Performance of Artificial Intelligence Models Designed for Automated Estimation of Age Using Dento-Maxillofacial Radiographs—A Systematic Review
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Strategy
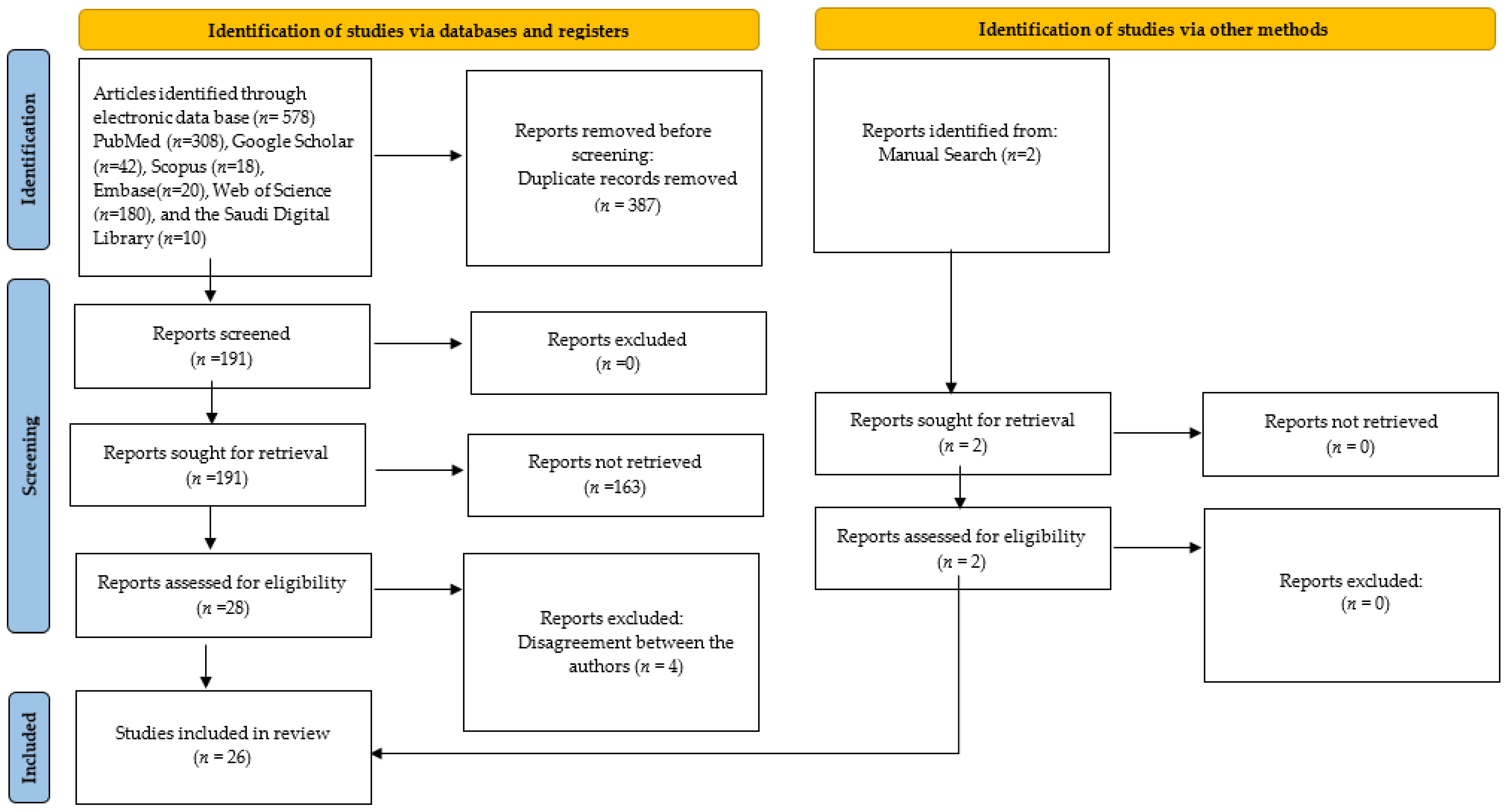
2.2. Study Selection
2.3. Eligibility Criteria
2.4. Data Extraction
3. Results
3.1. Qualitative Data of the Studies
3.2. Study Characteristics
3.3. Outcome Measures
3.4. Risk of Bias Assessment and Applicability Concern
3.5. Assessment of Strength of Evidence
4. Discussion
4.1. Effectiveness of AI in Automated Age Estimation Using Tooth Development Stages
4.2. Effectiveness of AI in Automated Age Estimation Using Tooth and Bone Parameters
4.3. Effectiveness of AI in Automated Age Estimation Using Bone Parameters
4.4. Effectiveness of AI in Pulp–Tooth Ratio
5. Challenges and Future Considerations in AI
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Limdiwala, P.; Shah, J. Age Estimation by Using Dental Radiographs. J. Forensic Dent. Sci. 2013, 5, 118. [Google Scholar] [CrossRef] [PubMed]
- Willems, G.; Moulin-Romsee, C.; Solheim, T. Non-Destructive Dental-Age Calculation Methods in Adults: Intra- and Inter-Observer Effects. Forensic Sci. Int. 2002, 126, 221–226. [Google Scholar] [CrossRef]
- Maltoni, R.; Ravaioli, S.; Bronte, G.; Mazza, M.; Cerchione, C.; Massa, I.; Balzi, W.; Cortesi, M.; Zanoni, M.; Bravaccini, S. Chronological Age or Biological Age: What Drives the Choice of Adjuvant Treatment in Elderly Breast Cancer Patients? Transl. Oncol. 2022, 15, 101300. [Google Scholar] [CrossRef] [PubMed]
- Franklin, D. Forensic Age Estimation in Human Skeletal Remains: Current Concepts and Future Directions. Leg. Med. 2010, 12, 1–7. [Google Scholar] [CrossRef]
- Vila-Blanco, N.; Varas-Quintana, P.; Tomás, I.; Carreira, M.J. A Systematic Overview of Dental Methods for Age Assessment in Living Individuals: From Traditional to Artificial Intelligence-Based Approaches. Int. J. Leg. Med. 2023, 137, 1117–1146. [Google Scholar] [CrossRef] [PubMed]
- Lewis, J.M.; Senn, D.R. Dental Age Estimation Utilizing Third Molar Development: A Review of Principles, Methods, and Population Studies Used in the United States. Forensic Sci. Int. 2010, 201, 79–83. [Google Scholar] [CrossRef]
- Celik, S.; Zeren, C.; Çelikel, A.; Yengil, E.; Altan, A. Applicability of the Demirjian Method for Dental Assessment of Southern Turkish Children. J. Forensic Leg. Med. 2014, 25, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Uzuner, F.D.; Kaygısız, E.; Darendeliler, N. Defining Dental Age for Chronological Age Determination. Post Mortem Exam. Autops. 2017, 6, 77–104. [Google Scholar] [CrossRef]
- Willems, G. A Review of the Most Commonly Used Dental Age Estimation Techniques. J. Forensic Odonto-Stomatol. 2001, 19, 9–17. [Google Scholar]
- Reesu, G.V.; Augustine, J.; Urs, A.B. Forensic Considerations When Dealing with Incinerated Human Dental Remains. J. Forensic Leg. Med. 2015, 29, 13–17. [Google Scholar] [CrossRef]
- Stavrianos, C.; Mastagas, D.; Stavrianou, I.; Karaiskou, O. Dental Age Estimation of Adults: A Review of Methods and Principles. Res. J. Med. Sci. 2008, 2, 258–268. [Google Scholar]
- Panchbhai, A. Dental Radiographic Indicators, a Key to Age Estimation. Dentomaxillofacial Radiol. 2011, 40, 199–212. [Google Scholar] [CrossRef] [PubMed]
- AlQahtani, S.J.; Hector, M.P.; Liversidge, H.M. Brief Communication: The London Atlas of Human Tooth Development and Eruption. Am. J. Phys. Anthropol. 2010, 142, 481–490. [Google Scholar] [CrossRef] [PubMed]
- Blenkin, M.; Taylor, J. Age Estimation Charts for a Modern Australian Population. Forensic Sci. Int. 2012, 221, 106–112. [Google Scholar] [CrossRef] [PubMed]
- Reppien, K.; Sejrsen, B.; Lynnerup, N. Evaluation of Post-Mortem Estimated Dental Age versus Real Age: A Retrospective 21-Year Survey. Forensic Sci. Int. 2006, 159, S84–S88. [Google Scholar] [CrossRef] [PubMed]
- McKenna, C.; James, H.; Taylor, J.; Townsend, G. Tooth Development Standards for South Australia. Aust. Dent. J. 2002, 47, 223–227. [Google Scholar] [CrossRef] [PubMed]
- Liversidge, H.M.; Smith, B.H.; Maber, M. Bias and Accuracy of Age Estimation Using Developing Teeth in 946 Children. Am. J. Phys. Anthropol. 2010, 143, 545–554. [Google Scholar] [CrossRef] [PubMed]
- Mani, S.A.; Naing, L.; John, J.; Samsudin, A.R. Comparison of Two Methods of Dental Age Estimation in 7–15-Year-Old Malays. Int. J. Paediatr. Dent. 2008, 18, 380–388. [Google Scholar] [CrossRef] [PubMed]
- Shah, P.; Venkatesh, R. Pulp/Tooth Ratio of Mandibular First and Second Molars on Panoramic Radiographs: An Aid for Forensic Age Estimation. J. Forensic Dent. Sci. 2016, 8, 112. [Google Scholar] [CrossRef]
- Lee, J.-H.; Kim, D.-H.; Jeong, S.-N.; Choi, S.-H. Detection and Diagnosis of Dental Caries Using a Deep Learning-Based Convolutional Neural Network Algorithm. J. Dent. 2018, 77, 106–111. [Google Scholar] [CrossRef]
- Chen, I.-H.; Lin, C.-H.; Lee, M.-K.; Chen, T.-E.; Lan, T.-H.; Chang, C.-M.; Tseng, T.-Y.; Wang, T.; Du, J.-K. Convolutional-Neural-Network-Based Radiographs Evaluation Assisting in Early Diagnosis of the Periodontal Bone Loss via Periapical Radiograph. J. Dent. Sci. 2024, 19, 550–559. [Google Scholar] [CrossRef]
- Yang, H.; Jo, E.; Kim, H.J.; Cha, I.; Jung, Y.-S.; Nam, W.; Kim, J.-Y.; Kim, J.-K.; Kim, Y.H.; Oh, T.G.; et al. Deep Learning for Automated Detection of Cyst and Tumors of the Jaw in Panoramic Radiographs. J. Clin. Med. 2020, 9, 1839. [Google Scholar] [CrossRef]
- Serindere, G.; Bilgili, E.; Yesil, C.; Ozveren, N. Evaluation of Maxillary Sinusitis from Panoramic Radiographs and Cone-Beam Computed Tomographic Images Using a Convolutional Neural Network. Imaging Sci. Dent. 2022, 52, 187. [Google Scholar] [CrossRef] [PubMed]
- Choi, E.; Kim, D.; Lee, J.-Y.; Park, H.-K. Artificial Intelligence in Detecting Temporomandibular Joint Osteoarthritis on Orthopantomogram. Sci. Rep. 2021, 11, 10246. [Google Scholar] [CrossRef]
- Merdietio Boedi, R.; Banar, N.; De Tobel, J.; Bertels, J.; Vandermeulen, D.; Thevissen, P.W. Effect of Lower Third Molar Segmentations on Automated Tooth Development Staging Using a Convolutional Neural Network. J. Forensic Sci. 2019, 65, 481–486. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. Br. Med. J. 2021, 372, n71. [Google Scholar] [CrossRef]
- Whiting, P.F. QUADAS-2: A Revised Tool for the Quality Assessment of Diagnostic Accuracy Studies. Ann. Intern. Med. 2011, 155, 529. [Google Scholar] [CrossRef] [PubMed]
- Bunyarit, S.S.; Nambiar, P.; Naidu, M.; Asif, M.K.; Poh, R.Y.Y. Dental Age Estimation of Malaysian Indian Children and Adolescents: Applicability of Chaillet and Demirjian’s Modified Method Using Artificial Neural Network. Ann. Hum. Biol. 2022, 49, 192–199. [Google Scholar] [CrossRef] [PubMed]
- Mualla, N.; Houssein, E.H.; Hassan, M.R. Dental Age Estimation Based on X-ray Images. Comput. Mater. Contin. 2020, 62, 591–605. [Google Scholar] [CrossRef]
- Galibourg, A.; Cussat-Blanc, S.; Dumoncel, J.; Telmon, N.; Monsarrat, P.; Maret, D. Comparison of Different Machine Learning Approaches to Predict Dental Age Using Demirjian’s Staging Approach. Int. J. Leg. Med. 2021, 135, 665–675. [Google Scholar] [CrossRef]
- Atas, İ.; Özdemir, C.; Atas, M.; Dogan, Y. Forensic Dental Age Estimation Using Modified Deep Learning Neural Network. Balk. J. Electr. Comput. Eng. 2023, 11, 298–305. [Google Scholar] [CrossRef]
- Wallraff, S.; Vesal, S.; Syben, C.; Lutz, R.; Maier, A. Age Estimation on Panoramic Dental X-Ray Images Using Deep Learning. In Bildverarbeitung für die Medizin 2021; Spinger: Berlin/Heidelberg, Germany, 2021; pp. 186–191. [Google Scholar] [CrossRef]
- Kim, S.; Lee, Y.-H.; Noh, Y.-K.; Park, F.C.; Auh, Q.-S. Age-Group Determination of Living Individuals Using First Molar Images Based on Artificial Intelligence. Sci. Rep. 2021, 11, 1073. [Google Scholar] [CrossRef]
- Shen, S.; Liu, Z.; Wang, J.; Fan, L.; Ji, F.; Tao, J. Machine Learning Assisted Cameriere Method for Dental Age Estimation. BMC Oral Health 2021, 21, 641. [Google Scholar] [CrossRef]
- Milošević, D.; Vodanović, M.; Galić, I.; Subašić, M. Automated Estimation of Chronological Age from Panoramic Dental X-Ray Images Using Deep Learning. Expert Syst. Appl. 2022, 189, 116038. [Google Scholar] [CrossRef]
- Han, M.; Du, S.; Ge, Y.; Zhang, D.; Chi, Y.; Long, H.; Yang, J.; Yang, Y.; Xin, J.; Chen, T.; et al. With or without Human Interference for Precise Age Estimation Based on Machine Learning? Int. J. Leg. Med. 2022, 136, 821–831. [Google Scholar] [CrossRef]
- Baydoğan, M.P.; Baybars, S.C.; Tuncer, S.A. Age Detection by Deep Learning from Dental Panoramic Radiographs. Artif. Intell. Theory Appl. 2022, 2, 51–58. [Google Scholar]
- Pintana, P.; Upalananda, W.; Saekho, S.; Yarach, U.; Wantanajittikul, K. Fully Automated Method for Dental Age Estimation Using the ACF Detector and Deep Learning. Egypt. J. Forensic Sci. 2022, 12, 54. [Google Scholar] [CrossRef]
- Saric, R.; Kevric, J.; Hadziabdic, N.; Osmanovic, A.; Kadic, M.; Saracevic, M.; Jokic, D.; Rajs, V. Dental Age Assessment Based on CBCT Images Using Machine Learning Algorithms. Forensic Sci. Int. 2022, 334, 111245. [Google Scholar] [CrossRef]
- Shen, S.; Yuan, X.; Wang, J.; Fan, L.; Zhao, J.; Tao, J. Evaluation of a Machine Learning Algorithms for Predicting the Dental Age of Adolescent Based on Different Preprocessing Methods. Front. Public Health 2022, 10, 1068253. [Google Scholar] [CrossRef]
- Wang, X.; Liu, Y.; Miao, X.; Chen, Y.; Cao, X.; Zhang, Y.; Li, S.; Zhou, Q. DENSEN: A Convolutional Neural Network for Estimating Chronological Ages from Panoramic Radiographs. BMC Bioinform. 2022, 23, 426. [Google Scholar] [CrossRef]
- Kumagai, A.; Jeong, S.; Kim, D.; Kong, H.-J.; Oh, S.; Lee, S.-S. Validation of Data Mining Models by Comparing with Conventional Methods for Dental Age Estimation in Korean Juveniles and Young Adults. Sci. Rep. 2023, 13, 726. [Google Scholar] [CrossRef]
- Yeom, H.-G.; Lee, B.-D.; Lee, W.; Lee, T.; Yun, J.P. Estimating Chronological Age through Learning Local and Global Features of Panoramic Radiographs in the Korean Population. Sci. Rep. 2023, 13, 21857. [Google Scholar] [CrossRef]
- Kahm, S.H.; Kim, J.-Y.; Yoo, S.; Bae, S.-M.; Kang, J.-E.; Lee, S.H. Application of Entire Dental Panorama Image Data in Artificial Intelligence Model for Age Estimation. BMC Oral Health 2023, 23, 1007. [Google Scholar] [CrossRef]
- Aljameel, S.S.; Althumairy, L.; Albassam, B.; Alsheikh, G.; Albluwi, L.; Althukair, R.; Alhareky, M.; Alamri, A.; Alabdan, A.; Shahin, S.Y. Predictive Artificial Intelligence Model for Detecting Dental Age Using Panoramic Radiograph Images. Big Data Cogn. Comput. 2023, 7, 8. [Google Scholar] [CrossRef]
- Kim, Y.-R.; Choi, J.-H.; Ko, J.; Jung, Y.-J.; Kim, B.; Nam, S.-H.; Chang, W.-D. Age Group Classification of Dental Radiography without Precise Age Information Using Convolutional Neural Networks. Healthcare 2023, 11, 1068. [Google Scholar] [CrossRef]
- Murray, J.; Heng, D.; Lygate, A.; Porto, L.; Abade, A.; Manica, S.; Franco, A. Applying Artificial Intelligence to Determination of Legal Age of Majority from Radiographic. Morphol. Bull. L’association Anat. 2023, 108, 100723. [Google Scholar] [CrossRef]
- Zaborowicz, M.; Zaborowicz, K.; Biedziak, B.; Garbowski, T. Deep Learning Neural Modelling as a Precise Method in the Assessment of the Chronological Age of Children and Adolescents Using Tooth and Bone Parameters. Sensors 2022, 22, 637. [Google Scholar] [CrossRef]
- Mu, C.C.; Li, G. Age Estimation Using Panoramic Radiographs by Transfer Learning. Chin. J. Dent. Res. 2022, 25, 119–124. [Google Scholar] [CrossRef]
- Wang, J.; Dou, J.; Han, J.; Li, G.; Tao, J. A Population-Based Study to Assess Two Convolutional Neural Networks for Dental Age Estimation. BMC Oral Health 2023, 23, 109. [Google Scholar] [CrossRef]
- Sharifonnasabi, F.; Jhanjhi, N.Z.; John, J.; Obeidy, P.; Band, S.S.; Alinejad-Rokny, H.; Baz, M. Hybrid HCNN-KNN Model Enhances Age Estimation Accuracy in Orthopantomography. Front. Public Health 2022, 10, 879418. [Google Scholar] [CrossRef]
- Pereira de Sousa, D.; Diniz Lima, E.; Souza Paulino, J.A.; Dos Anjos Pontual, M.L.; Meira Bento, P.; Melo, D.P. Age Determination on Panoramic Radiographs Using the Kvaal Method with the Aid of Artificial Intelligence. Dento Maxillo Facial Radiol. 2023, 52, 20220363. [Google Scholar] [CrossRef]
- Dogan, O.B.; Boyacioglu, H.; Goksuluk, D. Machine Learning Assessment of Dental Age Classification Based on Cone-Beam CT Images: A Different Approach. Dento Maxillo Facial Radiol. 2024, 53, 67–73. [Google Scholar] [CrossRef]
- Granholm, A.; Alhazzani, W.; Møller, M.H. Use of the GRADE Approach in Systematic Reviews and Guidelines. Br. J. Anaesth. 2019, 123, 554–559. [Google Scholar] [CrossRef]
- Costa, J.; Montero, J.; Serrano, S.; Albaladejo, A.; López-Valverde, A.; Bica, I. Accuracy in the Legal Age Estimation according to the Third Molars Mineralization among Mexicans and Columbians. Atención Primaria 2014, 46, 165–175. [Google Scholar] [CrossRef]
- Markovic, E.; Marinkovic, N.; Zelic, K.; Milovanovic, P.; Djuric, M.; Nedeljkovic, N. Dental Age Estimation according to European Formula and Willems Method: Comparison between Children with and without Cleft Lip and Palate. Cleft Palate Craniofacial J. 2021, 58, 612–618. [Google Scholar] [CrossRef]
- Shamim, T. Forensic Pediatric Dentistry. J. Forensic Dent. Sci. 2018, 10, 128. [Google Scholar] [CrossRef]
- Rath, H.; Rath, R.; Mahapatra, S.; Debta, T. Assessment of Demirjian’s 8-Teeth Technique of Age Estimation and Indian-Specific Formulas in an East Indian Population: A Cross-Sectional Study. J. Forensic Dent. Sci. 2017, 9, 45. [Google Scholar]
- Jayaraman, J.; Wong, H.M.; King, N.; Roberts, G. The French–Canadian Data Set of Demirjian for Dental Age Estimation: A Systematic Review and Meta-Analysis. J. Forensic Leg. Med. 2013, 20, 373–381. [Google Scholar] [CrossRef]
- Lee, S.-S.; Kim, D.; Lee, S.; Lee, U.-Y.; Seo, J.S.; Ahn, Y.W.; Han, S.-H. Validity of Demirjian’s and Modified Demirjian’s Methods in Age Estimation for Korean Juveniles and Adolescents. Forensic Sci. Int. 2011, 211, 41–46. [Google Scholar] [CrossRef]
- Zaborowicz, K.; Biedziak, B.; Olszewska, A.; Zaborowicz, M. Tooth and Bone Parameters in the Assessment of the Chronological Age of Children and Adolescents Using Neural Modelling Methods. Sensors 2021, 21, 6008. [Google Scholar] [CrossRef]
- Murray, P.E.; Stanley, H.R.; Matthews, J.B.; Sloan, A.J.; Smith, A.J. Age-Related Odontometric Changes of Human Teeth. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2002, 93, 474–482. [Google Scholar] [CrossRef] [PubMed]
- Verma, M.; Verma, N.; Sharma, R.; Sharma, A. Dental Age Estimation Methods in Adult Dentitions: An Overview. J. Forensic Dent. Sci. 2019, 11, 57. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Q.; Ge, Z.; Du, H.; Li, G. Age Estimation Based on 3D Pulp Chamber Segmentation of First Molars from Cone-Beam–Computed Tomography by Integrated Deep Learning and Level Set. Int. J. Leg. Med. 2020, 135, 365–373. [Google Scholar] [CrossRef] [PubMed]
- Putra, R.H.; Doi, C.; Yoda, N.; Astuti, E.R.; Sasaki, K. Current Applications and Development of Artificial Intelligence for Digital Dental Radiography. Dentomaxillofacial Radiol. 2021, 51, 20210197. [Google Scholar] [CrossRef] [PubMed]
- Demirjian, A.; Goldstein, H.; Tanner, J.M. A New System of Dental Age Assessment. Hum. Biol. 1973, 45, 211–227. [Google Scholar]

| Research question | What are the developments and performance of the artificial intelligence models that have been used in age estimation using dento-maxillofacial radiographs? |
| Population | Patients who underwent dento-maxillofacial radiographs |
| Intervention | AI-based models developed for age estimation |
| Comparison | Traditional methods of age estimation, expert opinion, other AI models |
| Outcome | Accuracy, sensitivity, specificity, precision, recall, receiver, area under the curve (AUC), F measure, mean absolute error (MAE), root mean squared error (RMSE), R squared (R2), root mean squared percentage error (RMSPE). |
| SI No. | Authors | Year of Publication | Study Design | Algorithm Architecture | Objective of the Study | No. of Patients/Images/Photographs for Testing [Datasets] | Study Factor | Modality | Comparison If Any | Evaluation Accuracy/Average Accuracy/Statistical Significance | Results (+) Effective, (−) Non-Effective (N) Neutral | Outcomes | Authors Suggestions/Conclusions |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bunyarit SS et al. [28] | 2018 | Retrospective cross-sectional study | ANN | To investigate the applicability of Chaillet and Demirjian’s scores for the age estimation of Malaysian Chinese children and adolescents | 1228 DPRs | Tooth development stages (permanent teeth from central incisor to third molar-left mandible). | DPRs | Chaillet and Demirjian’s method | Accurate estimation of age and the difference between CA and DA was −0.05 ± 0.92 years for boys and −0.06 ± 1.11 years for girls using ANN–MLP networking model. | Effective (+) | Chaillet and Demirjian’s method underestimated the DA. Population-specific prediction model was developed using the ANN–MLP networking model. | These ethnic-specific data can be used to estimate the DA of Malaysian Chinese children and adolescents in both clinical and forensic applications. |
| Mualla N et al. [29] | 2020 | Observational study | CNN | To implement automated dental age estimation using transfer learning | 1429 X-ray images | Tooth development stages | X-rays | ResNet | AlexNet (KNN classifier) Accuracy 98.8% Specificity 99.18 Precision 99.005 Recall 99.98 F-measure 98.99 ResNet Accuracy 98.8% Specificity 99.818 Precision 99.149 Recall 99.062 F-measure 99.104 | Effective (+) | AlexNet-based features were better than the ResNet-based features. | Transfer learning has proved its effectiveness in many machine learning and object recognition problems. This method needs to be evaluated more using a larger dataset and other classification models. |
| Galibourg A et al. [30] | 2021 | Comparative study | ML | To compare the predictive performance of ten machine learning algorithms for dental age estimation in children and young adults using left permanent mandibular teeth and third molars | 3570 OPGs | Tooth development stages (seven permanent mandibular teeth and four third molars) | OPG | Age estimation methods by Demirjian et al. and Willems et al. | AI method had a mean absolute error (MAE) under 0.811 years. With Demirjian’s and Willems’ methods, the MAE was 1.107 and 0.927 years, | Effective (+) | WILL method was significantly more accurate than DEM, and all ML methods were more accurate than the best reference method. | This study supported the use of ML algorithms instead of using standard population tables. |
| İsa AT al. [31] | 2021 | Comparative study | DL models | To estimate the forensic ages of individuals using an automated approach | 1332 DPRs Training 962 (85%), testing 170 (15%), and validating 200 (15%) | Tooth development stages (teeth, gingival tissue, and upper jaw) | DPR Images | InceptionV3 DenseNet201 EffcientNetB4MobileNetV2 VGG16, and ResNet50V2 | MAE = 3.13, RMSE =4.77, and correlation coefficient R2 was 87%. | Effective (+) | Modified InceptionV3 model delivered results faster and estimated ages more precisely compared to others. | This method proved to be potentially dependable and practical ancillary equipment in forensic sciences and dental medicine. |
| Wallraff S et al. [32] | 2021 | Comparative study | CNN | To automate age estimation of adolescents using a supervised regression-based deep learning method | 14000 DPRs 10,000 for training, 2500 for validation, and 244 for testing | Tooth development stages | DPR | Senior physician for oral and maxillofacial surgery and three dental students specially trained in age estimation | MAE of 1.08 years and error rate (ER) of 17.52%. | Effective (+) | This method achieved better results than manual estimation methods used by clinical experts. | This method will be useful in age estimation compared to manual methods. |
| Kim S et al. [33] | 2021 | Comparative study | CNN | To estimate age group by incorporating a CNN using dental X-ray image patches of the first molars extracted via panoramic radiography | DPRs of 1586 individuals Training: 1078 Validation: 190 Test: 318 | Tooth development stages (permanent first molars of both arches) | Panoramic radiographs | Subgroup comparison | The accuracy of the tooth-wise estimation was 89.05 to 90.27%. AUC scores ranged from 0.94 to 0.98 for all age groups. | Effective (+) | Study demonstrated the suitability of CNNs for accurately estimating the age groups of both the maxillary and mandibular first molars. | The prediction accuracy and heat map analyses support that this AI-based age-group determination model is plausible and useful. |
| Shen S et al. [34] | 2021 | Retrospective study | ML | To utilize seven lower left permanent teeth alongside three models (RF, SVM, and LR) based on the Cameriere method for predicting children’s dental age and assess their performance against Cameriere age estimation | 748 OPGs Training: 598 (80%) Test: 150 (20%) | Tooth development stages (7 lower left permanent teeth) | OPG | Traditional Cameriere formula | ME, MAE, MSE, and RMSE values of the SVM model (0.004, 0.489, 0.392, and 0.625, respectively) and the RF model (−0.004, 0.495, 0.389, and 0.623, respectively) had the highest accuracy. | Effective (+) | The research showed that the ML models have better accuracy than the traditional Cameriere formula. | All tested machine learning methods were significantly more accurate than the two Cameriere formulas for all metrics. |
| Milošević D et al. [35] | 2022 | Comparative study | CNN | To explore the applicability of deep learning in chronological age estimation using panoramic dental X-rays | 4035 DPRs training/validation/test size is 80%/10%/10% | Tooth development stages | DPR | State of art methods | Mean absolute error of 3.96 years, a median absolute error of 2.95 years, and an R2 of 0.8439. | (+) Effective | This research showcases the effectiveness of automated deep learning in dental imaging for precise age estimation. | The proposed approach attains the lowest estimation error in the literature for adult and senior subjects. |
| Hann et al. [36] | 2022 | Cross-sectional, descriptive, analytical study. | CNN | To assess the accuracy of a machine learning model for precise age estimation with or without relying on human interference | 10,257 OPGs Training set (80%), validation set (10%), and test set (10%). | Tooth development stages (8 permanent teeth of left mandible) | OPG | ADSE model based on specified manually defined features | MAE of the ADAE model is 0.83 years, being reduced by half that of the MDAE model. ADSE model for stage classification is 0.17 stages; its accuracy in dental age estimation is unsatisfactory. | (+) Effective | The ADSE model slightly improves accuracy with manually defined features and enhances evaluation efficiency. In contrast, the ADAE model, utilizing CNN, significantly boosts accuracy and efficiency without human intervention. | Fully automated feature extraction in a deep learning model without human interference performs better in dental age estimation, prominently increasing the accuracy and objectivity. |
| Baydoğan MP et al. [37] | 2022 | Observational study | CNN | To estimate age by deep learning using dental panoramic radiographs | 627 OPGs Training (70%) Test (30%) | Tooth development stages | OPG | Dentist | 84% accuracy, 85% F-score, and 76% sensitivity values were reached using the Alexnet architecture and k-nearest neighbor (k-NN) algorithm. | (+) Effective | The proposed system will ensure age determination in less time and abate the cost compared to the traditional age determination method. | The study will support dentists in the clinical environment and can be used in education |
| Pintana P et al. [38] | 2022 | Observational study | CNN | To develop and implement a fully automated system for estimating dental age utilizing the ACF detector and deep learning methodologies | 1000 OPGs Training: 800 Testing: 200 | Tooth development stages (lower left third molars) | OPG | Demirjian’s method | Localized the lower left mandibular third molar automatically with 99.5% accuracy and achieved 83.25% classifcation accuracy using the transfer learning strategy with the Resnet50 network. | (+) Effective | ACF detector and CNN model successfully localized third molars and identified the developmental stages automatically in order to estimate the age of the subjects. | The proposed method can be applied in clinical practice as a tool that helps clinicians reduce the time and subjectivity for dental age estimation. |
| Saric et al. [39] | 2022 | Observational study | ANN | To decide on the most desirable machine learning for dental age estimation based on buccal bone level | 150 CBCTs | Tooth development stages (lower seven mandibular teeth) | CBCT | Conventional ML models | Random forest classifier provided the greatest result with a correlation coefficient of 0.803 and a mean absolute error of 6.022. | (+) Effective | RF proved to be the best algorithm in our study, providing the most acceptable result for age estimation, using the three most important attributes. | We have also shown that considering sinus-related features can be a significant addition to the databases. |
| Shen et al. [40] | 2022 | Comparative study | ML | To compare of the accuracy of the Cameriere and Demirjian methods of dental age estimation using ML simultaneously | 748 OPGs Training: 80% Testing: 20% | Tooth development stages (seven mandibular teeth) | OPG | Demirjian method or Cameriere European formula | KNN model based on the Cameriere method had the highest accuracy (ME = 0.015, MAE = 0.473, MSE = 0.340, RMSE = 0.583, R2 = 0.94), | (+) Effective | KNN model based on the Cameriere method was able to infer dental age more accurately in a clinical setting. | ML can be used for dental age estimation in a larger geographical area and over a larger age range. |
| Wang et al. [41] | 2022 | Comparative study | CNN | To estimate chronological ages by using DENSEN for different age groups | 1903 OPGs | Tooth development stages | OPG | Bayesian CNN Net and DANet | DENSEN produced MAEs of 0.6885, 0.7615, 1.3502, and 2.8770 for children teens, young adults, and adults, respectively. | (+) Effective | DENSEN has lower errors for the adult group. The proposed model is memory-compact, consuming about 1.0 MB of memory overhead. | This approach required less laboratory work compared with existing methods. |
| Kumagai A et al. [42] | 2023 | Comparative study | ML | To validate data-mining-based dental age estimation by comparing its accuracy and classification performance at 18-year thresholds against conventional methods | 2657 DPRs Training: 900 Test sets: 857 | Tooth development stages (second and third molars of both jaws) | DPRs | Conventional method | The accuracy of the conventional method with the internal test set was slightly higher than that of the data mining models, with a slight difference (mean absolute error< 0.21 years, root mean square error< 0.24 years). | Neutral (N) | The threshold was also similar between the conventional method and the data mining models. | This method proved that conventional methods can be replaced by data mining models in forensic age estimation using second and third molar maturity of Korean juveniles and young adults. |
| Yeom HG et al. [43] | 2023 | Comparative study | CNN | To estimate chronological age using a hybrid method based on ResNet 50 and ViT | 9663 DPRs Training: 5861 Validation: 1916 Test data: 1886 | Tooth development stages | DPRs | ResNet50 or ViT. | Significant improvements were observed in both MAE and RMSE values across all network models (ResNet50, ViT, and Hybrid | (+) Effective | The age estimation model designed using the hybrid method performed better than those using only ResNet50 or ViT. | This model can perform better and be used effectively in the clinical field. |
| Kahm SH et al. [44] | 2023 | Comparative study | DL | To evaluate the efficiency of an AI model by applying the entire panoramic image for age estimation | 27,877 DPRs Training: 13,220 Validation: 1653 Test data: 1653 | Tooth development stages | DPRs | Two experienced dentists | Incorporating ± 3years of deviation, the accuracy of type 1 and 2 was 0.2716, 0.7323, respectively; and the F1 score was 0.1709 and 0.6437, respectively. | (+) Effective | The study showed significant accurate diagnosis in type 2 grouping with ±3years of deviation in both DN and WRN models. | In the future, the application of AI is expected to assist humans in clinical and dento-maxillofacial radiology fields. |
| Aljameel S et al. [47] | 2023 | Comparative study | CNN | To predict dental age using AI model | 529 DPRs 423 (80% for training) 106 (20% for testing) | Tooth development stages (7 left permanent teeth and 3 molars) | DPRs | Three dentists | Xception model had the best performance, with an error rate of 1.417 for the 6–11 age group. | (+) Effective | Xception model had the best performance, with an error rate of 1.417 for the 6–11 age group. | The proposed model can assist dentists in determining the appropriate treatment for patients based on their DA rather than their chronological age. |
| Rin Kim et al. [46] | 2023 | Observational study | CNN | To classify the age group using deep neural network when precise age information is not given | 10023 DPRs | Tooth development stages | DPRs | NM | The accuracies were 53.846% with a tolerance of ±5 years, 95.121% with ±15 years, and 99.581% with ±25 years, which means the probability of the estimation error being larger than one age group is 0.419%. | (+) Effective | This study confirmed the potential possibility of age estimation using AI in terms of clinical aspects of oral care, as well as forensic medicine, by determining the difference between the actual age and predicted age using panoramic radiographic images, which can be used to evaluate the overall oral conditions. | This study has the potential to be used as oral health education material using the difference between the actual age and the predicted age through AI in dental clinics. |
| Murray J et al. [47] | 2024 | Observational study | CNN | To apply AI in determination of legal age | 4003 DPRs Training: 80% Testing: 20% | Tooth development stages (third molars) | DPRs | Experts | Of the subjects over 18 years of age, 88% were correctly identified, and 87.0% of subjects under the age of majority were similarly predicted. | (+) Effective | AI-based methods could improve courtroom efficiency, stand as automated assessment methods, and contribute to our understanding of biological aging. | The present model may be used as an automated assessment tool for identifying legal age. The weightings generated by this architecture can also help researchers identify which patterns are most significant for understanding this challenging age group. |
| Zaborowicz M et al. [48] | 2022 | Observational study | DL | To utilize deep learning neural models for accurate assessment of chronological age in children and adolescents based on tooth and bone parameters | 619 OPGs | Tooth and bone parameters | OPG | Radial basis function networks | The MAE error of the produced models, depending on the learning set used, is between 2.34 and 4.61 months, while the RMSE error is between 5.58 and 7.49 months. The correlation coefficient R2 ranges from 0.92 to 0.96. | (+) Effective | The conducted research indicates that neural modeling methods are an appropriate tool for determining the metric age based on the developed proprietary tooth and bone indices. | The initial iteration of learning the network with all developed metrics already yields high-quality deep neural models. It is advisable to construct deep neural networks using the indicators from the initial research stage. |
| MU CC et al. [49] | 2022 | Comparative study | DL | To asess the accuracy of transfer learning models for age estimation from panoramic radiographs of permanent dentitions of patients | 3000 DPRs Training: 2400 Validating: 300 Test set: 300 | Tooth and bone parameters (teeth, maxillary sinus, and mandibular angle) | DPRs | ResNet VggNet DenseNet | MAE and RMSE of EfficentNet–B5 were 2.83 and 4.49, respectively | (+) Effective | This method of transfer learning proves to be applicable for age estimation utilizing panoramic radiographs. | This model can be used for age estimation with panoramic radiographs. |
| Wang J et al. [50] | 2023 | Comparative study | CNN | To investigate the possibility of using AI-based methods for age estimation in an eastern Chinese population | 9586 OPGs Training: 70% Testing: 30% | Tooth and bone parameters (molars, maxillary sinus, and nasal septum) | OPGs | ResNet101 | Accuracy of VGG16 model = 93.63%. Accuracy of ResNet101 network = 88.73%. | (+) Effective | VGG16 outperformed ResNet101 in terms of DA prediction performance. | CNNs such as VGG16 hold great promise for future use in clinical practice and forensic sciences. |
| Sharifonnasabi F et al. [51] | 2022 | Comparative study | CNN | To evaluate the accuracy of a hybrid HCNN-KNN model in age estimation | 1922 DPRs Training: 80% Testing: 20% | Bone age measurement | OPG | ResNet, CNN, GoogLeNet Inception | Successfully estimated the age in classified studies of - year-old, 6 months, 3 months, and 1-month-old cases with accuracies of 99.98, 99.96, 99.87, and 98.78 respectively. | (+) Effective | The evaluation of our model on a diverse dataset confirms its superior performance. | The benchmarking with current existing models also showed that the HCNN-KNN model is the best model for bone age measurement. |
| Pereira de Sousa et al. [52] | 2023 | Comparative study | ML | To assess and compare age estimation on panoramic radiography using the Kvaal method and machine learning | 554 DPRs Training: 85% Testing: 15% | Pulp–tooth ratio | DPRs | Kaval method | ML (MAE: 4.77 presented higher age estimation precision than the Kvaal method (MAE: 5.68). | (+) Effective | ML classifiers can improve age estimation when assessing panoramic radiography using the Kvaal method. | The use of ML on panoramic radiographs can improve age estimation. |
| Dogan B et al. [53] | 2024 | Observational study | ML | To use ML algorithms to evaluate the efficacy of pulp/tooth area ratio (PTR) in cone-beam CT (CBCT) images to predict dental age classification in adults | 236 CBCT Training: 70% Testing: 30% | Pulp/tooth area ratio | CBCT | CART SVM | The models’ highest accuracy and confidence intervals were found to belong to the RF algorithm. | Neutral (N) | The models’ performances were found to be low. | The models were found to be low in performance but were considered as a different approach. |
| Outcome | Inconsistency | Indirectness | Imprecision | Risk of Bias | Publication Bias | Strength of Evidence |
|---|---|---|---|---|---|---|
| Application of AI in automated age estimation using tooth development stages [28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47] | Not Present | Not Present | Not Present | Present | Not Present | ⨁⨁⨁◯ |
| Application of AI in automated age estimation using tooth and bone parameters [48,49,50] | Not Present | Not Present | Not Present | Not Present | Not Present | ⨁⨁⨁⨁ |
| Application of AI in automated age estimation using bone age measurements [51] | Not Present | Not Present | Not Present | Not Present | Not Present | ⨁⨁⨁⨁ |
| Application of AI in automated age estimation using pulp–tooth ratio [52,53] | Not Present | Not Present | Not Present | Not Present | Not Present | ⨁⨁⨁⨁ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khanagar, S.B.; Albalawi, F.; Alshehri, A.; Awawdeh, M.; Iyer, K.; Alsomaie, B.; Aldhebaib, A.; Singh, O.G.; Alfadley, A. Performance of Artificial Intelligence Models Designed for Automated Estimation of Age Using Dento-Maxillofacial Radiographs—A Systematic Review. Diagnostics 2024, 14, 1079. https://doi.org/10.3390/diagnostics14111079
Khanagar SB, Albalawi F, Alshehri A, Awawdeh M, Iyer K, Alsomaie B, Aldhebaib A, Singh OG, Alfadley A. Performance of Artificial Intelligence Models Designed for Automated Estimation of Age Using Dento-Maxillofacial Radiographs—A Systematic Review. Diagnostics. 2024; 14(11):1079. https://doi.org/10.3390/diagnostics14111079
Chicago/Turabian StyleKhanagar, Sanjeev B., Farraj Albalawi, Aram Alshehri, Mohammed Awawdeh, Kiran Iyer, Barrak Alsomaie, Ali Aldhebaib, Oinam Gokulchandra Singh, and Abdulmohsen Alfadley. 2024. "Performance of Artificial Intelligence Models Designed for Automated Estimation of Age Using Dento-Maxillofacial Radiographs—A Systematic Review" Diagnostics 14, no. 11: 1079. https://doi.org/10.3390/diagnostics14111079
APA StyleKhanagar, S. B., Albalawi, F., Alshehri, A., Awawdeh, M., Iyer, K., Alsomaie, B., Aldhebaib, A., Singh, O. G., & Alfadley, A. (2024). Performance of Artificial Intelligence Models Designed for Automated Estimation of Age Using Dento-Maxillofacial Radiographs—A Systematic Review. Diagnostics, 14(11), 1079. https://doi.org/10.3390/diagnostics14111079






