Analytical Evaluation of Point-of-Care Finecare™ Procalcitonin Rapid Quantitative Test in Sepsis Population as Compared with Elecsys® BRAHMS Procalcitonin Immunoassay
Abstract
1. Introduction
2. Materials and Methods
2.1. Overview of POC PCT Test Analyzer
2.1.1. Finecare FIA Meter
2.1.2. Cobas e411
2.2. Statistical Analysis
2.3. Details of Method
2.3.1. Accuracy
2.3.2. Precision
2.3.3. Linearity
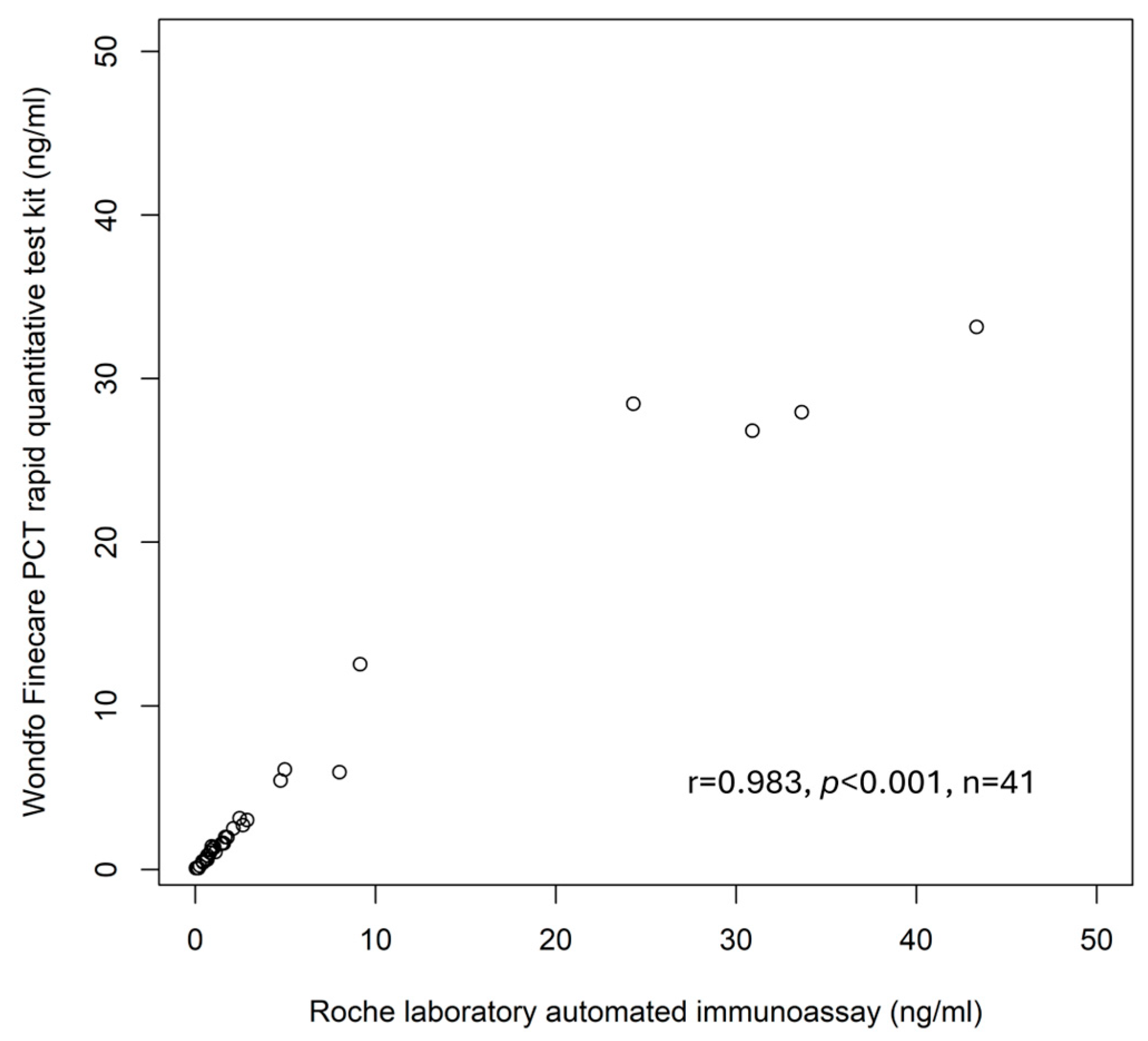
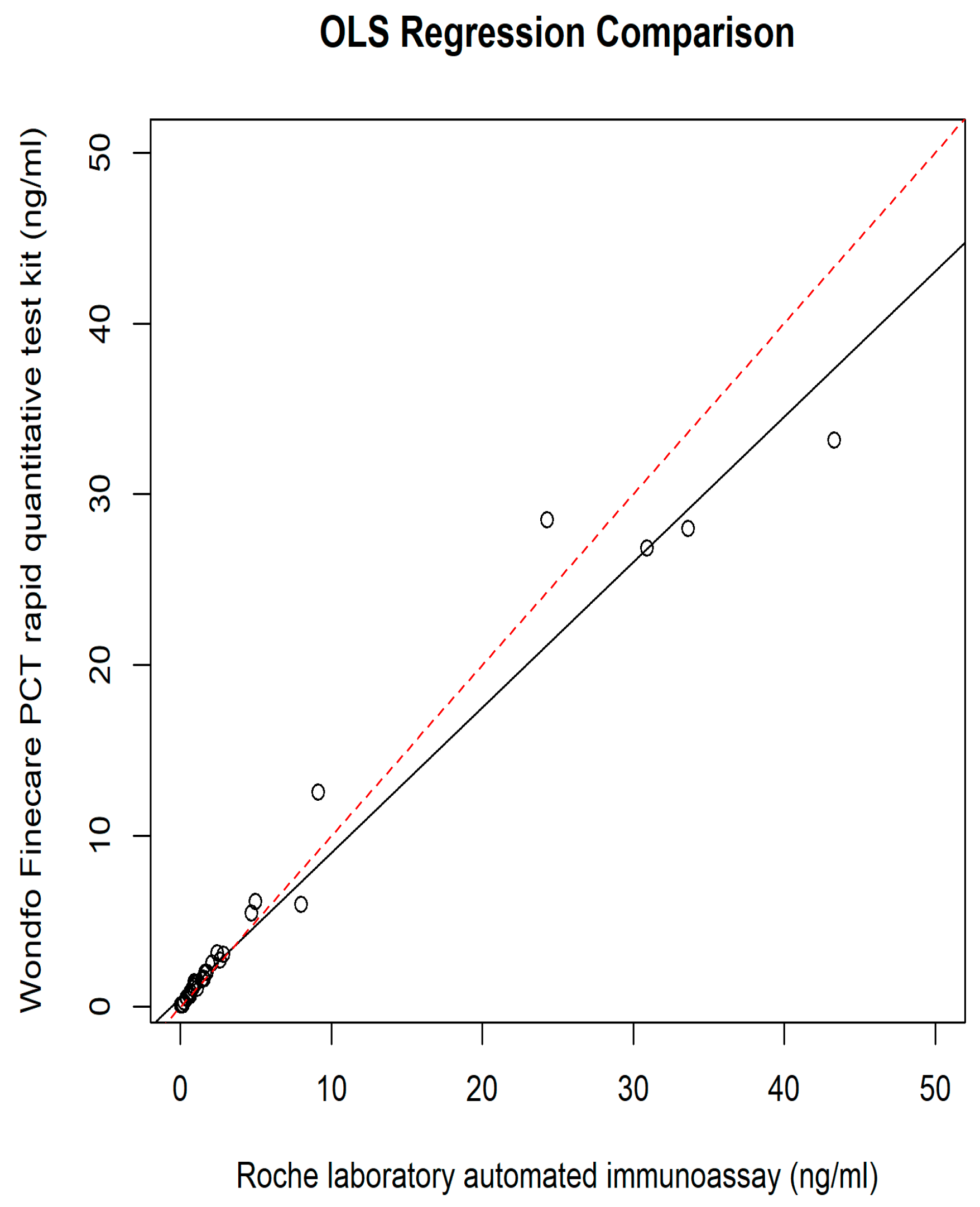
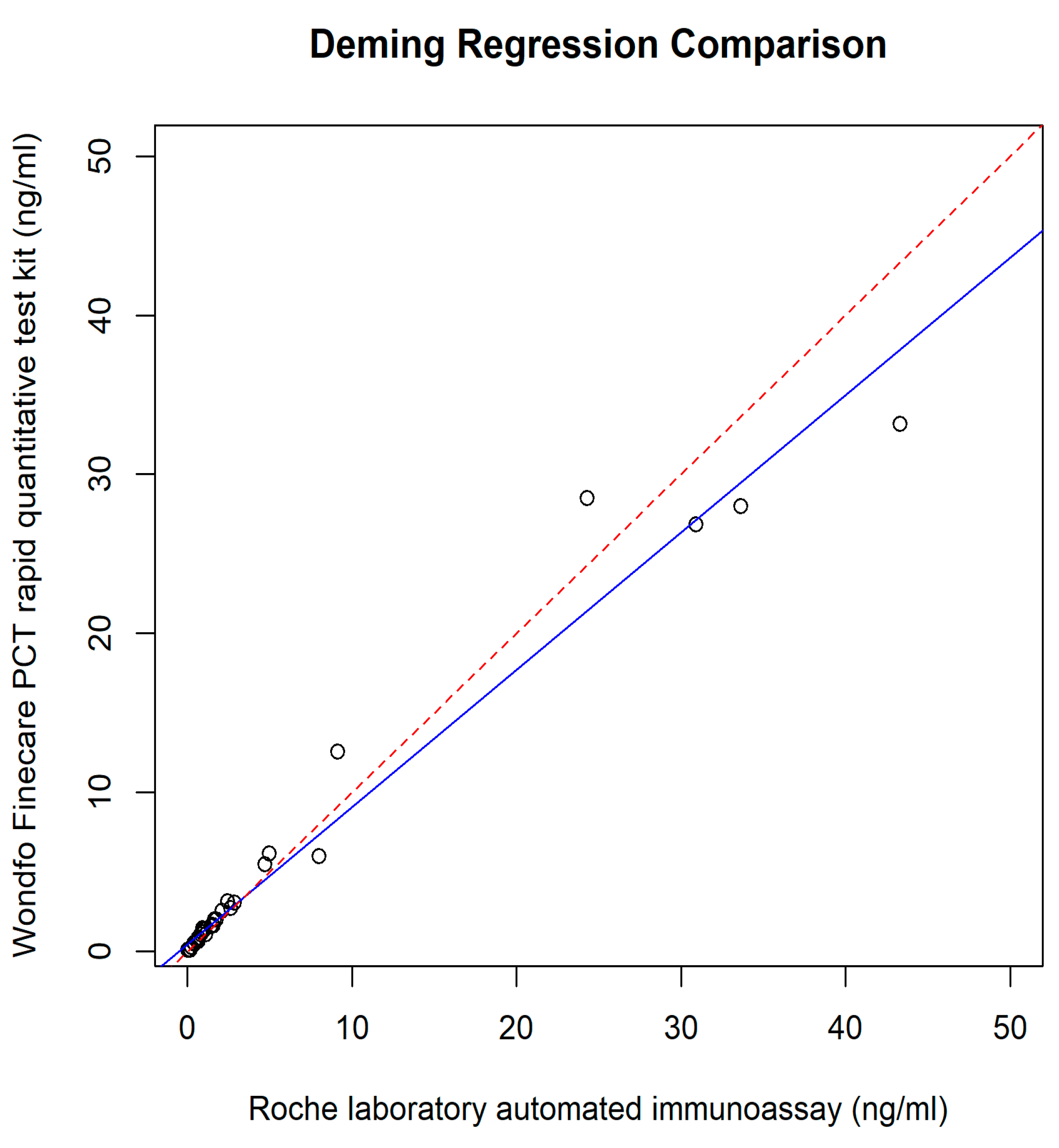
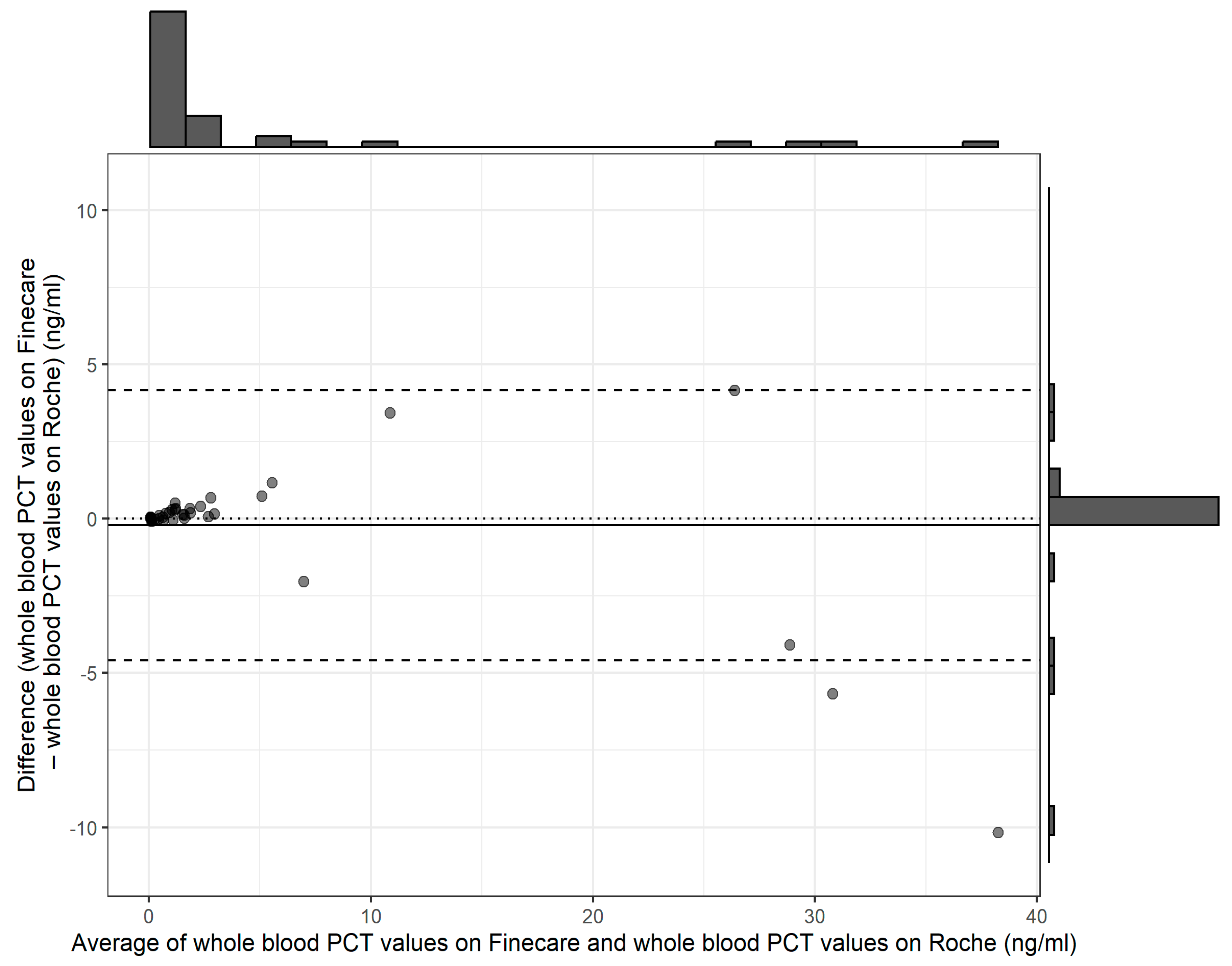
3. Results
4. Discussion
5. Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Assicot, M.; Bohuon, C.; Gendrel, D.; Raymond, J.; Carsin, H.; Guilbaud, J. High serum procalcitonin concentrations in patients with sepsis and infection. Lancet 1993, 341, 515–518. [Google Scholar] [CrossRef] [PubMed]
- Dandona, P.; Nix, D.; Wilson, M.F.; Aljada, A.; Love, J.; Assicot, M.; Bohuon, C. Procalcitonin increase after endotoxin injection in normal subjects. J. Clin. Endocrinol. Metab. 1994, 79, 1605–1608. [Google Scholar] [CrossRef] [PubMed]
- Hoeboer, S.H.; van der Geest, P.J.; Nieboer, D.; Groeneveld, A.J. The diagnostic accuracy of procalcitonin for bacteraemia: A sys-tematic review and meta-analysis. Clin. Microbiol. Infect. 2015, 21, 474–481. [Google Scholar] [CrossRef] [PubMed]
- Bouadma, L.; Luyt, C.-E.; Tubach, F.; Cracco, C.; Alvarez, A.; Schwebel, C.; Schortgen, F.; Lasocki, S.; Veber, B.; Dehoux, M.; et al. Use of procalcitonin to reduce patients’ exposure to antibiotics in intensive care units (PRORATA trial): A multicentre randomised controlled trial. Lancet 2010, 375, 463–474. [Google Scholar] [CrossRef] [PubMed]
- Evans, L.; Rhodes, A.; Alhazzani, W.; Antonelli, M.; Coopersmith, C.M.; French, C.; Machado, F.R.; Mcintyre, L.; Ostermann, M.; Prescott, H.C.; et al. Surviving sepsis campaign: International guidelines for management of sepsis and septic shock 2021. Crit. Care Med. 2021, 49, E1063–E1143. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Huang, Y.; Xu, Y. Determining procalcitonin at point-of-care; A method comparison study of four commercial PCT assays. Pr. Lab. Med. 2021, 25, e00214. [Google Scholar] [CrossRef] [PubMed]
- FDA. Clears BioMérieux’s VIDAS® B·R·A·H·M·S PCT® Assay A First Indication for Sepsis Risk Assessment in the ICU. Available online: https://www.biomerieux.com/corp/en/journalists/press-releases/fda-clears-biomerieux-s-vidas-brahms-pct-assay-first-indication-sepsis-risk-assessment-icu.html (accessed on 18 March 2024).
- BRAHMS AG Receives FDA Clearance to Market Automated Procalcitonin (PCT) Test. Available online: https://www.biospace.com/article/releases/brahms-ag-receives-fda-clearance-to-market-automated-procalcitonin-pct-test-197585/ (accessed on 18 March 2024).
- Dipalo, M.; Guido, L.; Micca, G.; Pittalis, S.; Locatelli, M.; Motta, A.; Bianchi, V.; Callegari, T.; Aloe, R.; Da Rin, G.; et al. Multicenter comparison of automated procalcitonin im-munoassays. Pract. Lab. Med. 2015, 2, 22–28. [Google Scholar] [CrossRef] [PubMed]
- Reitz, K.M.; Kennedy, J.; Li, S.R.; Handzel, R.; Tonetti, D.A.; Neal, M.D.; Zuckerbraun, B.S.; Hall, D.E.; Sperry, J.L.; Angus, D.C.; et al. Association Between Time to Source Control in Sepsis and 90-Day Mortality. JAMA Surg. 2022, 157, 817–826. [Google Scholar] [CrossRef]
- Masetto, T.; Eidizadeh, A.; Peter, C.; Grimmler, M. National external quality assessment and direct method comparison reflect crucial deviations of Procalcitonin measurements in Germany. Clin. Chim. Acta 2022, 529, 67–75. [Google Scholar] [CrossRef] [PubMed]
- Kari, K.A.; Shukeri, W.F.W.M.; Yaacob, N.M.; Li, A.Y.; Zaini, R.H.; Mazlan, M.Z. Prevalence and Outcome of Sepsis: Mortality and Prolonged Intensive Care Unit Stay among Sepsis Patients Admitted to a Tertiary Centre in Malaysia. Malays. J. Med. Sci. 2023, 30, 120. [Google Scholar] [CrossRef]
- Mazlan, M.Z.; Ghazali, A.G.; Omar, M.; Yaacob, N.M.; Mohamad, N.A.N.; Hassan, M.H.; Shukeri, W.F.W.M. Predictors of Treatment Failure and Mortality among Patients with Septic Shock Treated with Meropenem in the Intensive Care Unit. Malays. J. Med. Sci. 2024, 31, 76–90. [Google Scholar] [CrossRef]
- Kim, S.J.; Hwang, S.O.; Kim, Y.W.; Lee, J.H.; Cha, K.-C. Procalcitonin as a diagnostic marker for sepsis/septic shock in the emergency department; a study based on Sepsis-3 definition. Am. J. Emerg. Med. 2019, 37, 272–276. [Google Scholar] [CrossRef]
- AlRawahi, A.N.; AlHinai, F.A.; Doig, C.J.; Ball, C.G.; Dixon, E.; Xiao, Z.; Kirkpatrick, A.W. The prognostic value of serum procalcitonin meas-urements in critically injured patients: A systematic review. Crit. Care 2019, 23, 390. [Google Scholar] [CrossRef] [PubMed]
- Tan, M.; Lu, Y.; Jiang, H.; Zhang, L. The diagnostic accuracy of procalcitonin and C-reactive protein for sepsis: A systematic review and meta-analysis. J. Cell. Biochem. 2019, 120, 5852–5859. [Google Scholar] [CrossRef]
- Vijayan, A.L.; Vanimaya Ravindran, S.; Saikant, R.; Lakshmi, S.; Kartik, R. Procalcitonin: A promising diagnostic marker for sepsis and antibiotic therapy. J. Intensive Care 2017, 5, 51. [Google Scholar] [CrossRef] [PubMed]
- Schuetz, P.; Birkhahn, R.; Sherwin, R.; Jones, A.E.; Singer, A.; Kline, J.A.; Runyon, M.S.; Self, W.H.; Courtney, D.M.; Nowak, R.M.; et al. Serial procalcitonin predicts mortality in severe sepsis patients: Results from the Multicenter Procalcitonin Monitoring Sepsis (MOSES) study. Crit. Care Med. 2017, 45, 781–789. [Google Scholar] [CrossRef] [PubMed]
- Kutz, A.; Hausfater, P.; Oppert, M.; Alan, M.; Grolimund, E.; Gast, C.; Alonso, C.; Wissmann, C.; Kuehn, C.; Bernard, M.; et al. Comparison between B·R·A·H·M·S PCT direct, a new sensitive point-of-care testing device for rapid quantification of procalcitonin in emergency department patients and established reference methods—A prospective multinational trial. Clin. Chem. Lab. Med. 2016, 54, 577–584. [Google Scholar] [CrossRef]
- Ismail, M.A.; Mazlan, M.Z.; Hassan, S.K.; Azman, W.N.W.; Koon, T.S.; Yaacob, N.M.; Omar, M.; Mohamad, N.A.N. Evaluation of agreement between point-of-care and laboratory automated immunoassay in procalcitonin measurement among critically ill patients. Anaesth. Pain Intensiv. Care 2023, 27, 470–477. [Google Scholar] [CrossRef]
- Shim, B.-S.; Yoon, Y.-H.; Kim, J.-Y.; Cho, Y.-D.; Park, S.-J.; Lee, E.-S.; Choi, S.-H. Clinical Value of Whole Blood Procalcitonin Using Point of Care Testing, Quick Sequential Organ Failure Assessment Score, C-Reactive Protein and Lactate in Emergency Department Patients with Suspected Infection. J. Clin. Med. 2019, 8, 833. [Google Scholar] [CrossRef]
- Lippi, G.; Salvagno, G.L.; Gelati, M.; Pucci, M.; Cascio, C.L.; Demonte, D.; Faggian, D.; Plebani, M. Two-center comparison of 10 fully-automated commercial procalcitonin (PCT) immunoassays. Clin. Chem. Lab. Med. 2019, 58, 77–84. [Google Scholar] [CrossRef]
- Huynh, H.; Bœuf, A.; Pfannkuche, J.; Schuetz, P.; Thelen, M.; Nordin, G.; van der Hagen, E.; Kaiser, P.; Kesseler, D.; Badrick, T.; et al. Harmonization status of procalci-tonin measurements: What do comparison studies and EQA schemes tell us? Clin. Chem. Lab Med. 2021, 59, 1610–1622. [Google Scholar] [CrossRef] [PubMed]

| Variables | Mean (SD)/n(%) | |
| Age (Years) | 51.63 (16.55) | |
| Sex | ||
| Female | 14 (34.1) | |
| Male | 27 (65.9) | |
| BMI (Kg/m2) | 26.44 (9.20) | |
| AKI | ||
| No | 28 (68.3) | |
| Yes | 13 (31.7) | |
| ESRF | ||
| No | 37 (90.2) | |
| Yes | 4 (9.8) | |
| Total White Cell Count (×109/L) | 13.43 (4.85) | |
| Temperature (°C) | 36.74 (0.64) | |
| Noradrenaline | ||
| No | 25 (61.0) | |
| Yes | 16 (39.0) | |
| Ventilation | ||
| No | 4 (9.8) | |
| Yes | 37 (90.2) | |
| Characteristic | n = 41 a |
|---|---|
| Finecare™ PCT Rapid Quantitative Test (ng/mL) | 4.46 (8.68) |
| Elecsys® BRAHMS PCT (ng/mL) | 4.67 (10.03) |
| Method | Mean (SD) ng/mL | Mean Different | t (df) | p Value |
|---|---|---|---|---|
| Finecare™ PCT Rapid Quantitative Test (ng/mL) | 4.46 (8.68) | −0.21 (−0.91, 0.49) | −0.59 (39) | 0.556 |
| Elecsys® BRAHMS PCT (ng/mL) | 4.67 (10.03) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mazlan, M.Z.; Wan Azman, W.N.; Yaacob, N.M.; Koon, T.S.; Yahya, N.K. Analytical Evaluation of Point-of-Care Finecare™ Procalcitonin Rapid Quantitative Test in Sepsis Population as Compared with Elecsys® BRAHMS Procalcitonin Immunoassay. Diagnostics 2024, 14, 1080. https://doi.org/10.3390/diagnostics14111080
Mazlan MZ, Wan Azman WN, Yaacob NM, Koon TS, Yahya NK. Analytical Evaluation of Point-of-Care Finecare™ Procalcitonin Rapid Quantitative Test in Sepsis Population as Compared with Elecsys® BRAHMS Procalcitonin Immunoassay. Diagnostics. 2024; 14(11):1080. https://doi.org/10.3390/diagnostics14111080
Chicago/Turabian StyleMazlan, Mohd Zulfakar, Wan Norlina Wan Azman, Najib Majdi Yaacob, Tan Say Koon, and Nurul Khaiza Yahya. 2024. "Analytical Evaluation of Point-of-Care Finecare™ Procalcitonin Rapid Quantitative Test in Sepsis Population as Compared with Elecsys® BRAHMS Procalcitonin Immunoassay" Diagnostics 14, no. 11: 1080. https://doi.org/10.3390/diagnostics14111080
APA StyleMazlan, M. Z., Wan Azman, W. N., Yaacob, N. M., Koon, T. S., & Yahya, N. K. (2024). Analytical Evaluation of Point-of-Care Finecare™ Procalcitonin Rapid Quantitative Test in Sepsis Population as Compared with Elecsys® BRAHMS Procalcitonin Immunoassay. Diagnostics, 14(11), 1080. https://doi.org/10.3390/diagnostics14111080






