Associations of Brain Arteriovenous Malformation-Related Factors with Epileptic Seizure Presentations
Abstract
1. Introduction
2. Methods
2.1. Study Design
2.2. Variables
2.3. Statistical Analysis
3. Results
3.1. Study Subjects
3.2. Association Study
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Ding, D.; Quigg, M.; Starke, R.M.; Yen, C.-P.; Przybylowski, C.J.; Dodson, B.K.; Sheehan, J.P. Cerebral Arteriovenous Malformations and Epilepsy, Part 2: Predictors of Seizure Outcomes Following Radiosurgery. World Neurosurg. 2015, 84, 653–662. [Google Scholar] [CrossRef] [PubMed]
- Germans, M.R.; Sun, W.; Sebök, M.; Keller, A.; Regli, L. Molecular Signature of Brain Arteriovenous Malformation Hemorrhage: A Systematic Review. World Neurosurg. 2022, 157, 143–151. [Google Scholar] [CrossRef] [PubMed]
- Bustuchina, V.M. New approaches for brain arteriovenous malformations-related epilepsy. Rev. Neurol. 2023, 179, 188–200. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Li, R.; Ma, L.; Zhao, Y.; Yu, T.; Wang, H.; Ye, X.; Wang, R.; Chen, X.; Zhao, Y. Single-Stage Combined Embolization and Resection for Spetzler-Martin Grade III/IV/V Arteriovenous Malformations: A Single-Center Experience and Literature Review. Front. Neurol. 2020, 11, 570198. [Google Scholar] [CrossRef] [PubMed]
- Crawford, P.M.; West, C.R.; Shaw, M.D.M. Cerebral Arteriovenous Malformations and Epilepsy: Factors in the Development of Epilepsy. Epilepsia 1986, 27, 270–275. [Google Scholar] [CrossRef] [PubMed]
- Garcin, B.; Houdart, E.; Porcher, R.; Manchon, E.; Saint-Maurice, J.; Bresson, D.; Stapf, C. Epileptic seizures at initial presentation in patients with brain arteriovenous malformation. Neurology 2012, 78, 626–631. [Google Scholar] [CrossRef] [PubMed]
- Jiang, P.; Lv, X.; Wu, Z.; Li, Y.; Jiang, C.; Yang, X.; Zhang, Y. Characteristics of Brain Arteriovenous Malformations Presenting with Seizures without Acute or Remote Hemorrhage. Neuroradiol. J. 2011, 24, 886–888. [Google Scholar] [CrossRef] [PubMed]
- Spetzler, R.F.; Martin, N.A. A proposed grading system for arteriovenous malformations. J. Neurosurg. 1986, 65, 476–483. [Google Scholar] [CrossRef] [PubMed]
- Turjman, F.; Massoud, T.F.; Sayre, J.W.; Viñuela, F.; Guglielmi, G.; Duckwiler, G. Epilepsy associated with cerebral arteriovenous malformations: A multivariate analysis of angioarchitectural characteristics. Am. J. Neuroradiol. 1995, 16, 345–350. [Google Scholar] [PubMed]
- Hoh, B.L.; Chapman, P.H.; Loeffler, J.S.; Carter, B.S.; Ogilvy, C.S. Results of multimodality treatment for 141 patients with brain arteriovenous malformations and seizures: Factors associated with seizure incidence and seizure outcomes. Neurosurgery 2002, 51, 303–311. [Google Scholar] [CrossRef] [PubMed]
- Pandey, P.; Marks, M.P.; Harraher, C.D.; Westbroek, E.M.; Chang, S.D.; Do, H.M.; Levy, R.P.; Dodd, R.L.; Steinberg, G.K. Multimodality management of Spetzler-Martin Grade III arteriovenous malformations. J. Neurosurg. 2012, 116, 1279–1288. [Google Scholar] [CrossRef] [PubMed]
- Sebök, M.; Germans, M.R.; van Niftrik, C.H.B.; Kulcsár, Z.; Regli, L.; Fierstra, J. More pronounced hemodynamic alterations in patients with brain arteriovenous malformation–associated epilepsy. Neurosurg. Focus 2022, 53, E4. [Google Scholar] [CrossRef] [PubMed]
- Dicpinigaitis, A.J.; Ogulnick, J.V.; Mayer, S.A.; Gandhi, C.D.; Al-Mufti, F. Increase in Ruptured Cerebral Arteriovenous Malformations and Mortality in the United States: Unintended Consequences of the ARUBA Trial? Stroke Vasc. Int. Neurol. 2022, 3, e000442. [Google Scholar] [CrossRef]
- Capocci, R.; Bustuchina, V.M.; Shotar, E.; Mathon, B.; Delaitre, M.; Premat, K.; Talaat, M.; Talbi, A.; Boch, A.L.; Lenck, S.; et al. Benefits from Exclusion Treatment of Unruptured Brain Arteriovenous Malformations on Epilepsy in Adults. Clin. Neuroradiol. 2022, 32, 749–760. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.J.; Shabo, L.M.; Ding, D.; Ironside, N.; Kano, H.; Mathieu, D.; Kondziolka, D.; Feliciano, C.; Rodriguez-Mercado, R.; Grills, I.S.; et al. Seizure Presentation in Patients with Brain Arteriovenous Malformations Treated with Stereotactic Radiosurgery: A Multicenter Study. World Neurosurg. 2019, 126, e634–e640. [Google Scholar] [CrossRef] [PubMed]
- Galletti, F.; Costa, C.; Cupini, L.M.; Eusebi, P.; Hamam, M.; Caputo, N.; Siliquini, S.; Conti, C.; Moschini, E.; Lunardi, P.; et al. Brain arteriovenous malformations and seizures: An Italian study. J. Neurol. Neurosurg. Psychiatry 2013, 85, 284–288. [Google Scholar] [CrossRef] [PubMed]
- Bokhari, M.R.; Bokhari, S.R.A. Arteriovenous Malformation (AVM) of the Brain. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar] [PubMed]
- Sen, R.D.; Nistal, D.; McGrath, M.; Barros, G.; Shenoy, V.S.; Sekhar, L.N.; Levitt, M.R.; Kim, L.J. De Novo epilepsy after microsurgical resection of brain arteriovenous malformations. Neurosurg. Focus 2022, 53, E6. [Google Scholar] [CrossRef] [PubMed]
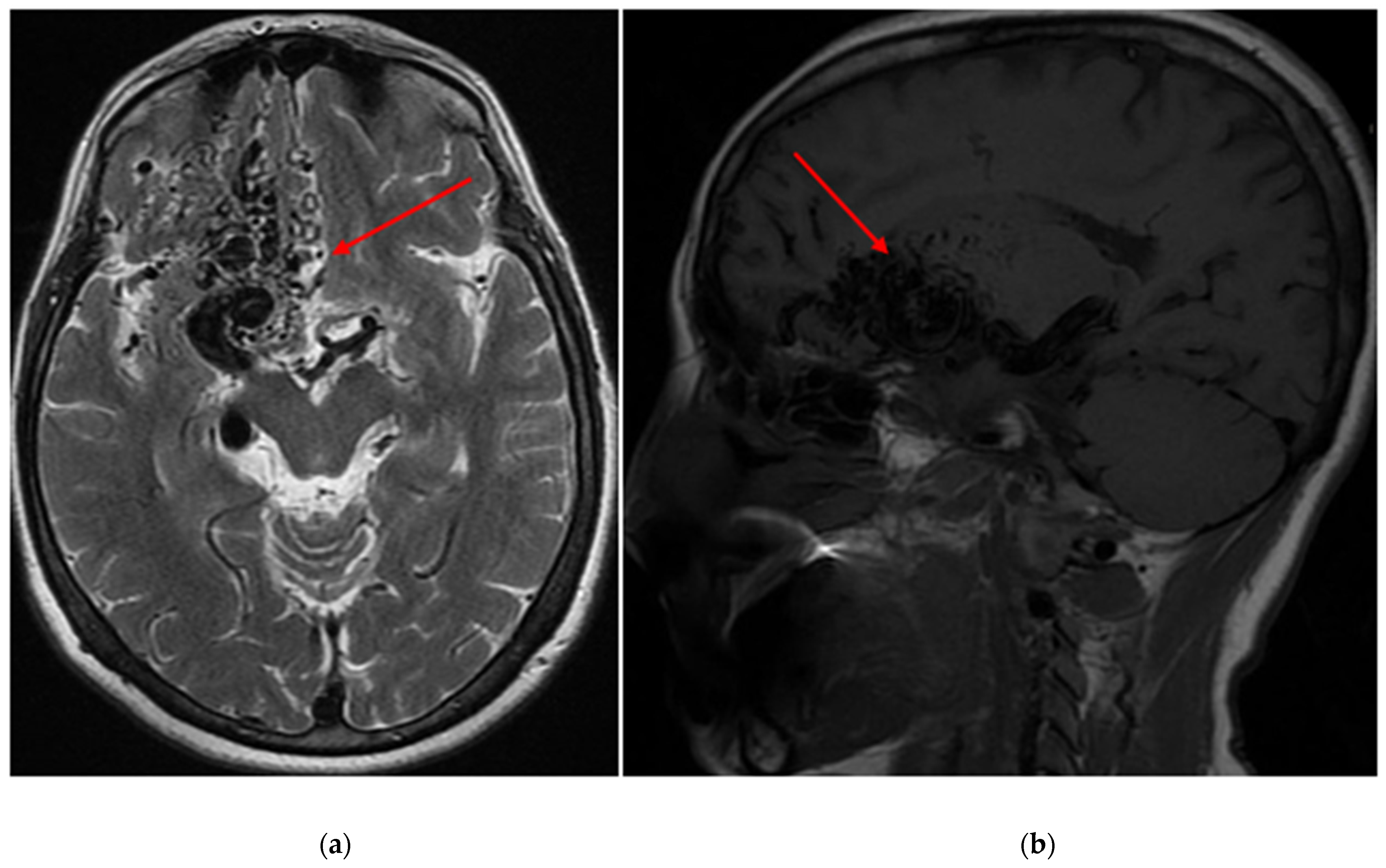
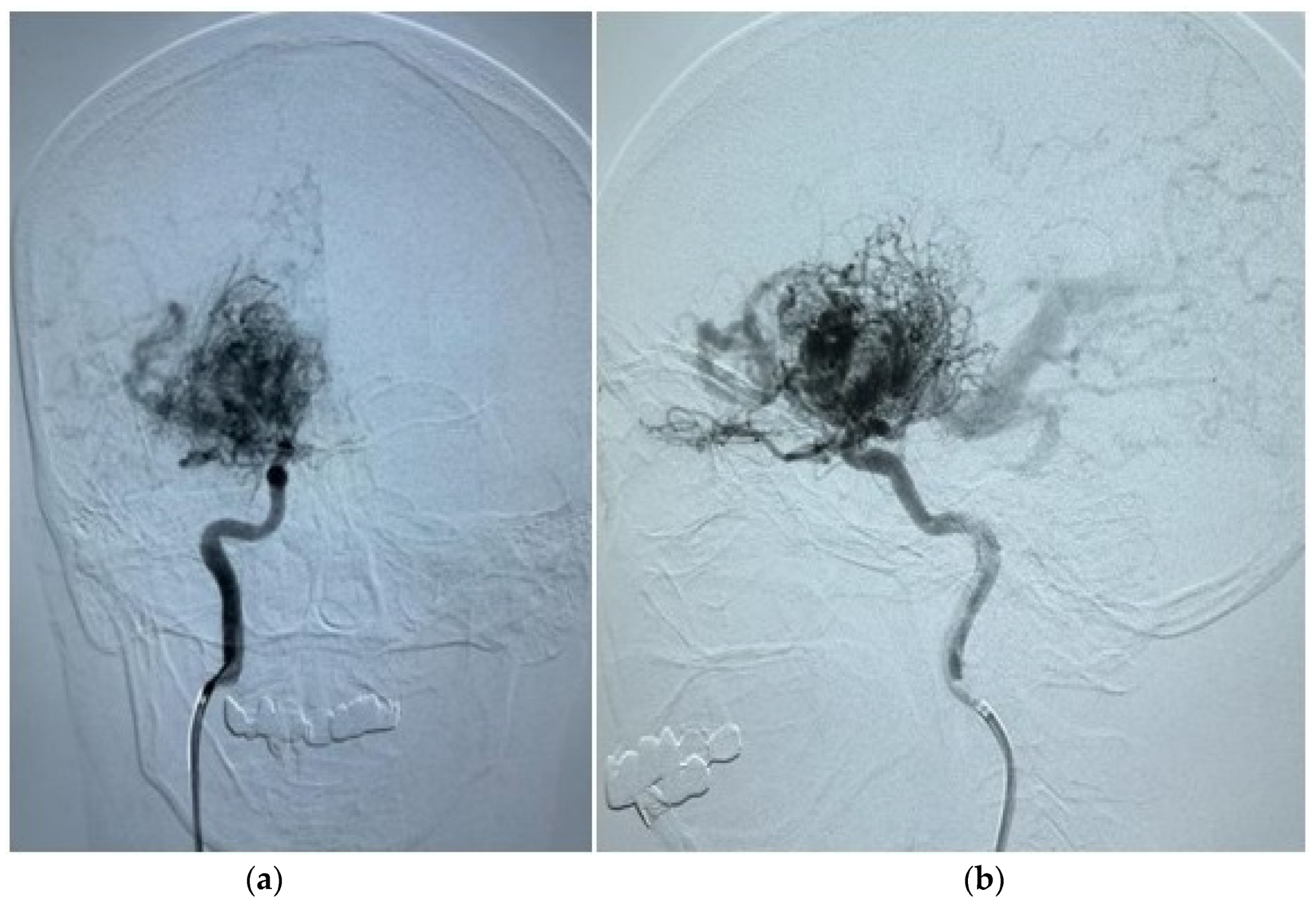
| Variables | Total (n = 163) | Seizures (n = 70) | No Seizures (n = 93) | p-Value | |||
|---|---|---|---|---|---|---|---|
| Age | 39.21 ± 12.75 | 39.91 ± 11.49 | 38.63 ± 13.69 | 0.397 | |||
| Gender | 0.875 | ||||||
| male | 71 | 43.56% | 31 | 44.29% | 40 | 43.01% | |
| female | 92 | 56.44% | 39 | 55.71% | 53 | 56.99% | |
| Height | 167.44 ± 9.11 | 168.03 ± 8.91 | 166.97 ± 9.32 | 0.489 | |||
| Weight | 68.9 ± 14.42 | 69.84 ± 14.23 | 68.15 ± 14.68 | 0.161 | |||
| BMI | 24.68 ± 4.74 | 24.97 ± 4.35 | 24.45 ± 5.05 | 0.248 | |||
| Arterial hypertension | 32 | 19.63% | 11 | 15.71% | 21 | 22.58% | 0.322 |
| Smoke | 44 | 26.99% | 21 | 30% | 23 | 24.73% | 0.480 |
| Intracranial hemorrhage in relatives | 40 | 32.79% | 16 | 32% | 24 | 33.33% | 1.000 |
| Rupture | 68 | 41.98% | 18 | 26.09% | 50 | 53.76% | 0.000 ** |
| AVM relatives | 3 | 2.38% | 0 | 0.00% | 3 | 4.11% | 0.263 |
| Aneurysm | 15 | 9.2% | 6 | 8.57% | 9 | 9.68% | 1.000 |
| Cephalgic syndrome | 17 | 10.49% | 5 | 7.25% | 12 | 12.9% | 0.305 |
| Hemiparesis | 22 | 13.58% | 3 | 4.35% | 19 | 20.43% | 0.004 * |
| Variables | Total (n = 163) | Seizures (n = 70) | No Seizures (n = 93) | p-Value | |||
|---|---|---|---|---|---|---|---|
| Size of AVM | 0.000 *** | ||||||
| 0–3 | 47 | 29.01% | 11 | 15.71% | 36 | 39.13% | |
| 3–6 | 97 | 59.88% | 54 | 77.14% | 43 | 46.74% | |
| 6+ | 18 | 11.11% | 5 | 7.14% | 13 | 14.13% | |
| Location in cortex | 134 | 83.75% | 68 | 97.14% | 66 | 73.33% | 0.000 *** |
| Frontal lobe | 45 | 28.13% | 30 | 42.86% | 15 | 16.67% | 0.000 *** |
| Parietal lobe | 62 | 38.75% | 37 | 52.86% | 25 | 27.78% | 0.002 ** |
| Temporal lobe | 49 | 30.63% | 27 | 38.57% | 22 | 24.44% | 0.060 |
| Occipital lobe | 31 | 19.38% | 7 | 10% | 24 | 26.67% | 0.009 ** |
| Cerebellum | 12 | 7.5% | 1 | 1.43% | 11 | 12.22% | 0.013 * |
| Brain stem | 4 | 2.5% | 0 | 0.0% | 4 | 4.44% | 0.132 |
| Basal ganglia | 24 | 15% | 7 | 10% | 17 | 18.89% | 0.180 |
| Vein drainage | 0.411 | ||||||
| deep | 71 | 47.65% | 27 | 43.55% | 44 | 50.57% | |
| surface | 78 | 52.35% | 35 | 56.45% | 43 | 49.43% | |
| Spetzler-Martin Score | 0.029 * | ||||||
| Low (I–II) | 47 | 29.19% | 13 | 18.57% | 34 | 37.36% | |
| Medium (III) | 55 | 34.16% | 29 | 41.43% | 26 | 28.57% | |
| High (IV–V) | 59 | 36.65% | 28 | 40.00% | 31 | 34.07% | |
| Location | 0.869 | ||||||
| Left | 88 | 58.28% | 40 | 57.14% | 48 | 59.26% | |
| Right | 63 | 41.72% | 30 | 42.86% | 33 | 40.74% | |
| Prior embolization | 143 | 94.08% | 67 | 98.53% | 76 | 90.48% | 0.043 * |
| Prior SRS | 14 | 9.21% | 4 | 5.88% | 10 | 11.90% | 0.264 |
| Prior craniotomy | 12 | 7.89% | 6 | 8.82% | 6 | 7.14% | 0.768 |
| Variable | OR (95% CI) | p-Value |
|---|---|---|
| Ruptured | ||
| yes | 0.36 (0.14–0.91) | 0.032 * |
| no | Reference | |
| Spetzler-Martin score | ||
| Low (I–II) | Reference | |
| Medium (III) | 6.16 (2.14–17.69) | 0.001 *** |
| High (IV–V) | 3.05 (1.08–8.68) | 0.036 * |
| Frontal Lobe | 6.16 (2.04–18.54) | 0.001 *** |
| Parietal Lobe | 9.37 (3.26–26.91) | 0.000 *** |
| Temporal Lobe | 4.57 (1.56–13.36) | 0.005 ** |
| Occipital Lobe | 0.27 (0.08–0.91) | 0.035 * |
| Hemiparesis | ||
| yes | 0.12 (0.02–0.66) | 0.015 * |
| no | Reference | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mukhtarova, K.; Nurimanov, C.; Zholdybayeva, E.; Makhambetov, Y.; Akshulakov, S. Associations of Brain Arteriovenous Malformation-Related Factors with Epileptic Seizure Presentations. Diagnostics 2024, 14, 1077. https://doi.org/10.3390/diagnostics14111077
Mukhtarova K, Nurimanov C, Zholdybayeva E, Makhambetov Y, Akshulakov S. Associations of Brain Arteriovenous Malformation-Related Factors with Epileptic Seizure Presentations. Diagnostics. 2024; 14(11):1077. https://doi.org/10.3390/diagnostics14111077
Chicago/Turabian StyleMukhtarova, Kymbat, Chingiz Nurimanov, Elena Zholdybayeva, Yerbol Makhambetov, and Serik Akshulakov. 2024. "Associations of Brain Arteriovenous Malformation-Related Factors with Epileptic Seizure Presentations" Diagnostics 14, no. 11: 1077. https://doi.org/10.3390/diagnostics14111077
APA StyleMukhtarova, K., Nurimanov, C., Zholdybayeva, E., Makhambetov, Y., & Akshulakov, S. (2024). Associations of Brain Arteriovenous Malformation-Related Factors with Epileptic Seizure Presentations. Diagnostics, 14(11), 1077. https://doi.org/10.3390/diagnostics14111077






