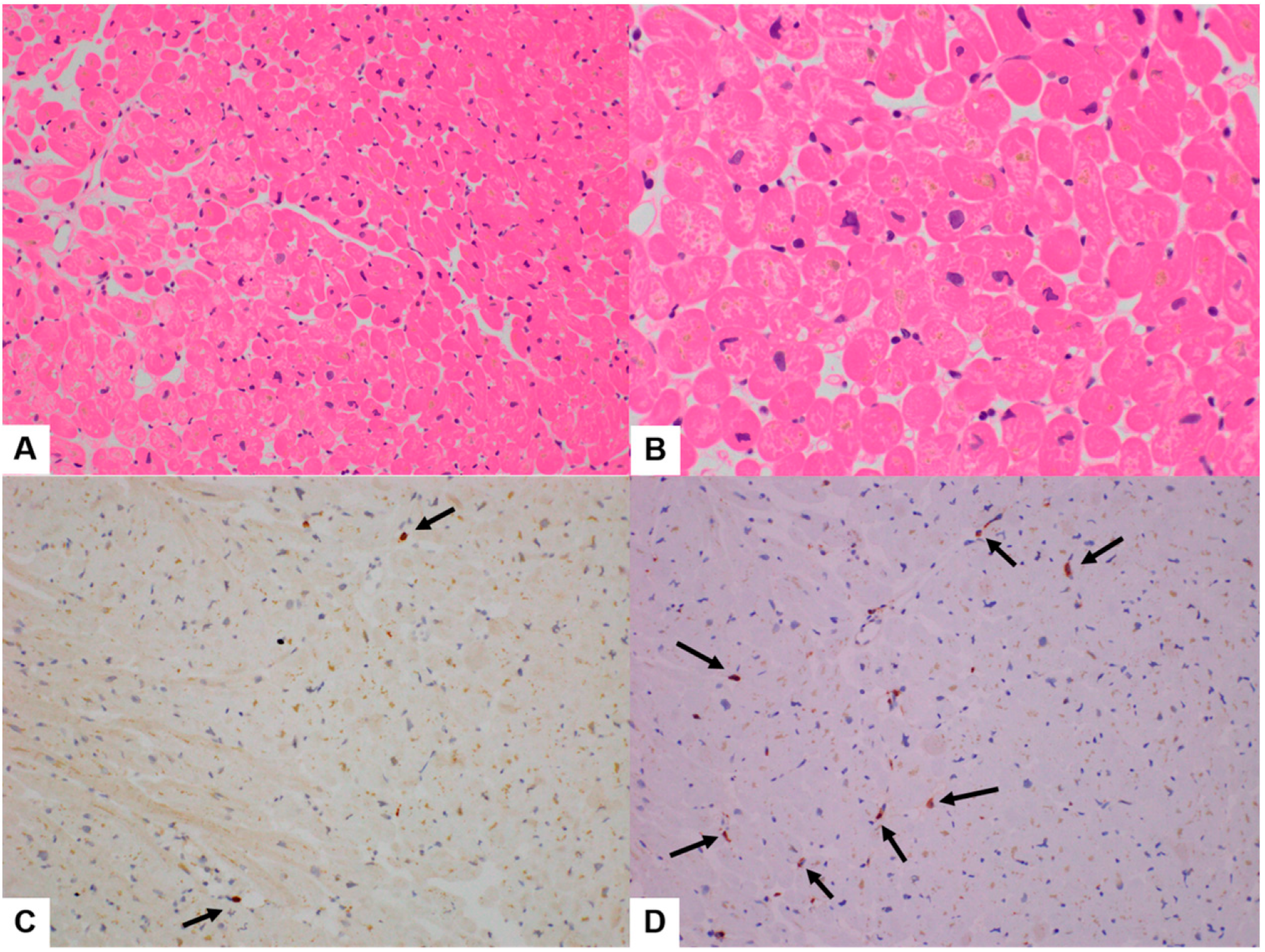
3.1. Clinicopathologic Features
3.2. Quantitative Analysis of Immunohistochemical Stains
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Basso, C. Myocarditis. N. Engl. J. Med. 2022, 387, 1488–1500. [Google Scholar] [CrossRef] [PubMed]
- Albert, E.; Aurigemma, G.; Saucedo, J.; Gerson, D.S. Myocarditis following COVID-19 vaccination. Radiol. Case Rep. 2021, 16, 2142–2145. [Google Scholar] [CrossRef] [PubMed]
- Bozkurt, B.; Kamat, I.; Hotez, P.J. Myocarditis with COVID-19 mRNA vaccines. Circulation 2021, 144, 471–484. [Google Scholar] [CrossRef] [PubMed]
- Abbate, A.; Gavin, J.; Madanchi, N.; Kim, C.; Shah, P.R.; Klein, K.; Boatman, J.; Roberts, C.; Patel, S.; Danielides, S. Fulminant myocarditis and systemic hyperinflammation temporally associated with BNT162b2 mRNA COVID-19 vaccination in two patients. Int. J. Cardiol. 2021, 340, 119–121. [Google Scholar] [CrossRef] [PubMed]
- Larson, K.F.; Ammirati, E.; Adler, E.D.; Cooper, L.T., Jr.; Hong, K.N.; Saponara, G.; Couri, D.; Cereda, A.; Procopio, A.; Cavalotti, C.; et al. Myocarditis After BNT162b2 and mRNA-1273 Vaccination. Circulation 2021, 144, 506–508. [Google Scholar] [CrossRef] [PubMed]
- Rosner, C.M.; Genovese, L.; Tehrani, B.N.; Atkins, M.; Bakhshi, H.; Chaudhri, S.; Damluji, A.A.; De Lemos, J.A.; Desai, S.S.; Emaminia, A. Myocarditis temporally associated with COVID-19 vaccination. Circulation 2021, 144, 502–505. [Google Scholar] [CrossRef] [PubMed]
- Chow, B.T.; Lai, C.K. Lymphohistiocytic Myocarditis Possibly Due to Moderna mRNA-1273 VaccineA Case Report. Am. J. Clin. Pathol. 2022, 158, 167–172. [Google Scholar] [CrossRef] [PubMed]
- Kiblboeck, D.; Klingel, K.; Genger, M.; Traxler, S.; Braunsteiner, N.; Steinwender, C.; Kellermair, J. Myocarditis following mRNA COVID-19 vaccination: Call for endomyocardial biopsy. ESC Heart Fail. 2021, 9, 1996–2002. [Google Scholar] [CrossRef] [PubMed]
- Sung, K.; McCain, J.; King, K.R.; Hong, K.; Aisagbonhi, O.; Adler, E.D.; Urey, M.A. Biopsy-Proven Giant Cell Myocarditis Following the COVID-19 Vaccine. Circ. Heart Fail. 2022, 15, e009321. [Google Scholar] [CrossRef] [PubMed]
- Ujueta, F.; Azimi, R.; Lozier, M.R.; Poppiti, R.; Ciment, A. Lymphohistocytic myocarditis after Ad26.COV2.S viral vector COVID-19 vaccination. Int. J. Cardiol. Heart Vasc. 2021, 36, 100869. [Google Scholar] [CrossRef] [PubMed]
- Verma, A.K.; Lavine, K.J.; Lin, C.-Y. Myocarditis after COVID-19 mRNA vaccination. N. Engl. J. Med. 2021, 385, 1332–1334. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, M.; Tajiri, K.; Ayuzawa, S.; Ieda, M. Pathological Findings of Clinically Suspected Myocarditis Temporally Associated with COVID-19 Vaccination. Eur. J. Heart Fail. 2022, 24, 1132–1138. [Google Scholar] [CrossRef] [PubMed]
- Leone, O.; Pieroni, M.; Rapezzi, C.; Olivotto, I. The spectrum of myocarditis: From pathology to the clinics. Virchows Arch. 2019, 475, 279–301. [Google Scholar] [CrossRef] [PubMed]
- Yoon, B.K.; Oh, T.G.; Bu, S.; Seo, K.J.; Kwon, S.H.; Lee, J.Y.; Kim, Y.; Kim, J.W.; Ahn, H.S.; Fang, S. The Peripheral Immune Landscape in a Patient with Myocarditis after the Administration of BNT162b2 mRNA Vaccine. Mol. Cells 2022, 45, 738–748. [Google Scholar] [CrossRef] [PubMed]
- Ilonze, O.J.; Guglin, M.E. Myocarditis following COVID-19 vaccination in adolescents and adults: A cumulative experience of 2021. Heart Fail. Rev. 2022, 27, 2033–2043. [Google Scholar] [CrossRef] [PubMed]
- Khan, T.; Selvakumar, D.; Trivedi, S.; Rao, K.; Harapoz, M.; Thiagalingam, A.; Denniss, A.R.; Varikatt, W. The value of endomyocardial biopsy in diagnosis and guiding therapy. Pathology 2017, 49, 750–756. [Google Scholar] [CrossRef] [PubMed]
- Leone, O.; Veinot, J.P.; Angelini, A.; Baandrup, U.T.; Basso, C.; Berry, G.; Bruneval, P.; Burke, M.; Butany, J.; Calabrese, F.; et al. 2011 Consensus statement on endomyocardial biopsy from the Association for European Cardiovascular Pathology and the Society for Cardiovascular Pathology. Cardiovasc. Pathol. 2012, 21, 245–274. [Google Scholar] [CrossRef] [PubMed]
- Thomas Aretz, H. Myocarditis: The Dallas criteria. Hum. Pathol. 1987, 18, 619–624. [Google Scholar] [CrossRef] [PubMed]

| Case Series [Reference No.] | Age (Yrs) | Sex | Cardiac Function LVEF (%) | Symptoms | MRI Findings | CK-MB | Cardiac Troponin * I or # T | Pathologic Myocarditis Type | Specimen Type | Vaccine Type (Dose) | Symptoms Onset after Vaccination (Days) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Rosner et al. [6] | 28 | M | 51 | chest pain, no fever or coughing | Patchy LGE, no ED | U | * 17.08 (ng/mL) | U | ND | J (1st) | 5 |
| Rosner et al. [6] | 39 | M | 35–40 | chest pain, no fever or coughing | Patchy LGE, no ED | U | * 11.01 (ng/mL) | U | ND | P (2nd) | 3 |
| Rosner et al. [6] | 39 | M | 60 | chest pain, fever, chills, and shortness of breath | Multifocal LGE, no ED | U | * 13.01 (ng/mL) | U | ND | P (2nd) | 4 |
| Rosner et al. [6] | 24 | M | 53 | chest pain | Midmyocardial LGE, ED | U | * 0.37 (ng/mL) | U | ND | P (1st) | 7 |
| Rosner et al. [6] | 19 | M | 55 | chest pain | Multifocal LGE, ED | U | * 44.8 (ng/mL) | U | ND | P (2nd) | 2 |
| Rosner et al. [6] | 20 | M | 50–55 | chest pain | Subepicardial LGE, ED | U | * 8.36 (ng/mL) | U | ND | P (2nd) | 3 |
| Rosner et al. [6] | 23 | M | 58 | subjective fevers, chest pain, and myalgia | Midmyocardial LGE, no ED | U | U | U | ND | P (2nd) | 3 |
| Abbate et al. [4] | 27 | M | 20 | nausea and vomiting | U | 252 (ng/ml) | U | U | ND | P (1st) | 2 |
| Abbate et al. [4] | 34 | F | 15 | fever, cough, chest pain, nausea, and vomiting | U | 42.4 (ng/ml) | U | U | ND | P (2nd) | 9 |
| Larson et al. [5] | 22 | M | 50 | fever, chills, and myalgia | LGE | U | 285 FUL | U | ND | M (2nd) | 3 |
| Larson et al. [5] | 31 | M | 34 | fever, chills, and myalgia | LGE | U | 46 FUL | U | ND | M (2nd) | 3 |
| Larson et al. [5] | 40 | M | 47 | chest pain | ED, LGE, PE | U | 520 FUL | U | ND | P (2nd) | 2 |
| Larson et al. [5] | 56 | M | 60 | chest pain | ED, LGE | U | 37 FUL | U | ND | P (2nd) | 3 |
| Larson et al. [5] | 26 | M | 60 | cough, fever | ED, LGE, PE | U | 100 FUL | U | ND | P (2nd) | 3 |
| Larson et al. [5] | 35 | M | 50 | fever and chest pain | ED, LGE | U | 29 FUL | U | ND | P (2nd) | 2 |
| Larson et al. [5] | 21 | M | 54 | fever and chest pain | ED, LGE, PE | U | 1164 FUL | U | ND | P (2nd) | 4 |
| Larson et al. [5] | 22 | M | 53 | chest pain | ED, LGE | U | 1433 FUL | U | ND | M (2nd) | 2 |
| Verma et al. [11] | 45 | F | 15–20 | dyspnea and dizziness | LGE | U | * 10.453 (ng/mL) | LM admixed with eosinophils, B cells, and plasma cells | EMB | P (1st) | 10 |
| Verma et al. [11] | 42 | M | 15 | dyspnea and chest pain | Not available | U | * 44.30 (ng/mL) | An inflammatory infiltrate admixed with macrophages, T cells, eosinophils, and B cells | Autopsy | M (2nd) | 2wks |
| Ujueta et al. [10] | 62 | F | 29 | progressive body aches, weakness, and fatigue | U | U | * 6.4 (ng/mL) | LM with sparse eosinophils | Autopsy | J (1st) | 4 |
| Sung et al. [9] | 63 | M | 35 | fever, fatigue, and cough | U | U | # 5.816 (ng/mL) | GCM | EMB | P (2nd) | 7 |
| Kiblboek et al. [8] | 18 | M | 33 | chest pain, fever, and fatigue | ED, LGE | U | # 1.386 (ng/mL) | LM with sparse eosinophils | EMB | P (1st) | 3 |
| Kiblboek et al. [8] | 22 | M | 40 | chest pain, no fever, and fatigue | ED, LGE | U | * 38.735 (ng/mL) | LM with sparse eosinophils | EMB | P (2nd) | 1 |
| Kiblboek et al. [8] | 38 | M | 48 | chest pain, no fever, and fatigue | ED, LGE | U | # 1.104 (ng/mL) | LM with sparse eosinophils | EMB | P (1st) | 4 |
| Yamamoto et al. [12] | 41 | M | 15 | chest pain, myalgia, and fever | U | 236 (U/L) | # 15 (ng/mL) | Severe LM predominantly composed of cytotoxic T cells (CD8+) and macrophages (CD68+) admixed with B cells (CD20+) and a few eosinophils | EMB | M (2nd) | 19 |
| Yamamoto et al. [12] | 18 | M | 27 | fever, chest pain, and fatigue | U | 32 (U/L) | # 3.09 (ng/mL) | Severe LM predominantly composed of cytotoxic T cells (CD8+) and macrophages (CD68+) | EMB | P (1st) | 9 |
| Yamamoto et al. [12] | 18 | M | 46 | fever and chest pain | U | 72 (U/L) | # 1.3 (ng/mL) | Inflammatory cell infiltration is trivial, obvious cardiomyocyte damage (loss of nuclei and mild vacuolar degeneration) and perivascular and interstitial fibrosis | EMB | M (2nd) | 2 |
| Yamamoto et al. [12] | 18 | M | 62 | fever and chest pain | U | 32 (U/L) | # 0.515 (ng/mL) | Same as the above | EMB | M (2nd) | 3 |
| Chow et al. [7] | 45 | F | 40 | progressive decline of exercise capacity, palpitation, fatigue, and exertional dyspnea | Multifocal LGE | U | U | LM and a focal histiocytic collection suggestive of an ill-defined granuloma | EMB | M (1st) | 7 |
| Our case 1 | 59 | M | 24 | dyspnea, leg edema | Patchy midmyocardial LGE | 22.7 (ng/mL) | # 0.019 (ng/mL) | LM | EMB | P (1st) | 3 |
| Our case 2 | 52 | M | 35 | cardiac arrest d/t ventricular fibrillation | ND | 31.3 (ng/mL) | # 2.00 (ng/mL) | LM | EMB | P (2nd) | 19 |
| Our case 3 | 19 | M | 65 | chest pain | LGE | 11.7 (ng/mL) | # 0.085 (ng/mL) | LM | EMB | M (2nd) | 3 |
| Our case 4 | 83 | M | 35 | dyspnea, palpitation due to ventricular tachycardia | LGE | 18.7 (ng/mL) | # 0.82 (ng/mL) | LM | EMB | P (3rd) | 2 |
| Our case 5 | 69 | F | 60 | dyspnea, chest discomfort | Patchy subepicardial and midmyocardial LGE | 23.4 (ng/mL) | # 0.667 (ng/mL) | LM | EMB | M (3rd) | 2 |
| Our case 6 | 38 | M | 18 | myalgia, dyspnea | Patchy subepicardial and midmyocardial LGE and PE | 15.96 (ng/mL) | # 0.233 (ng/mL) | LM | EMB | P (3rd) | 12 |
| Case [Reference No.] | Age (Years) | Sex | Myocarditis Type | CD3 (Cells/mm2) | CD68 (Cells/mm2) |
|---|---|---|---|---|---|
| Kiblboeck et al. [8] | 18 | M | LM with sparse eosinophils | >50 | >100 |
| Kiblboeck et al. [8] | 22 | M | LM with sparse eosinophils | 5 | 14 |
| Kiblboeck et al. [8] | 38 | M | LM with sparse eosinophils | 30 | 34 |
| Our case 1 | 59 | M | LM (* myocarditis) | 6 | 10 |
| Our case 2 | 52 | M | LM (* borderline myocarditis) | 5 | 5 |
| Our case 3 | 19 | M | LM (* myocarditis) | 10 | 5 |
| Our case 4 | 83 | M | LM (* borderline myocarditis) | 2 | 7 |
| Our case 5 | 69 | F | LM (* borderline myocarditis) | 3 | 0 |
| Our case 6 | 38 | M | LM (* myocarditis) | 10 | 5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahn, H.-S.; Ahn, Y.; Jang, J.; Bu, S.; Lim, S.; Kim, C.; Lee, J.-M.; Lee, K.; Seo, K.-J. Post-COVID-19 Vaccination Myocarditis: A Histopathologic Study on a Monocentric Series of Six Cases. Diagnostics 2024, 14, 219. https://doi.org/10.3390/diagnostics14020219
Ahn H-S, Ahn Y, Jang J, Bu S, Lim S, Kim C, Lee J-M, Lee K, Seo K-J. Post-COVID-19 Vaccination Myocarditis: A Histopathologic Study on a Monocentric Series of Six Cases. Diagnostics. 2024; 14(2):219. https://doi.org/10.3390/diagnostics14020219
Chicago/Turabian StyleAhn, Hyo-Suk, Yuran Ahn, Jaehyuk Jang, Seonghyun Bu, Sungmin Lim, Chanjoon Kim, Jong-Min Lee, Kyungji Lee, and Kyung-Jin Seo. 2024. "Post-COVID-19 Vaccination Myocarditis: A Histopathologic Study on a Monocentric Series of Six Cases" Diagnostics 14, no. 2: 219. https://doi.org/10.3390/diagnostics14020219
APA StyleAhn, H.-S., Ahn, Y., Jang, J., Bu, S., Lim, S., Kim, C., Lee, J.-M., Lee, K., & Seo, K.-J. (2024). Post-COVID-19 Vaccination Myocarditis: A Histopathologic Study on a Monocentric Series of Six Cases. Diagnostics, 14(2), 219. https://doi.org/10.3390/diagnostics14020219








