Laboratory Tests, Bacterial Resistance, and Treatment Options in Adult Patients Hospitalized with a Suspected Urinary Tract Infection
Abstract
1. Introduction
2. Tests Can Only Rule out a Urinary Tract Infection but Not Confirm the Diagnosis
3. The Role of the Dipstick to Rule out a Urinary Tract Infection
4. A Positive Dipstick Should Not Be Confirmed by a Microscopic Urinalysis
5. Positive Findings on the Dipstick Can Lead to Inappropriate Further Testing
5.1. The Disutility of Finding Blood by Dipstick Analysis
5.2. Proteinuria
6. Other Tests to Rule out a Urinary Tract Infection
7. Urine Cultures
7.1. Urine Cultures Are Overutilized and Have Clinical Disutility (Table 2)
| 1. Unnecessary use of antibiotics |
| a. Adverse drug events |
| b. Clostridium difficile infection |
| c. Increased bacterial resistance rates |
| 2. Delay in definitive interventions |
| 3. Introduction of a urine catheter to obtain a specimen |
7.2. Bacterial Sensitivities and Antibiotic Therapy
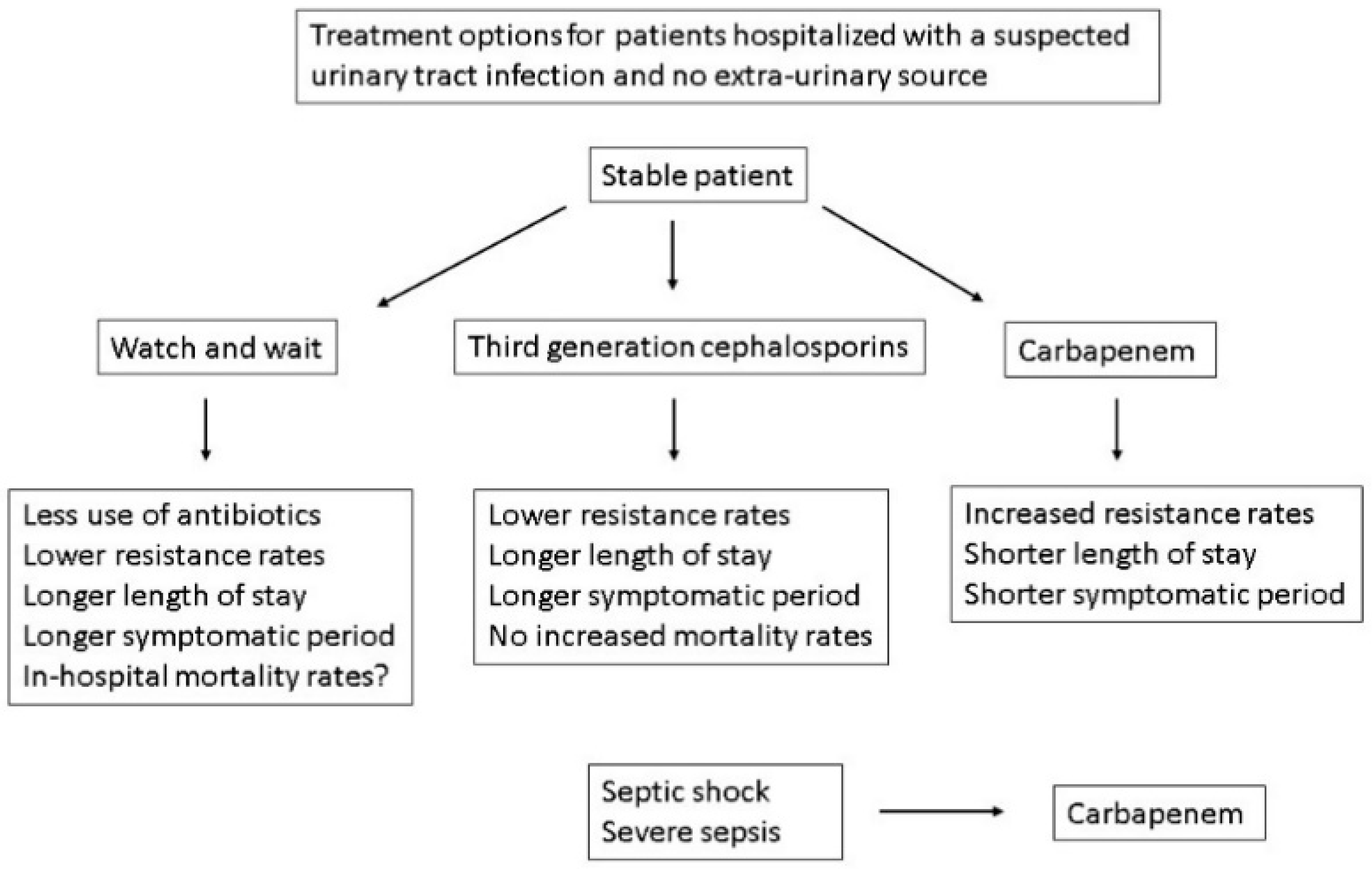
8. Should All Hospitalized Patients with a Suspected Systemic UTI Be Treated with Drugs That Have <10% Resistance Rates?
9. Watch and Wait
10. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Froom, P.; Shimoni, Z. The uncertainties of the diagnosis and treatment of a suspected urinary tract infection in elderly hospitalized patients. Expert. Rev. Anti. Infect. Ther. 2018, 16, 763–770. [Google Scholar] [CrossRef]
- Advani, S.D.; Polage, C.R.; Fakih, M.G. Deconstructing the urinalysis: A novel approach to diagnostic and antimicrobial stewardship. Antimicrob. Steward Healthc. Epidemiol. 2021, 1, e6. [Google Scholar] [CrossRef]
- Schulz, L.; Hoffman, R.J.; Pothof, J.; Fox, B. Top ten myths regarding the diagnosis and treatment of urinary tract infections. J. Emerg. Med. 2016, 51, 25–30. [Google Scholar] [CrossRef]
- Nicolle, L.E.; Gupta, K.; Bradley, S.F.; Colgan, R.; DeMuri, G.P.; Drekonja, D.; Eckert, L.O.; Geerlings, S.E.; Köves, B.; Hooton, T.M.; et al. Clinical practice guideline for the management of asymptomatic bacteriuria: 2019 update by the Infectious Diseases Society of America. Clin. Infect. Dis. 2019, 68, e83–e110. [Google Scholar] [CrossRef] [PubMed]
- Nicolle, L.E.; Gupta, K.; Bradley, S.F.; Colgan, R.; DeMuri, G.P.; Drekonja, D.; Eckert, L.O.; Geerlings, S.E.; Köves, B.; Hooton, T.M.; et al. Clinical practice guideline for the management of asymptomatic bacteriuria: 2019 update by the infectious diseases society of America. Clin. Infect. Dis. 2019, 68, 1611–1615. [Google Scholar] [CrossRef]
- Mody, L.; Juthani-Mehta, M. Urinary tract infections in older women: A clinical review. JAMA 2014, 311, 844–854. [Google Scholar] [CrossRef]
- Shimoni, Z.; Cohen, R.; Avdiaev, R.; Froom, P. Treatment of febrile geriatric patients with suspected urinary tract infections in a hospital with high rates of ESBL producing bacteria: A cohort study. BMJ. Open 2016, 6, e013696. [Google Scholar] [CrossRef]
- Shimoni, Z.; Avdiaev, R.; Froom, P. Urine cultures in hospitalized geriatric patients presenting with fever. Am. J. Med. Sci. 2017, 353, 17–21. [Google Scholar] [CrossRef] [PubMed]
- Juthani-Mehta, M.; Tinetti, M.; Perrelli, E.; Towle, V.; Van Ness, P.H.; Quagliarello, V. Diagnostic accuracy of criteria for urinary tract infection in a cohort of nursing home residents. J. Am. Geriatr. Soc. 2007, 55, 1072–1077. [Google Scholar] [CrossRef]
- Beveridge, L.A.; Davey, P.G.; Phillips, G.; McMurdo, M.E. Optimal management of urinary tract infections in older people. Clin. Interv. Aging. 2011, 6, 173–180. [Google Scholar] [CrossRef]
- Nicolle, L.E. Urinary infections in the elderly: Symptomatic or asymptomatic. Int. J. Antimicrob. Agents. 1999, 11, 265–268. [Google Scholar] [CrossRef] [PubMed]
- Loeb, M.; Brazil, K.; Lohfeld, L.; McGeer, A.; Simor, A.; Stevenson, K.; Zoutman, D.; Smith, S.; Liu, X.; Walter, S.D. Effect of a multifaceted intervention on number of antimicrobial prescriptions for suspected urinary tract infections in residents of nursing homes: Cluster randomised controlled trial. BMJ 2005, 331, 669. [Google Scholar] [CrossRef] [PubMed]
- Rowe, T.A.; Juthani-Mehta, M. Diagnosis and management of urinary tract infection in older adults. Infect. Dis. Clin. N. Am. 2014, 28, 75–89. [Google Scholar] [CrossRef]
- Stone, N.D.; Ashraf, M.S.; Calder, J.; Crnich, C.J.; Crossley, K.; Drinka, P.J.; Gould, C.V.; Juthani-Mehta, M.; Lautenbach, E.; Loeb, M.; et al. Society for healthcare epidemiology long-term care special interest group. Surveillance definitions of infections in long-term care facilities: Revisiting the McGeer criteria. Infect. Control Hosp. Epidemiol. 2012, 33, 965–977. [Google Scholar] [CrossRef]
- Garcia, R.; Spitzer, E.D. Promoting appropriate urine culture management to improve health care outcomes and the accuracy of catheter-associated urinary tract infections. Am. J. Infect. Control 2017, 45, 1143–1153. [Google Scholar] [CrossRef]
- Ninan, S.; Walton, C.; Barlow, G. Investigation of suspected urinary tract infection in older people. BMJ 2014, 349, g4070. [Google Scholar] [CrossRef] [PubMed]
- Nicolle, L.E.; SHEA Long-Term-Care-Committee. Urinary tract infections in long-term-care facilities. Infect. Control Hosp. Epidemiol. 2001, 22, 167–175. [Google Scholar] [CrossRef] [PubMed]
- Detweiler, K.; Mayers, D.; Fletcher, S.G. Bacteriuria and urinary tract infections in the elderly. Urol. Clin. N. Am. 2015, 42, 561–568. [Google Scholar] [CrossRef] [PubMed]
- Woodford, H.J.; Graham, C.; Meda, M.; Miciuleviciene, J. Bacteremic urinary tract infection in hospitalized older patients-are any currently available diagnostic criteria sensitive enough? J. Am. Geriatr. Soc. 2011, 59, 567–568. [Google Scholar] [CrossRef]
- Barkham, T.M.S.; Martin, F.C.; Eykyn, S.J. Delay in diagnosis of bacteraemic urinary tract infection in elderly patients. Age Ageing. 1996, 25, 130–132. [Google Scholar] [CrossRef]
- Shimoni, Z.; Kasem, A.; Froom, P. The influence of mental status on reported local urinary tract symptoms in patients with bacteraemic urinary tract infections. Int. J. Clin. Pract. 2021, 75, e13741. [Google Scholar] [CrossRef] [PubMed]
- Ouslander, J.G.; Schapira, M.; Schnelle, J.F.; Fingold, S. Pyuria among chronically incontinent but otherwise asymptomatic nursing home residents. J. Am. Geriatr. Soc. 1996, 44, 420–423. [Google Scholar] [CrossRef]
- Shimoni, Z.; Glick, J.; Hermush, V.; Froom, P. Sensitivity of the dipstick in detecting bacteremic urinary tract infections in elderly hospitalized patients. PLoS ONE 2017, 12, e0187381. [Google Scholar] [CrossRef] [PubMed]
- Monane, M.; Gurwitz, J.H.; Lipsitz, L.A.; Glynn, R.J.; Choodnovskiy, I.; Avorn, J. Epidemiologic and diagnostic aspects of bacteriuria: A longitudinal study in older women. J. Am. Geriatr. Soc. 1995, 43, 618–622. [Google Scholar] [CrossRef] [PubMed]
- Kaye, D.; Boscia, J.A.; Abrutyn, E.; Levison, M.E. Asymptomatic bacteriuria in the elderly. Trans. Am. Clin. Climatol. Assoc. 1989, 100, 155–162. [Google Scholar] [PubMed]
- Nicolle, L.E. Asymptomatic bacteriuria in the elderly. Infect. Dis. Clin. N. Am. 1997, 11, 647–662. [Google Scholar] [CrossRef]
- Hedin, K.; Petersson, C.; Widebäck, K.; Kahlmeter, G.; Mölstad, S. Asymptomatic bacteriuria in a population of elderly in municipal institutional care. Scand. J. Prim. Health Care 2002, 20, 166–168. [Google Scholar] [CrossRef] [PubMed]
- Kayalp, D.; Dogan, K.; Ceylan, G.; Senes, M.; Yucel, D. Can routine automated urinalysis reduce culture requests? Clin. Biochem. 2013, 46, 1285–1289. [Google Scholar] [CrossRef] [PubMed]
- Jones, C.W.; Culbreath, K.D.; Mehrotra, A.; Gilligan, P.H. Reflect urine culture cancellation in the emergency department. J. Emerg. Med. 2014, 46, 71–76. [Google Scholar] [CrossRef]
- Shimoni, Z.; Hermush, V.; Glick, J.; Froom, P. No need for a urine culture in elderly hospitalized patients with a negative dipstick test result. Eur. J. Clin. Microbiol. Infect. Dis. 2018, 37, 1459–1464. [Google Scholar] [CrossRef]
- Laan, B.J.; van Horrik, T.M.Z.X.K.; Nanayakkara, P.W.B.; Geerlings, S.E. How many urinalysis and urine cultures are necessary? Eur. J. Intern. Med. 2021, 83, 58–61. [Google Scholar] [CrossRef]
- Devillé, W.L.; Yzermans, J.C.; van Duijn, N.P.; Bezemer, P.D.; van der Windt, D.A.; Bouter, L.M. The urine dipstick test useful to rule out infections. A meta-analysis of the accuracy. BMC Urol. 2004, 4, 4. [Google Scholar] [CrossRef] [PubMed]
- Froom, P.; Bieganiec, B.; Ehrenrich, Z.; Barak, M. Stability of common analytes in urine refrigerated for 24 h before automated analysis by test strips. Clin. Chem. 2000, 46, 1384–1386. [Google Scholar] [CrossRef] [PubMed]
- Gambke, B.; Kouri, T.; Kutter, D.; Nagel, D.; Vukovich, T.; Wefers, A. Multicentre evaluation of the urine analyser miditron junior. Scand. J. Clin. Lab. Invest. 1997, 57, 605–611. [Google Scholar] [CrossRef] [PubMed]
- Bonnardeaux, A.; Somerville, P.; Kaye, M. A study on the reliability of dipstick urinalysis. Clin. Nephrol. 1994, 41, 167–172. [Google Scholar] [PubMed]
- Gadeholt, H. Quantitative estimation of urinary sediment, with special regard to sources of error. Br. Med. J. 1964, 1, 1547–1549. [Google Scholar] [CrossRef]
- Oyaert, M.; Delanghe, J. Progress in Automated Urinalysis. Ann. Lab. Med. 2019, 39, 15–22. [Google Scholar] [CrossRef]
- Wald, R.; Bell, C.M.; Nisenbaum, R.; Perrone, S.; Liangos, O.; Laupacis, A.; Jaber, B.L. Interobserver reliability of urine sediment interpretation. Clin. J. Am. Soc. Nephrol. 2009, 4, 567–571. [Google Scholar] [CrossRef] [PubMed]
- Howard-Anderson, J.R.; Ashraf, S.; Overton, E.C.; Reif, L.; Murphy, D.J.; Jacob, J.T. Sustained decrease in urine culture utilization after implementing a reflex urine culture intervention: A multicenter quasi-experimental study. Infect. Control Hosp. Epidemiol. 2020, 41, 369–371. [Google Scholar] [CrossRef]
- Mark, D.G.; Hung, Y.Y.; Salim, Z.; Tarlton, N.J.; Torres, E.; Frazee, B.W. Third-Generation Cephalosporin Resistance and Associated Discordant Antibiotic Treatment in Emergency Department Febrile Urinary Tract Infections. Ann. Emerg. Med. 2021, 78, 357–369. [Google Scholar] [CrossRef]
- Froom, P.; Barak, M. Cessation of dipstick urinalysis reflex testing and physician ordering behavior. Am. J. Clin. Pathol. 2012, 137, 486–489. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Eintracht, S.; MacNamara, E. Successful protocol for eliminating excessive urine microscopies: Quality improvement and cost savings with physician support. Clin. Biochem. 2017, 50, 88–93. [Google Scholar] [CrossRef]
- Perazella, M.A.; O’Leary, M.P.; Etiology and Evaluation of Hematuria in Adults. UpToDate Version December 2022. Available online: https://www.medilib.ir/uptodate/show/7208 (accessed on 22 November 2022).
- Adams, E.C., Jr.; Fetter, M.C.; Free, H.M.; Free, A.H. Hemolysis in hematuria. J. Urol. 1962, 88, 427–430. [Google Scholar] [CrossRef]
- Penders, J.; Fiers, T.; Delanghe, J.R. Quantitative evaluation of urinalysis test strips. Clin. Chem. 2002, 48, 2236–2241. [Google Scholar] [CrossRef] [PubMed]
- Froom, P.; Shimoni, Z.; Dusseldorp, N.; Benbassat, J. Asymptomatic Microscopic Hematuria in Inpatient Nonsurgical Adults. Am. J. Clin. Pathol. 2023, 159, 221–224. [Google Scholar] [CrossRef]
- Shimoni, Z.; Froom, P.; Dusseldorp, N.; Benbassat, J. Stop routine microscopic urinalysis in hospitalized patients with dipstick abnormalities? J. Eval. Clin. Pract. 2022, 28, 566–568. [Google Scholar] [CrossRef] [PubMed]
- Shimoni, Z.; Froom, P.; Benbassat, J. Proteinuria in hospitalised internal medicine adult patients. Postgrad. Med. J. 2022, 98, 369–371. [Google Scholar] [CrossRef] [PubMed]
- Webster, A.C.; Nagler, E.V.; Morton, R.L.; Masson, P. Chronic kidney disease. Lancet 2017, 389, 1238–1252. [Google Scholar] [CrossRef]
- de Boer, F.J.; Gieteling, E.; van Egmond-Kreileman, H.; Moshaver, B.; van der Leur, S.J.; Stegeman, C.A.; Groeneveld, P.H. Accurate and fast urinalysis in febrile patients by flow cytometry. Infect. Dis. 2017, 49, 380–387. [Google Scholar] [CrossRef]
- Moshaver, B.; de Boer, F.; van Egmond-Kreileman, H.; Kramer, E.; Stegeman, C.; Groeneveld, P. Fast and accurate prediction of positive and negative urine cultures by flow cytometry. BMC Infect. Dis. 2016, 16, 211. [Google Scholar] [CrossRef]
- Broeren, M.A.; Bahçeci, S.; Vader, H.L.; Arents, N.L. Screening for urinary tract infection with the Sysmex UF-1000i urine flow cytometer. J. Clin. Microbiol. 2011, 49, 1025–1029. [Google Scholar] [CrossRef] [PubMed]
- Manoni, F.; Fornasiero, L.; Ercolin, M.; Tinello, A.; Ferrian, M.; Hoffer, P.; Valverde, S.; Gessoni, G. Cutoff values for bacteria and leukocytes for urine flow cytometer Sysmex UF-1000i in urinary tract infections. Diagn. Microbiol. Infect. Dis. 2009, 65, 103–107. [Google Scholar] [CrossRef] [PubMed]
- Íñigo, M.; Coello, A.; Fernández-Rivas, G.; Carrasco, M.; Marcó, C.; Fernández, A.; Casamajor, T.; Ausina, V. Evaluation of the SediMax automated microscopy sediment analyzer and the Sysmex UF-1000i flow cytometer as screening tools to rule out negative urinary tract infections. Clin. Chim. Acta 2016, 456, 31–35. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Mora, C.; Acevedo, D.; Porres, M.A.; Chaqués, A.M.; Zapardiel, J.; Gallego-Cabrera, A.; López, J.M.; Maesa, J.M. Comparison of automated devices UX-2000 and SediMAX/AutionMax for urine samples screening: A multicenter Spanish study. Clin. Biochem. 2017, 50, 714–718. [Google Scholar] [CrossRef] [PubMed]
- Bilsen, M.P.; Conroy, S.P.; Schneeberger, C.; Platteel, T.N.; van Nieuwkoop, C.; Mody, L.; Caterino, J.M.; Geerlings, S.E.; Köves, B.; Wagenlehner, F.; et al. A reference standard for urinary tract infection research: A multidisciplinary Delphi consensus study. Lancet Infect. Dis. 2024. [Google Scholar] [CrossRef] [PubMed]
- Previtali, G.; Ravasio, R.; Seghezzi, M.; Buoro, S.; Alessio, M.G. Performance evaluation of the new fully automated urine particle analyser UF-5000 compared to the reference method of the Fuchs-Rosenthal chamber. Clin. Chim. Acta 2017, 472, 123–130. [Google Scholar] [CrossRef] [PubMed]
- Chant, C.; Dos Santos, C.C.; Saccucci, P.; Smith, O.M.; Marshall, J.C.; Friedrich, J.O. Discordance between perception and treatment practices associated with intensive care unit-acquired bacteriuria and funguria: A Canadian physician survey. Crit. Care Med. 2008, 36, 1158–1167. [Google Scholar] [CrossRef] [PubMed]
- Petty, L.A.; Vaughn, V.M.; Flanders, S.A.; Malani, A.N.; Conlon, A.; Kaye, K.S.; Thyagarajan, R.; Osterholzer, D.; Nielsen, D.; Eschenauer, G.A.; et al. Risk Factors and outcomes associated with treatment of asymptomatic bacteriuria in hospitalized patients. JAMA Intern. Med. 2019, 179, 1519–1527. [Google Scholar] [CrossRef] [PubMed]
- Castañeda, N.R.; Doodlesack, A.; Fyfe, W.; Edlow, J.; Grossman, S.A. The data and the reality: Urine cultures and emergency medicine physicians. Intern. Emerg. Med. 2022, 17, 2349–2355. [Google Scholar] [CrossRef]
- Horstman, M.J.; Spiegelman, A.; Naik, A.D.; Trautner, B.W. National patterns of urine testing during inpatient admission. Clin. Infect. Dis. 2017, 65, 1199–1205. [Google Scholar] [CrossRef]
- Flokas, M.E.; Andreatos, N.; Alevizakos, M.; Kalbasi, A.; Onur, P.; Mylonakis, E. Inappropriate management of asymptomatic patients with positive urine cultures: A systematic review and meta-analysis. Open Forum Infect. Dis. 2017, 24, ofx207. [Google Scholar] [CrossRef]
- Shehab, N.; Lovegrove, M.C.; Geller, A.I.; Rose, K.O.; Weidle, N.J.; Budnitz, D.S. US emergency department visits for outpatient adverse drug events, 2013–2014. JAMA 2016, 316, 2115–2125. [Google Scholar] [CrossRef]
- Tamma, P.D.; Avdic, E.; Li, D.X.; Dzintars, K.; Cosgrove, S.E. Association of adverse events with antibiotic use in hospitalized patients. JAMA Intern. Med. 2017, 177, 1308–1315. [Google Scholar] [CrossRef] [PubMed]
- Malik, U.; Armstrong, D.; Ashworth, M.; Dregan, A.; L’Esperance, V.; McDonnell, L.; Molokhia, M.; White, P. Association between prior antibiotic therapy and subsequent risk of community-acquired infections: A systematic review. J. Antimicrob. Chemother. 2018, 73, 287–296. [Google Scholar] [CrossRef] [PubMed]
- Chalmers, J.D.; Akram, A.R.; Singanayagam, A.; Wilcox, M.H.; Hill, A.T. Risk factors for Clostridium difficile infection in hospitalized patients with community-acquired pneumonia. J. Infect. 2016, 73, 45–53. [Google Scholar] [CrossRef]
- Spellberg, B. The new antibiotic mantra-“Shorter Is Better. JAMA Intern. Med. 2016, 176, 1254–1255. [Google Scholar] [CrossRef]
- Sutton, J.D.; Stevens, V.W.; Chang, N.-C.N.; Khader, K.; Timbrook, T.T.; Spivak, E.S. Oral b-lactam antibiotics vs fluoroquinolones or trimethoprim-sulfamethoxazole for definitive treatment of Enterobacterales bacteremia from a urine source. JAMA Netw. Open 2020, 3, e2020166. [Google Scholar] [CrossRef] [PubMed]
- Meddings, J.; Saint, S.; Fowler, K.E.; Gaies, E.; Hickner, A.; Krein, S.L.; Bernstein, S.J. The Ann Arbor criteria for appropriate urinary catheter use in hospitalized medical patients: Results obtained by using the RAND/UCLA appropriateness method. Ann. Intern. Med. 2015, 162, S1–S34. [Google Scholar] [CrossRef]
- Malmros, K.; Huttner, B.D.; McNulty, C.; Rodríguez-Baño, J.; Pulcini, C.; Tängdén, T.; ESGAP UTI Working Group. Comparison of antibiotic treatment guidelines for urinary tract infections in 15 European countries—results of an online survey. Int. J. Antimicrob. Agents 2019, 54, 478–486. [Google Scholar] [CrossRef]
- Gupta, K.; Hooton, T.M.; Naber, K.G.; Wullt, B.; Colgan, R.; Miller, L.G.; Moran, G.J.; Nicolle, L.E.; Raz, R.; Schaeffer, A.J.; et al. International clinical practice guidelines for the treatment of acute uncomplicated cystitis and pyelonephritis in women: A 2010 update by the Infectious Diseases Society of America and the European Society for Microbiology and Infectious Diseases. Clin. Infect. Dis. 2011, 52, e103–e120. [Google Scholar] [CrossRef]
- de Cueto, M.; Aliaga, L.; Alós, J.I.; Canut, A.; Los-Arcos, I.; Martínez, J.A.; Mensa, J.; Pintado, V.; Rodriguez-Pardo, D.; Yuste, J.R.; et al. Executive summary of the diagnosis and treatment of urinary tract infection: Guidelines of the Spanish Society of Clinical Microbiology and Infectious Diseases (SEIMC). Enferm. Infecc. Microbiol. Clin. 2017, 35, 314–320. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.J.; Hsu, P.C.; Yang, C.C.; Kuo, A.J.; Chia, J.H.; Wu, T.L.; Lee, M.H. Risk factors and outcomes of carbapenem-nonsusceptible Escherichia coli bacteremia: A matched case-control study. J. Microbiol. Immunol. Infect. 2011, 44, 125–130. [Google Scholar] [CrossRef] [PubMed]
- Armand-Lefevre, L.; Angebault, C.; Barbier, F. Emergence of imipenem-resistant Gram-negative bacilli in intestinal flora of intensive care patients. Antimicrob. Agents Chemother. 2013, 57, 1488–1495. [Google Scholar] [CrossRef] [PubMed]
- Harris, P.N.; Tambyah, P.A.; Paterson, D.L. β-Lactam and β-lactamase inhibitor combinations in the treatment of extended-spectrum βlactamase producing Enterobacteriaceae: Time for a reappraisal in the era of few antibiotic options? Lancet Infect. Dis. 2015, 15, 475–485. [Google Scholar] [CrossRef] [PubMed]
- Oteo, J.; Pérez-Vázquez, M.; Extended-Spectrum, C.J. [beta]-lactamase producing Escherichia coli: Changing epidemiology and clinical impact. Curr. Opin. Infect. Dis. 2010, 23, 320–326. [Google Scholar] [CrossRef]
- Goodman, K.E.; Lessler, J.; Cosgrove, S.E.; Harris, A.D.; Lautenbach, E.; Han, J.H.; Milstone, A.M.; Massey, C.J.; Tamma, P.D.; Antibacterial Resistance Leadership Group. Antibacterial resistance leadership group. A clinical decision tree to predict whether a bacteremic patient is infected with an extended-spectrum β-lactamase-producing organism. Clin. Infect. Dis. 2016, 1, 896–903. [Google Scholar] [CrossRef] [PubMed]
- Shimoni, Z.; Salah, M.; Kasem, A.; Hermush, V.; Froom, P. Bacterial Resistance to Cephalosporin Treatment in Elderly Stable Patients Hospitalized with a Urinary Tract Infection. Am. J. Med. Sci. 2020, 360, 243–247. [Google Scholar] [CrossRef]
- Li, N.Y.; Poh, G.Q.; Teng, G.C.W.; Chen, H.H.; Chan, D.S.G.; Chan, S.P.; Tambyah, P.A.; Bagdasarian, N.; Wu, J.E. A Prediction Tool for the Presence of Ceftriaxone-Resistant Uropathogens upon Hospital Admission. Antibiotics 2020, 9, 316. [Google Scholar] [CrossRef] [PubMed]
- Shimoni, Z.; Froom, P. Ceftriaxone Usage and Resistance Rates in Internal Medicine Departments. Qeios 2023. [Google Scholar] [CrossRef]
- World Health Organization Global Antimicrobial Resistance and Use Surveillance System (GLASS). 2021. Available online: https://www.who.int/health-topics/antimicrobial-resistance (accessed on 31 December 2023).
- Kayaaslan, B.; Oktay, Z.; Hasanoglu, I.; Kalem, A.K.; Eser, F.; Ayhan, M.; Guner, R. Increasing rates of extended-spectrum B-lactamase-producing Escherichia coli and Klebsiella pneumoniae in uncomplicated and complicated acute pyelonephritis and evaluation of empirical treatments based on culture results. Eur. J. Clin. Microbiol. Infect. Dis. 2022, 41, 421–430. [Google Scholar] [CrossRef]
- Zavala-Cerna, M.G.; Segura-Cobos, M.; Gonzalez, R.; Zavala-Trujillo, I.G.; Navarro-Perez, S.F.; Rueda-Cruz, J.A.; Satoscoy-Tovar, F.A. The Clinical Significance of High Antimicrobial Resistance in Community-Acquired Urinary Tract Infections. Can. J. Infect. Dis. Med. Microbiol. 2020, 2020, 2967260. [Google Scholar] [CrossRef] [PubMed]
- Talan, D.A.; Takhar, S.S.; Krishnadasan, A.; Mower, W.R.; Pallin, D.J.; Garg, M.; Femling, J.; Rothman, R.E.; Moore, J.C.; Jones, A.E.; et al. Emergence of Extended-Spectrum β-Lactamase Urinary Tract Infections Among Hospitalized Emergency Department Patients in the United States. Ann. Emerg. Med. 2021, 77, 32–43. [Google Scholar] [CrossRef] [PubMed]
- Tocut, M.; Zohar, I.; Schwartz, O.; Yossepowitch, O.; Maor, Y. Short- and long-term mortality in patients with urosepsis caused by Escherichia coli susceptible and resistant to 3rd generation cephalosporins. BMC Infect. Dis. 2022, 22, 571. [Google Scholar] [CrossRef]
- Israeli Infection Prevention Unit Antibiotic Supplied to the Hospitalized Patients. 2021. Available online: https://www.gov.il/BlobFolder/dynamiccollectorresultitem/ic-rep-ant-hosp-2021/he/files_publications_units_infection-control_ant_hosp_2021.pdf (accessed on 31 December 2023).
- Wenzler, E.; Danziger, L.H. Urinary Tract Infections: Resistance Is Futile. Antimicrob. Agents Chemother. 2016, 60, 2596–2597. [Google Scholar] [CrossRef] [PubMed]
- Chastain, D.B.; King, S.T.; Stover, K.R. Rethinking urinary antibiotic breakpoints: Analysis of urinary antibiotic concentrations to treat multidrug resistant organisms. BMC Res. Notes 2018, 11, 497. [Google Scholar] [CrossRef]
- Asakura, T.; Ikeda, M.; Nakamura, A.; Kodera, S. Efficacy of empirical therapy with non-carbapenems for urinary tract infections with extended-spectrum beta-lactamase-producing Enterobacteriaceae. Int. J. Infect. Dis. 2014, 29, 91–95. [Google Scholar] [CrossRef] [PubMed]
- Wie, S.H.; Kim, H.W.; Chang, U.I. Effects of gentamicin monotherapy for the initial treatment of community-onset complicated non-obstructive acute pyelonephritis due to Enterobacteriaceae in elderly and non-elderly women. Clin. Microbiol. Infect. 2014, 20, 1211–1218. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.S.; Kim, Y.; Chung, D.R. Impact of discordant empirical therapy on outcome of community-acquired bacteremic acute pyelonephritis. J. Infect. 2011, 62, 159–164. [Google Scholar] [CrossRef] [PubMed]
- Eliakim-Raz, N.; Babitch, T.; Shaw, E.; Addy, I.; Wiegand, I.; Vank, C.; Torre-Vallejo, L.; Joan-Miquel, V.; Steve, M.; Grier, S.; et al. Risk factors for treatment failure and mortality among hospitalized patients with complicated urinary tract infection: A multicenter retrospective cohort study (RESCUING Study Group). Clin. Infect. Dis. 2019, 68, 29–36. [Google Scholar] [CrossRef]
- Wiggers, J.B.; Sehgal, P.; Pinto, R.; MacFadden, D.; Daneman, N. The association of adequate empirical treatment and time to recovery from bacteraemic urinary tract infections: A retrospective cohort study. Clin. Microbiol. Infect. 2019, 25, 1253–1258. [Google Scholar] [CrossRef]
- Babich, T.; Zusman, O.; Elbaz, M.; Ben-Zvi, H.; Paul, M.; Leibovici, L.; Avni, T. Empirical antibiotic therapy does not improve outcomes in catheter-associated urinary tract infection: Prospective cohort study. Clin. Infect. Dis. 2017, 65, 1799–1805. [Google Scholar] [CrossRef] [PubMed]
- Parienti, J.J.; Lucet, J.C.; Lefort, A.; Armand-Lefèvre, L.; Wolff, M.; Caron, F.; Cattoir, V.; Yazdanpanah, Y. Empirical therapies among adults hospitalized for community-acquired upper urinary tract infections: A decision-tree analysis of mortality, costs, and resistance. Am. J. Infect. Control 2015, 43, e53–e59. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Song, D.Y.; Cho, S.H.; Kwon, K.T. Impact of extended-spectrum beta-lactamase on acute pyelonephritis treated with empirical ceftriaxone. Microb. Drug Resist. 2014, 20, 39–44. [Google Scholar] [CrossRef]
- Jeon, J.H.; Kim, K.; Han, W.D.; Song, S.H.; Park, K.U.; Rhee, J.E.; Song, K.H.; Park, W.B.; Kim, E.S.; Park, S.W.; et al. Empirical use of ciprofloxacin for acute uncomplicated pyelonephritis caused by Escherichia coli in communities where the prevalence of fluoroquinolone resistance is high. Antimicrob. Agents Chemother. 2012, 56, 3043–3046. [Google Scholar] [CrossRef]
- Wagenlehner, F.M.; Gasink, L.B.; McGovern, P.C.; Moeck, G.; McLeroth, P.; Dorr, M.; Dane, A.; Henkel, T.; CERTAIN-1 Study Team. Cefepime-Taniborbactam in Complicated Urinary Tract Infection. N. Engl. J. Med. 2024, 390, 611–622. [Google Scholar] [CrossRef]
- Leung, Y.Y.; Ma, K.K.; Tsang, W.T.; Lau, C.L.; Ko, S.; Chan, W.L.; Ng, F. A retrospective study of geriatric patients presenting with fever to an accident and emergency department in Hong Kong. Hong Kong J. Emerg. Med. 2008, 15, 88–95. [Google Scholar] [CrossRef]
- Keating, H.J., 3rd; Klimek, J.J.; Levine, D.S.; Kiernan, F.J. Effect of aging on the clinical significance of fever in ambulatory adult patients. J. Am. Geriatr. Soc. 1984, 32, 282–287. [Google Scholar] [CrossRef] [PubMed]
- Limper, M.; Eeftinck Schattenkerk, D.; de Kruif, M.D.; van Wissen, M.; Brandjes, D.P.; Duits, A.J.; van Gorp, E.C. One-year epidemiology of fever at the emergency department. Neth. J. Med. 2011, 69, 124–128. [Google Scholar] [PubMed]
- Bilsen, M.P.; Jongeneel, R.M.H.; Schneeberger, C.; Platteel, T.N.; van Nieuwkoop, C.; Mody, L.; Caterino, J.M.; Geerlings, S.E.; Köves, B.; Wagenlehner, F.; et al. Definitions of Urinary Tract Infection in Current Research: A Systematic Review. Open Forum Infect. Dis. 2023, 10, ofad332. [Google Scholar] [CrossRef]
- Singer, M.; Deutschman, C.S.; Seymour, C.W.; Shankar-Hari, M.; Annane, D.; Bauer, M.; Bellomo, R.; Bernard, G.R.; Chiche, J.D.; Coopersmith, C.M.; et al. The third international consensus definitions for sepsis and septic shock (sepsis-3). JAMA 2016, 315, 801–810. [Google Scholar] [CrossRef]
- Seymour, C.W.; Liu, V.X.; Iwashyna, T.J.; Brunkhorst, F.M.; Rea, T.D.; Scherag, A.; Rubenfeld, G.; Kahn, J.M.; Shankar-Hari, M.; Singer, M.; et al. Assessment of clinical criteria for sepsis: For the third international consensus definitions for sepsis and septic shock (sepsis-3). JAMA 2016, 315, 762–774. [Google Scholar] [CrossRef] [PubMed]
- Sepsis: Recognition, diagnosis and early management. BJU Int. 2017, 2018, 497–514. [CrossRef] [PubMed]
| Variation in volume under the coverslip—3–10-fold |
| Variation in the discarded supernatant |
| Variation in mixing before and after centrifugation |
| Loss of cells during centrifugation |
| Intra and inter-observer variation |
| 1. Limit urine cultures if the dipstick is negative. |
| 2. Do not confirm the dipstick results with a microscopic urinalysis. |
| 3. Limit reflexing incidental findings on dipstick (blood, protein). |
| 4. Limit urine catheterization to obtain a urine sample. |
| 5. Consider a watch and wait policy for stable patients with nonspecific symptoms. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Froom, P.; Shimoni, Z. Laboratory Tests, Bacterial Resistance, and Treatment Options in Adult Patients Hospitalized with a Suspected Urinary Tract Infection. Diagnostics 2024, 14, 1078. https://doi.org/10.3390/diagnostics14111078
Froom P, Shimoni Z. Laboratory Tests, Bacterial Resistance, and Treatment Options in Adult Patients Hospitalized with a Suspected Urinary Tract Infection. Diagnostics. 2024; 14(11):1078. https://doi.org/10.3390/diagnostics14111078
Chicago/Turabian StyleFroom, Paul, and Zvi Shimoni. 2024. "Laboratory Tests, Bacterial Resistance, and Treatment Options in Adult Patients Hospitalized with a Suspected Urinary Tract Infection" Diagnostics 14, no. 11: 1078. https://doi.org/10.3390/diagnostics14111078
APA StyleFroom, P., & Shimoni, Z. (2024). Laboratory Tests, Bacterial Resistance, and Treatment Options in Adult Patients Hospitalized with a Suspected Urinary Tract Infection. Diagnostics, 14(11), 1078. https://doi.org/10.3390/diagnostics14111078







