A Novel Automatic Algorithm to Support Lung Ultrasound Non-Expert Physicians in Interstitial Pneumonia Evaluation: A Single-Center Study
Abstract
1. Introduction
2. Patients and Methods
2.1. Patients
2.2. Imaging
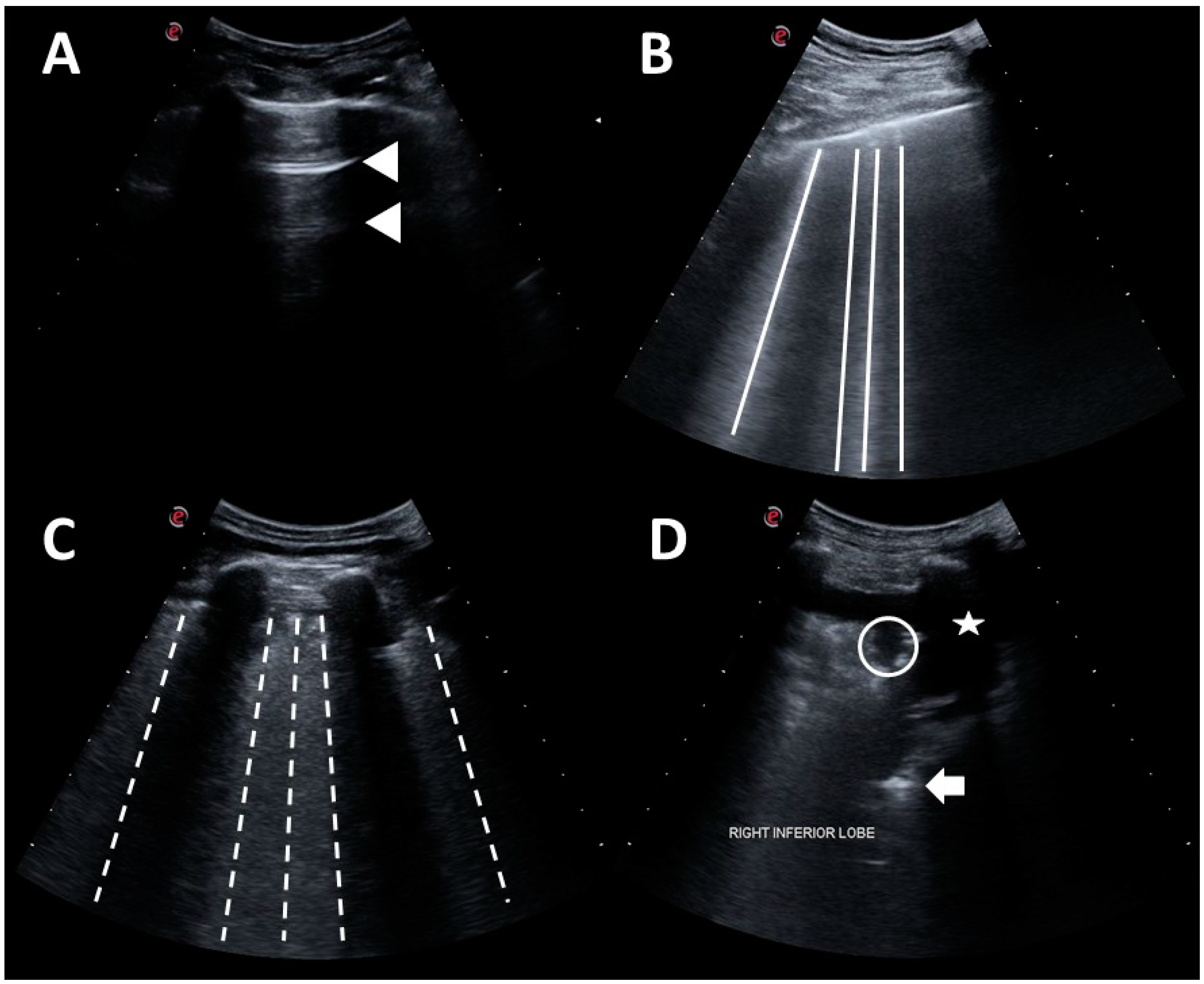
2.3. Lung Ultrasound
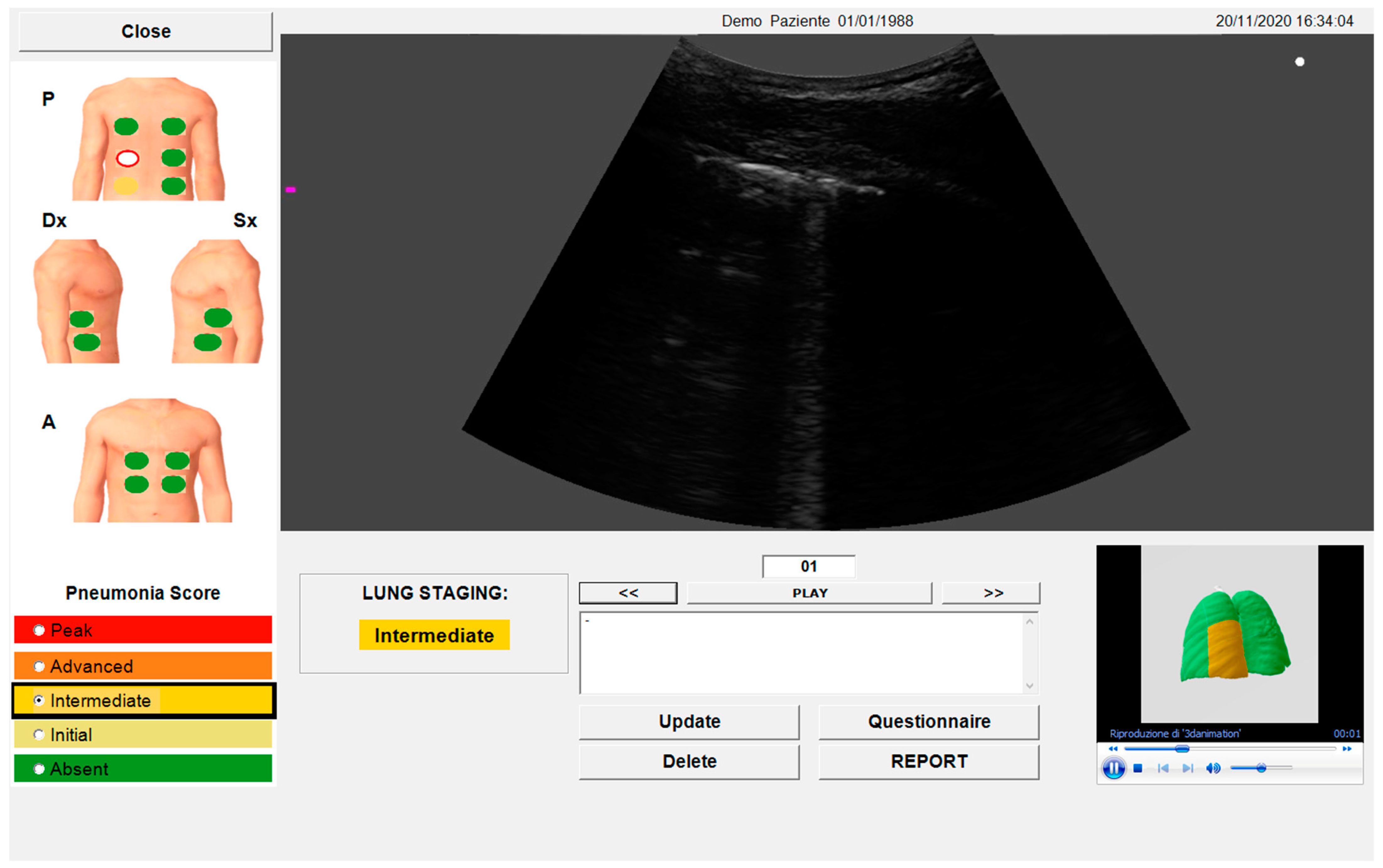
2.4. Automated Algorithm
2.5. Flow-Volume Spirometry and Diffusion Lung Carbon Monoxide
2.6. Statistics
3. Results
3.1. Patients
3.2. Algorithm Evaluation Compared to Human Evaluation
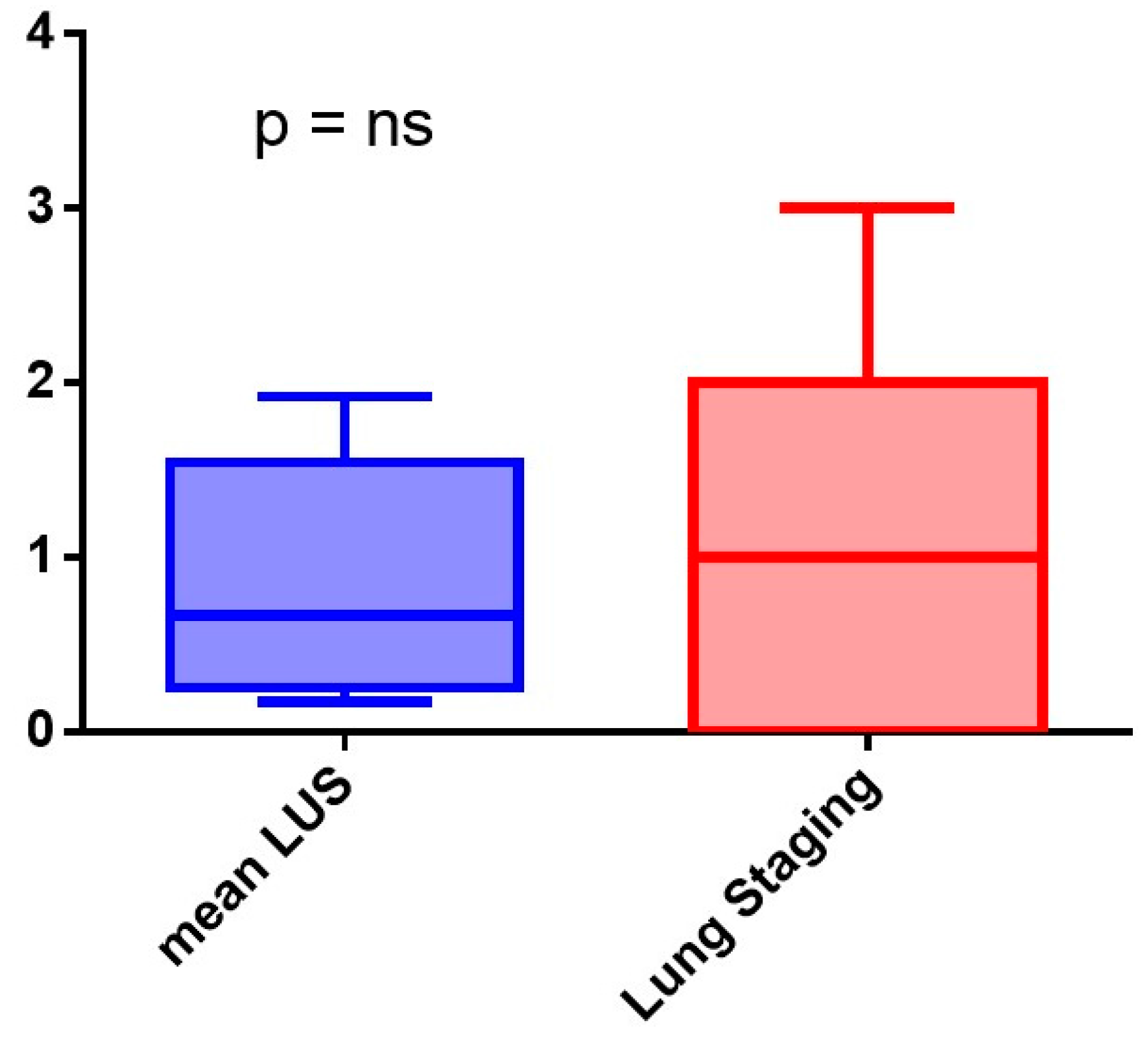
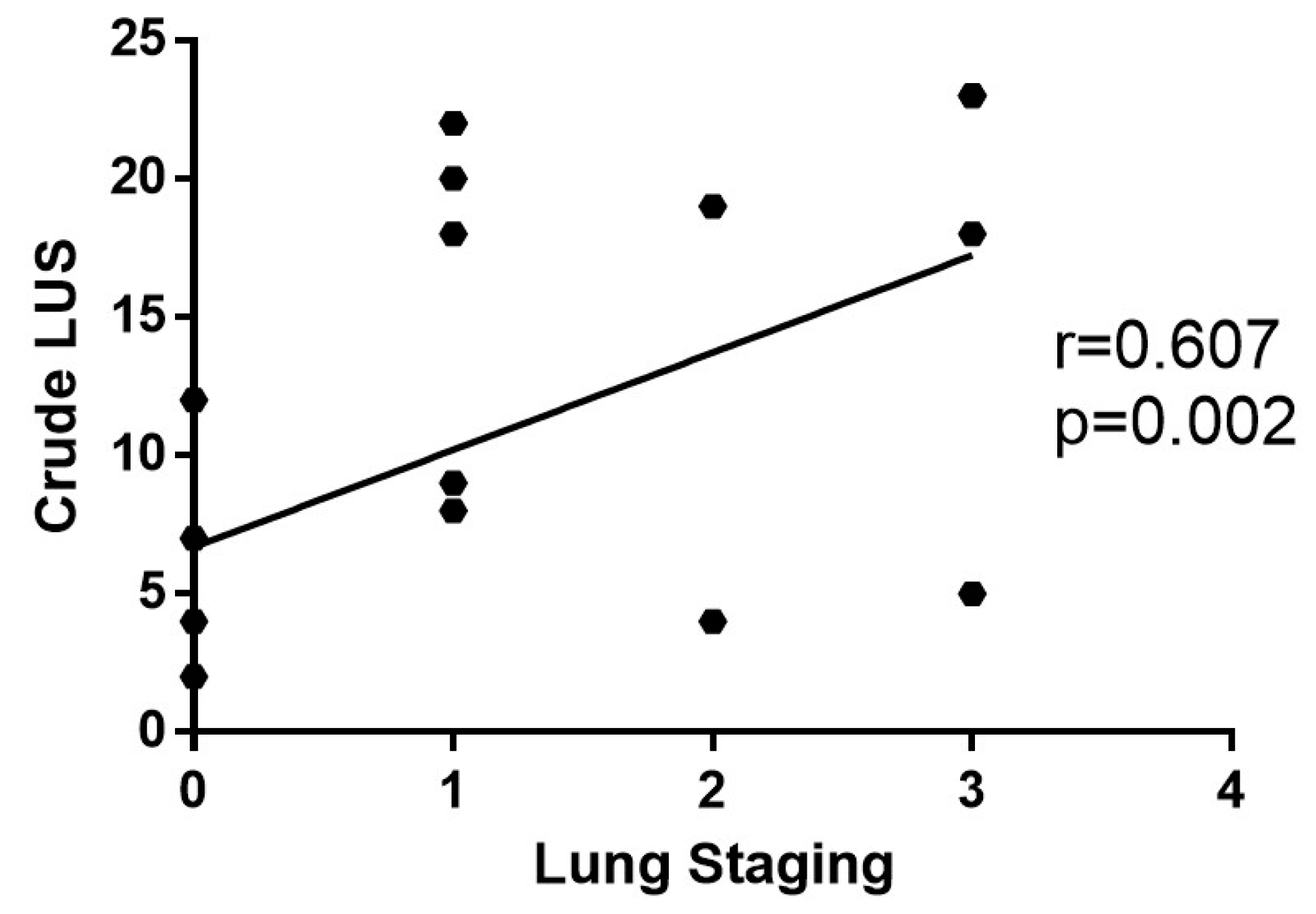
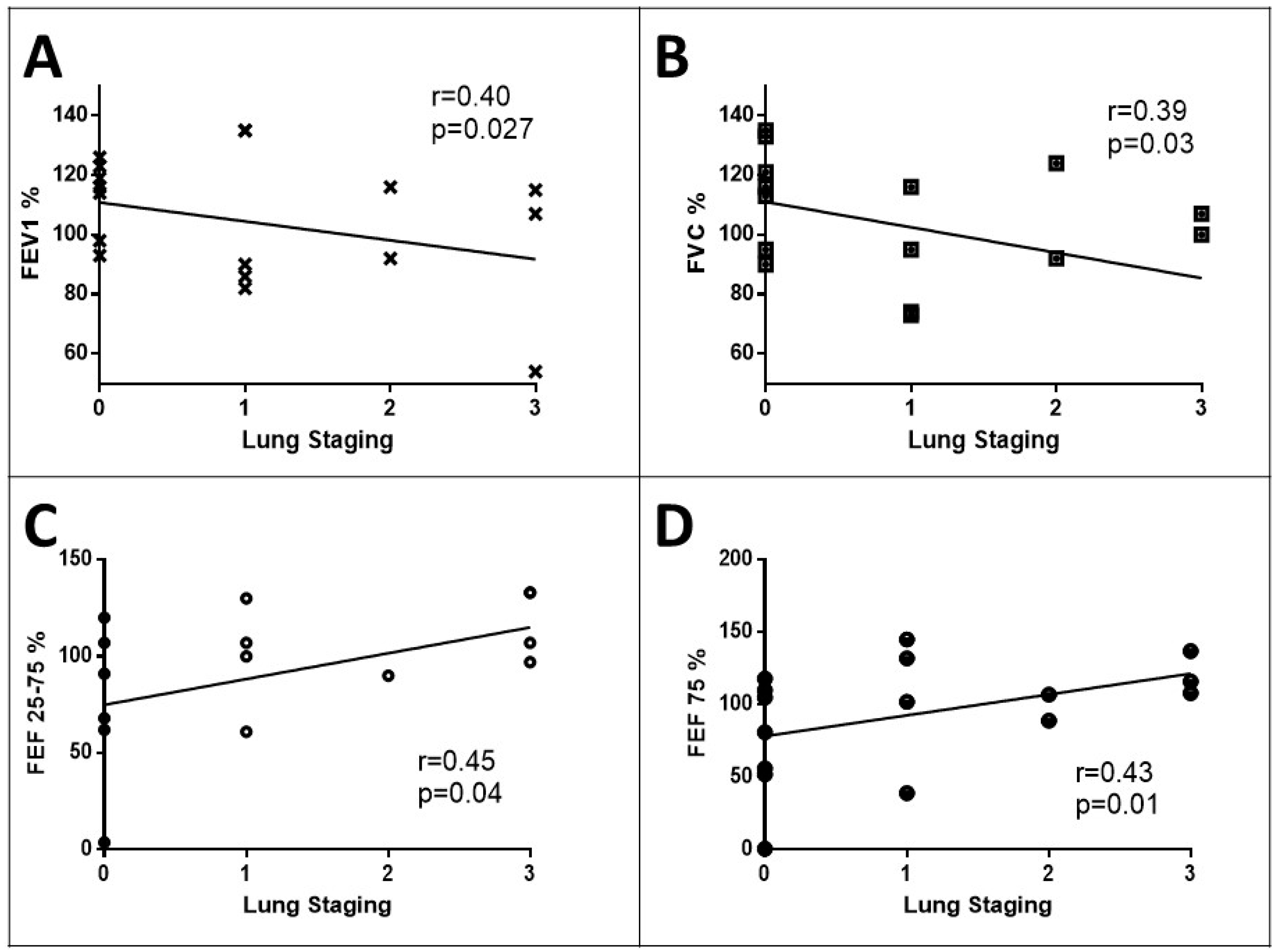
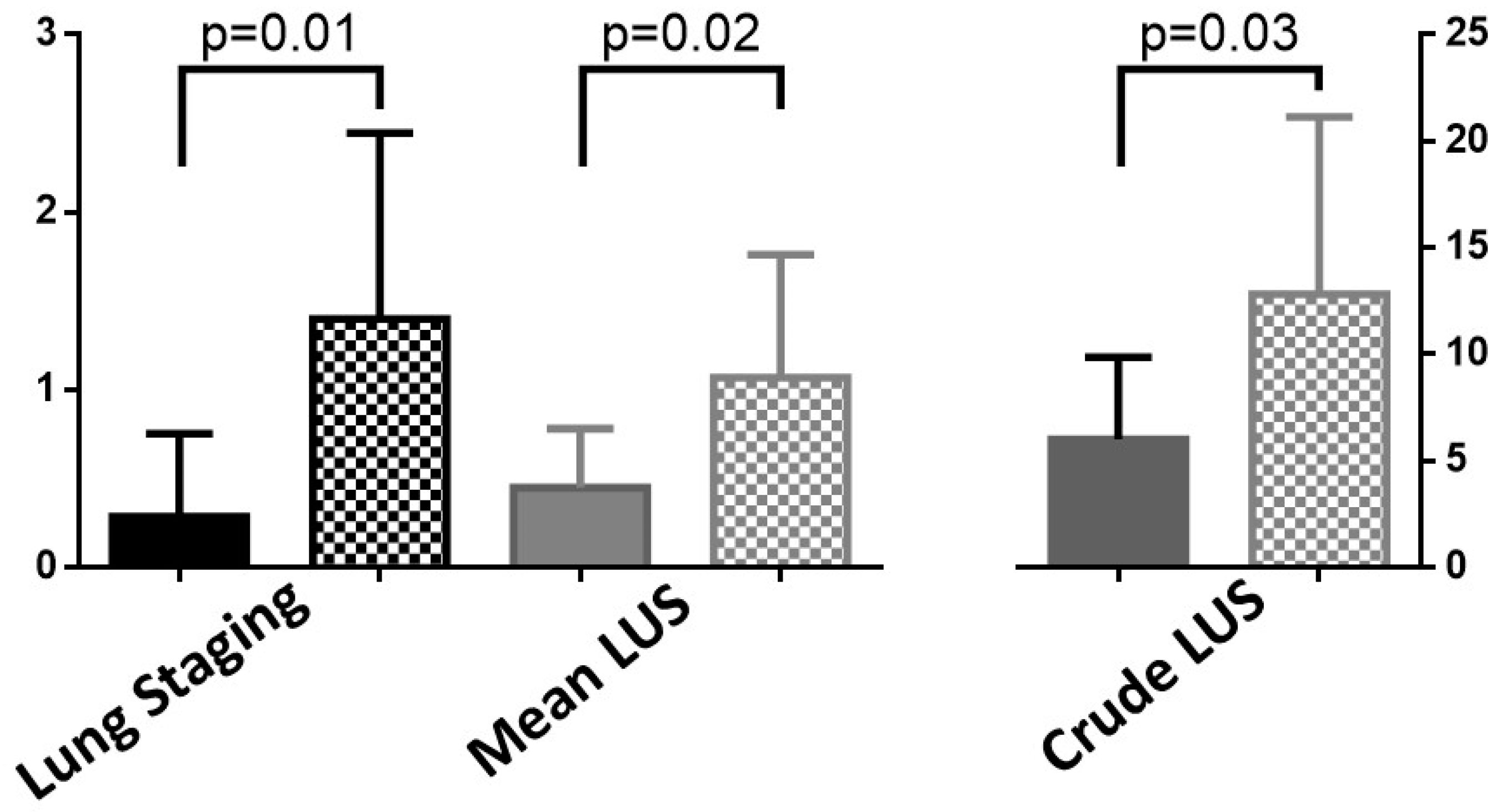
3.3. Clinical Meaning of the SensUS Lung Device Evaluation
4. Discussion
4.1. Limitations
4.2. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sippel, S.; Muruganandan, K.; Levine, A.; Shah, S. Review Article: Use of Ultrasound in the Developing World. Int. J. Emerg. Med. 2011, 4, 72. [Google Scholar] [CrossRef]
- Vetrugno, L.; Mojoli, F.; Boero, E.; Berchialla, P.; Bignami, E.G.; Orso, D.; Cortegiani, A.; Forfori, F.; Corradi, F.; Cammarota, G.; et al. Level of Diffusion and Training of Lung Ultrasound during the COVID-19 Pandemic—A National Online Italian Survey (ITALUS) from the Lung Ultrasound Working Group of the Italian Society of Anesthesia, Analgesia, Resuscitation, and Intensive Care (SIAARTI). Ultraschall Med. 2022, 43, 464–472. [Google Scholar] [CrossRef] [PubMed]
- Dell’Aquila, P.; Raimondo, P.; Racanelli, V.; De Luca, P.; De Matteis, S.; Pistone, A.; Melodia, R.; Crudele, L.; Lomazzo, D.; Solimando, A.G.; et al. Integrated Lung Ultrasound Score for Early Clinical Decision-Making in Patients with COVID-19: Results and Implications. Ultrasound J. 2022, 14, 21. [Google Scholar] [CrossRef] [PubMed]
- Cicco, S.; Vacca, A.; Cariddi, C.; Carella, R.; Altamura, G.; Solimando, A.; Lauletta, G.; Pappagallo, F.; Cirulli, A.; Stragapede, A.; et al. Imaging Evaluation of Pulmonary and Non-Ischaemic Cardiovascular Manifestations of COVID-19. Diagnostics 2021, 11, 1271. [Google Scholar] [CrossRef]
- Cicco, S.; Marozzi, M.S.; Palumbo, C.A.; Sturdà, E.; Fusillo, A.; Scarilli, F.; Albanese, F.; Morelli, C.; Bavaro, D.F.; Diella, L.; et al. Lung Ultrasound Is Useful for Evaluating Lung Damage in COVID-19 Patients Treated with Bamlanivimab and Etesevimab: A Single-Center Pilot Study. Medicina 2023, 59, 203. [Google Scholar] [CrossRef] [PubMed]
- Schäfer, V.S.; Recker, F.; Kretschmer, E.; Putensen, C.; Ehrentraut, S.F.; Staerk, C.; Fleckenstein, T.; Mayr, A.; Seibel, A.; Schewe, J.-C.; et al. Lung Ultrasound in Predicting Outcomes in Patients with COVID-19 Treated with Extracorporeal Membrane Oxygenation. Viruses 2023, 15, 1796. [Google Scholar] [CrossRef]
- Calamai, I.; Greco, M.; Bertolini, G.; Spina, R. Current Adoption of Lung Ultrasound in Intensive Care Units: An Italian Multi-Center Survey. Minerva Anestesiol. 2017, 83, 720–727. [Google Scholar] [CrossRef]
- Wallis, A.; Spinks, K. The Diagnosis and Management of Interstitial Lung Diseases. BMJ 2015, 350, h2072. [Google Scholar] [CrossRef]
- Luppi, F.; Sebastiani, M.; Salvarani, C.; Bendstrup, E.; Manfredi, A. Acute Exacerbation of Interstitial Lung Disease Associated with Rheumatic Disease. Nat. Rev. Rheumatol. 2022, 18, 85–96. [Google Scholar] [CrossRef]
- Ferro, F.; Delle Sedie, A. The Use of Ultrasound for Assessing Interstitial Lung Involvement in Connective Tissue Diseases. Clin. Exp. Rheumatol. 2018, 36 (Suppl. S114), 165–170. [Google Scholar]
- Wang, Y.; Gargani, L.; Barskova, T.; Furst, D.E.; Cerinic, M.M. Usefulness of Lung Ultrasound B-Lines in Connective Tissue Disease-Associated Interstitial Lung Disease: A Literature Review. Arthritis Res. Ther. 2017, 19, 206. [Google Scholar] [CrossRef] [PubMed]
- Demi, L.; Wolfram, F.; Klersy, C.; De Silvestri, A.; Ferretti, V.V.; Muller, M.; Miller, D.; Feletti, F.; Wełnicki, M.; Buda, N.; et al. New International Guidelines and Consensus on the Use of Lung Ultrasound. J. Ultrasound Med. 2023, 42, 309–344. [Google Scholar] [CrossRef] [PubMed]
- Umapathy, V.R.; Raj, R.D.S.; Yadav, S.; Munavarah, S.A.; Anandapandian, P.A.; Mary, A.V.; Padmavathy, K.; Akshay, R.; Rajkumar, D.S.R.; Anandapandian, I.V.; et al. Perspective of Artificial Intelligence in Disease Diagnosis: A Review of Current and Future Endeavours in the Medical Field. Cureus 2023, 15, e45684. [Google Scholar] [CrossRef]
- Miller, D.D.; Brown, E.W. Artificial Intelligence in Medical Practice: The Question to the Answer? Am. J. Med. 2018, 131, 129–133. [Google Scholar] [CrossRef]
- Lombardi, F.A.; Franchini, R.; Morello, R.; Casciaro, E.; Ianniello, S.; Serra, M.; Satriano, F.; Mojoli, F.; Mongodi, S.; Pignatelli, D.; et al. A New Standard Scoring for Interstitial Pneumonia Based on Quantitative Analysis of Ultrasonographic Data: A Study on COVID-19 Patients. Respir. Med. 2021, 189, 106644. [Google Scholar] [CrossRef]
- Marozzi, M.S.; Mancini, F.; Loponte, L.; Solimando, A.G.; Vacca, A.; Cicco, S. Block of the Angiotensin Pathways Affects Flow-Volume Spirometry in Patients with SARS-CoV-2 Infection. In Oxygen Transport to Tissue XLIV; Advances in Experimental Medicine and Biology; Scholkmann, F., LaManna, J., Wolf, U., Eds.; Springer International Publishing: Cham, Switzerland, 2023; Volume 1438, pp. 223–229. ISBN 978-3-031-42002-3. [Google Scholar]
- Walsh, S.L.F.; Mackintosh, J.A.; Calandriello, L.; Silva, M.; Sverzellati, N.; Larici, A.R.; Humphries, S.M.; Lynch, D.A.; Jo, H.E.; Glaspole, I.; et al. Deep Learning–Based Outcome Prediction in Progressive Fibrotic Lung Disease Using High-Resolution Computed Tomography. Am. J. Respir. Crit. Care Med. 2022, 206, 883–891. [Google Scholar] [CrossRef]
- Cazzato, G.; Colagrande, A.; Cimmino, A.; Arezzo, F.; Loizzi, V.; Caporusso, C.; Marangio, M.; Foti, C.; Romita, P.; Lospalluti, L.; et al. Artificial Intelligence in Dermatopathology: New Insights and Perspectives. Dermatopathology 2021, 8, 418–425. [Google Scholar] [CrossRef]
- Cazzato, G.; Massaro, A.; Colagrande, A.; Lettini, T.; Cicco, S.; Parente, P.; Nacchiero, E.; Lospalluti, L.; Cascardi, E.; Giudice, G.; et al. Dermatopathology of Malignant Melanoma in the Era of Artificial Intelligence: A Single Institutional Experience. Diagnostics 2022, 12, 1972. [Google Scholar] [CrossRef]
- Uegami, W.; Bychkov, A.; Ozasa, M.; Uehara, K.; Kataoka, K.; Johkoh, T.; Kondoh, Y.; Sakanashi, H.; Fukuoka, J. MIXTURE of Human Expertise and Deep Learning—Developing an Explainable Model for Predicting Pathological Diagnosis and Survival in Patients with Interstitial Lung Disease. Mod. Pathol. 2022, 35, 1083–1091. [Google Scholar] [CrossRef]
- Ienghong, K.; Cheung, L.W.; Tiamkao, S.; Bhudhisawasdi, V.; Apiratwarakul, K. Development and Remodeling of Point-of-Care Ultrasound Education for Emergency Medicine Residents in Resource Limited Countries during the COVID-19 Pandemic. Tomography 2021, 7, 721–733. [Google Scholar] [CrossRef]
- Di Napoli, A.; Tagliente, E.; Pasquini, L.; Cipriano, E.; Pietrantonio, F.; Ortis, P.; Curti, S.; Boellis, A.; Stefanini, T.; Bernardini, A.; et al. 3D CT-Inclusive Deep-Learning Model to Predict Mortality, ICU Admittance, and Intubation in COVID-19 Patients. J. Digit. Imaging 2022, 36, 603–616. [Google Scholar] [CrossRef] [PubMed]
- Fontanellaz, M.; Ebner, L.; Huber, A.; Peters, A.; Löbelenz, L.; Hourscht, C.; Klaus, J.; Munz, J.; Ruder, T.; Drakopoulos, D.; et al. A Deep-Learning Diagnostic Support System for the Detection of COVID-19 Using Chest Radiographs: A Multireader Validation Study. Investig. Radiol. 2021, 56, 348–356. [Google Scholar] [CrossRef]
- Anderson, K.L.; Fields, J.M.; Panebianco, N.L.; Jenq, K.Y.; Marin, J.; Dean, A.J. Inter-Rater Reliability of Quantifying Pleural B-Lines Using Multiple Counting Methods. J. Ultrasound Med. 2013, 32, 115–120. [Google Scholar] [CrossRef] [PubMed]
- Soldati, G.; Testa, A.; Sher, S.; Pignataro, G.; La Sala, M.; Silveri, N.G. Occult Traumatic Pneumothorax. Chest 2008, 133, 204–211. [Google Scholar] [CrossRef] [PubMed]
- Stewart, J.E.; Goudie, A.; Mukherjee, A.; Dwivedi, G. Artificial Intelligence-enhanced Echocardiography in the Emergency Department. Emerg. Med. Australas. 2021, 33, 1117–1120. [Google Scholar] [CrossRef] [PubMed]
- Asch, F.M.; Poilvert, N.; Abraham, T.; Jankowski, M.; Cleve, J.; Adams, M.; Romano, N.; Hong, H.; Mor-Avi, V.; Martin, R.P.; et al. Automated Echocardiographic Quantification of Left Ventricular Ejection Fraction Without Volume Measurements Using a Machine Learning Algorithm Mimicking a Human Expert. Circ. Cardiovasc. Imaging 2019, 12, e009303. [Google Scholar] [CrossRef] [PubMed]
- Badano, L.P.; Boccalini, F.; Muraru, D.; Bianco, L.D.; Peluso, D.; Bellu, R.; Zoppellaro, G.; Iliceto, S. Current Clinical Applications of Transthoracic Three-Dimensional Echocardiography. J. Cardiovasc. Ultrasound 2012, 20, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Handa, T. The Potential Role of Artificial Intelligence in the Clinical Practice of Interstitial Lung Disease. Respir. Investig. 2023, 61, 702–710. [Google Scholar] [CrossRef] [PubMed]
- Dack, E.; Christe, A.; Fontanellaz, M.; Brigato, L.; Heverhagen, J.T.; Peters, A.A.; Huber, A.T.; Hoppe, H.; Mougiakakou, S.; Ebner, L. Artificial Intelligence and Interstitial Lung Disease: Diagnosis and Prognosis. Investig. Radiol. 2023, 58, 602–609. [Google Scholar] [CrossRef]
- Soffer, S.; Morgenthau, A.S.; Shimon, O.; Barash, Y.; Konen, E.; Glicksberg, B.S.; Klang, E. Artificial Intelligence for Interstitial Lung Disease Analysis on Chest Computed Tomography: A Systematic Review. Acad. Radiol. 2022, 29, S226–S235. [Google Scholar] [CrossRef]
- Furukawa, T.; Oyama, S.; Yokota, H.; Kondoh, Y.; Kataoka, K.; Johkoh, T.; Fukuoka, J.; Hashimoto, N.; Sakamoto, K.; Shiratori, Y.; et al. A Comprehensible Machine Learning Tool to Differentially Diagnose Idiopathic Pulmonary Fibrosis from Other Chronic Interstitial Lung Diseases. Respirology 2022, 27, 739–746. [Google Scholar] [CrossRef]
- Hua, D.; Nguyen, K.; Petrina, N.; Young, N.; Cho, J.-G.; Yap, A.; Poon, S.K. Benchmarking the Diagnostic Test Accuracy of Certified AI Products for Screening Pulmonary Tuberculosis in Digital Chest Radiographs: Preliminary Evidence from a Rapid Review and Meta-Analysis. Int. J. Med. Inform. 2023, 177, 105159. [Google Scholar] [CrossRef] [PubMed]
- Fanni, S.C.; Marcucci, A.; Volpi, F.; Valentino, S.; Neri, E.; Romei, C. Artificial Intelligence-Based Software with CE Mark for Chest X-Ray Interpretation: Opportunities and Challenges. Diagnostics 2023, 13, 2020. [Google Scholar] [CrossRef] [PubMed]
- Jiang, B.; Li, N.; Shi, X.; Zhang, S.; Li, J.; De Bock, G.H.; Vliegenthart, R.; Xie, X. Deep Learning Reconstruction Shows Better Lung Nodule Detection for Ultra–Low-Dose Chest CT. Radiology 2022, 303, 202–212. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Lee, D.; Lu, M.T.; Raghu, V.K.; Park, C.M.; Goo, J.M.; Choi, S.H.; Kim, H. Deep Learning to Optimize Candidate Selection for Lung Cancer CT Screening: Advancing the 2021 USPSTF Recommendations. Radiology 2022, 305, 209–218. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Zhang, J.; Zhuang, W.; Gao, Z.; Kuang, K.; Tian, D.; Deng, C.; Wu, H.; Chen, R.; Lu, G.; et al. Improving the Efficiency of Identifying Malignant Pulmonary Nodules before Surgery via a Combination of Artificial Intelligence CT Image Recognition and Serum Autoantibodies. Eur. Radiol. 2022, 33, 3092–3102. [Google Scholar] [CrossRef] [PubMed]
- Nhat, P.T.H.; Van Hao, N.; Tho, P.V.; Kerdegari, H.; Pisani, L.; Thu, L.N.M.; Phuong, L.T.; Duong, H.T.H.; Thuy, D.B.; McBride, A.; et al. Clinical Benefit of AI-Assisted Lung Ultrasound in a Resource-Limited Intensive Care Unit. Crit. Care 2023, 27, 257. [Google Scholar] [CrossRef]
- Moshavegh, R.; Hansen, K.L.; Moller-Sorensen, H.; Nielsen, M.B.; Jensen, J.A. Automatic Detection of B-Lines in In Vivo Lung Ultrasound. IEEE Trans. Ultrason. Ferroelect. Freq. Contr. 2019, 66, 309–317. [Google Scholar] [CrossRef]
- Moore, C.L.; Wang, J.; Battisti, A.J.; Chen, A.; Fincke, J.; Wang, A.; Wagner, M.; Raju, B.; Baloescu, C. Interobserver Agreement and Correlation of an Automated Algorithm for B-Line Identification and Quantification With Expert Sonologist Review in a Handheld Ultrasound Device. J. Ultrasound Med. 2022, 41, 2487–2495. [Google Scholar] [CrossRef]
- Baloescu, C.; Rucki, A.A.; Chen, A.; Zahiri, M.; Ghoshal, G.; Wang, J.; Chew, R.; Kessler, D.; Chan, D.K.I.; Hicks, B.; et al. Machine Learning Algorithm Detection of Confluent B-Lines. Ultrasound Med. Biol. 2023, 49, 2095–2102. [Google Scholar] [CrossRef]
- Gottlieb, M.; Patel, D.; Viars, M.; Tsintolas, J.; Peksa, G.D.; Bailitz, J. Comparison of Artificial Intelligence versus Real-Time Physician Assessment of Pulmonary Edema with Lung Ultrasound. Am. J. Emerg. Med. 2023, 70, 109–112. [Google Scholar] [CrossRef]
- Siebert, J.N.; Hartley, M.-A.; Courvoisier, D.S.; Salamin, M.; Robotham, L.; Doenz, J.; Barazzone-Argiroffo, C.; Gervaix, A.; Bridevaux, P.-O. Deep Learning Diagnostic and Severity-Stratification for Interstitial Lung Diseases and Chronic Obstructive Pulmonary Disease in Digital Lung Auscultations and Ultrasonography: Clinical Protocol for an Observational Case–Control Study. BMC Pulm. Med. 2023, 23, 191. [Google Scholar] [CrossRef]
- Wang, J.; Yang, X.; Zhou, B.; Sohn, J.J.; Zhou, J.; Jacob, J.T.; Higgins, K.A.; Bradley, J.D.; Liu, T. Review of Machine Learning in Lung Ultrasound in COVID-19 Pandemic. J. Imaging 2022, 8, 65. [Google Scholar] [CrossRef] [PubMed]
- Van Sloun, R.J.G.; Demi, L. Localizing B-Lines in Lung Ultrasonography by Weakly Supervised Deep Learning, In-Vivo Results. IEEE J. Biomed. Health Inform. 2020, 24, 957–964. [Google Scholar] [CrossRef]
| Parameter | All Patients | Preserved DLCO | Reduced DLCO | p-Values |
|---|---|---|---|---|
| Demography | ||||
| Numbers of patients | 33 | 17 | 16 | |
| Age (years) | 69 ± 17 | 62 ± 7 | 72 ± 16 | Ns |
| Females/males | 16 F/17 M | 9 F/8 M | 7 F/9 M | Ns |
| Weight (kg) | 68.1 ± 13.66 | 67.6 ± 5.07 | 66.41 ± 13.54 | Ns |
| Height (m) | 1.64 ± 0.09 | 1.68 ± 0.07 | 1.63 ± 0.09 | Ns |
| Body Mass Index (kg/m2) | 25.0 ± 3.47 | 25.4 ± 3.39 | 24.6 ± 3.62 | Ns |
| Smokers (former) | 1 (9) | 0 (3) | 1 (6) | Ns |
| Clinical | ||||
| Hypertension | 15 | 9 | 6 | Ns |
| Diabetes (type 2) | 7 | 4 | 3 | Ns |
| Dyslipidemia | 7 | 3 | 4 | Ns |
| SARS-CoV-2 infection | 14 | 9 | 5 | Ns |
| Parameter | All Patients | Preserved DLCO | Reduced DLCO | p-Values |
|---|---|---|---|---|
| FEV1 (%) | 101.60 ± 26.83 | 121.00 ± 9.66 | 104.18 ± 26.40 | Ns |
| FVC (%) | 101.50 ± 26.80 | 123.00 ± 10.22 | 99.90 ± 28.47 | 0.03 |
| FEV1/FVC (%) | 103.90 ± 13.61 | 100.00 ± 12.02 | 107.10 ± 12.02 | Ns |
| FEF25 (%) | 90.79 ± 42.29 | 118.80 ± 49.47 | 76.95 ± 35.95 | Ns |
| FEF50 (%) | 87.56 ± 30.53 | 97.00 ± 24.96 | 87.07 ± 30.20 | Ns |
| FEF25–75 (%) | 78.75 ± 35.74 | 79.66 ± 15.50 | 84.05 ± 45.05 | Ns |
| FEF75 (%) | 88.32 ± 39.53 | 97.80 ± 31.97 | 100.16 ± 41.40 | Ns |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marozzi, M.S.; Cicco, S.; Mancini, F.; Corvasce, F.; Lombardi, F.A.; Desantis, V.; Loponte, L.; Giliberti, T.; Morelli, C.M.; Longo, S.; et al. A Novel Automatic Algorithm to Support Lung Ultrasound Non-Expert Physicians in Interstitial Pneumonia Evaluation: A Single-Center Study. Diagnostics 2024, 14, 155. https://doi.org/10.3390/diagnostics14020155
Marozzi MS, Cicco S, Mancini F, Corvasce F, Lombardi FA, Desantis V, Loponte L, Giliberti T, Morelli CM, Longo S, et al. A Novel Automatic Algorithm to Support Lung Ultrasound Non-Expert Physicians in Interstitial Pneumonia Evaluation: A Single-Center Study. Diagnostics. 2024; 14(2):155. https://doi.org/10.3390/diagnostics14020155
Chicago/Turabian StyleMarozzi, Marialuisa Sveva, Sebastiano Cicco, Francesca Mancini, Francesco Corvasce, Fiorella Anna Lombardi, Vanessa Desantis, Luciana Loponte, Tiziana Giliberti, Claudia Maria Morelli, Stefania Longo, and et al. 2024. "A Novel Automatic Algorithm to Support Lung Ultrasound Non-Expert Physicians in Interstitial Pneumonia Evaluation: A Single-Center Study" Diagnostics 14, no. 2: 155. https://doi.org/10.3390/diagnostics14020155
APA StyleMarozzi, M. S., Cicco, S., Mancini, F., Corvasce, F., Lombardi, F. A., Desantis, V., Loponte, L., Giliberti, T., Morelli, C. M., Longo, S., Lauletta, G., Solimando, A. G., Ria, R., & Vacca, A. (2024). A Novel Automatic Algorithm to Support Lung Ultrasound Non-Expert Physicians in Interstitial Pneumonia Evaluation: A Single-Center Study. Diagnostics, 14(2), 155. https://doi.org/10.3390/diagnostics14020155









