Ultrasound Imaging of Thoracolumbar Fascia Thickness: Chronic Non-Specific Lower Back Pain versus Healthy Subjects; A Sign of a “Frozen Back”?
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Participants
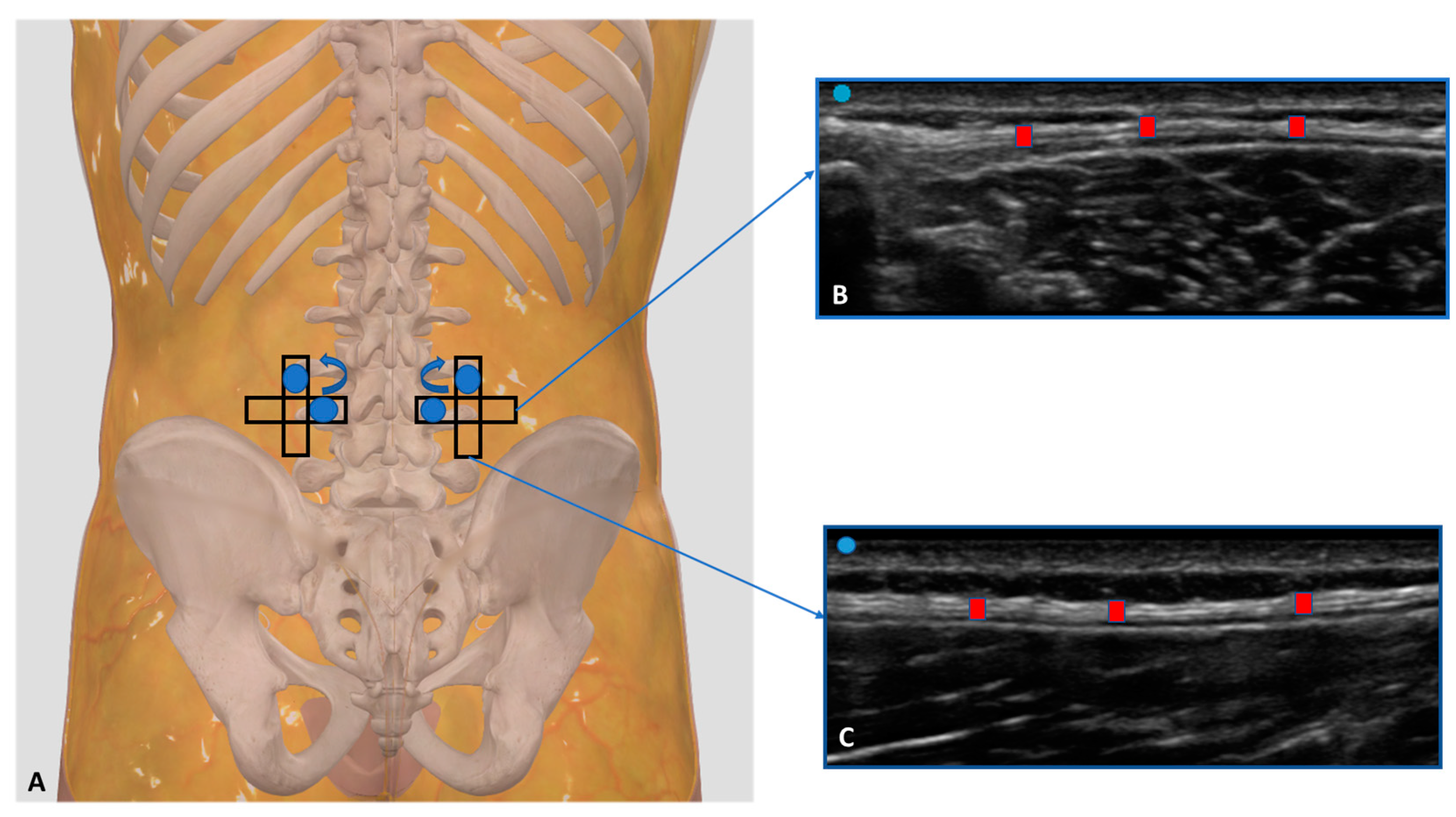
2.3. Ultrasound Examination Measurements
2.4. Statistical Analysis
3. Results
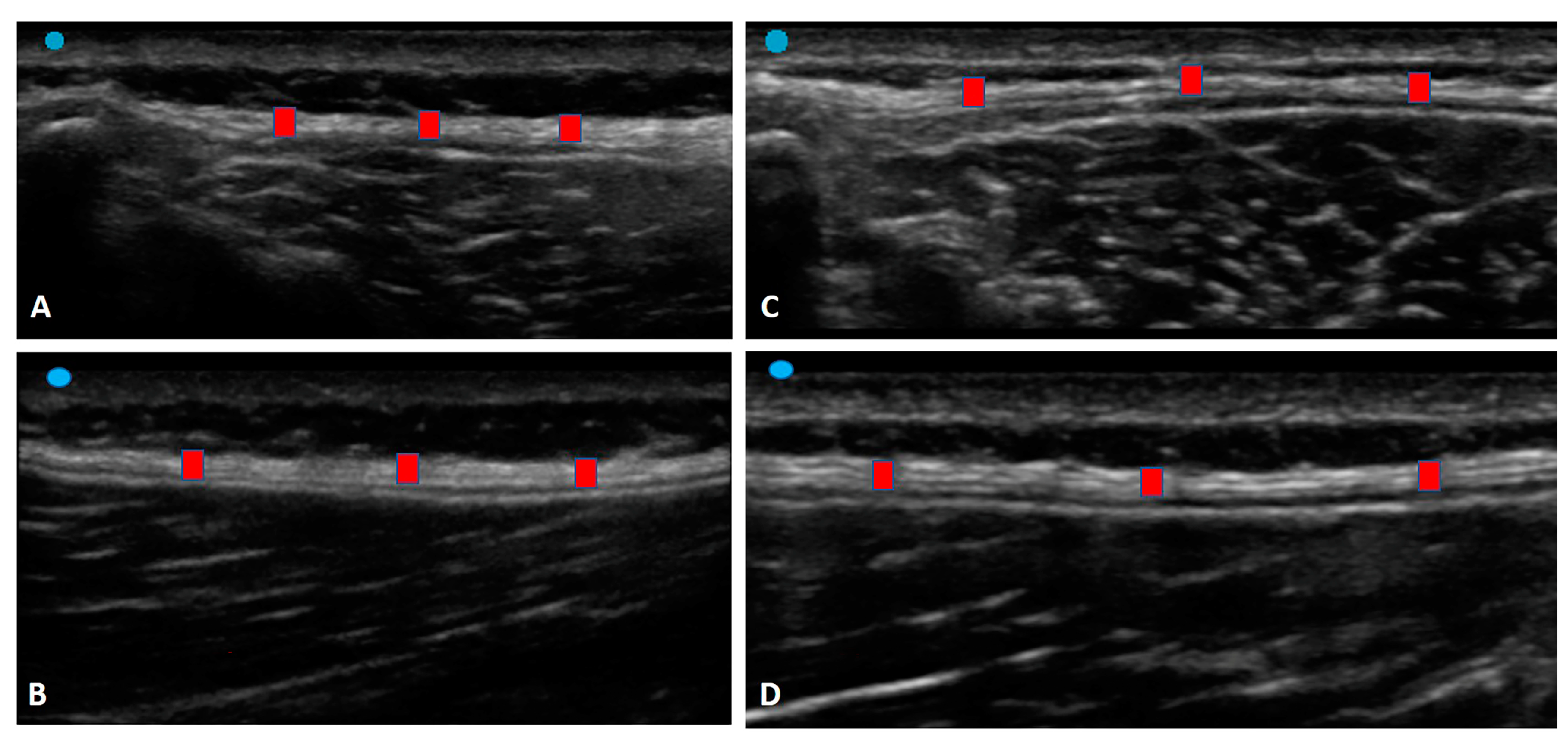
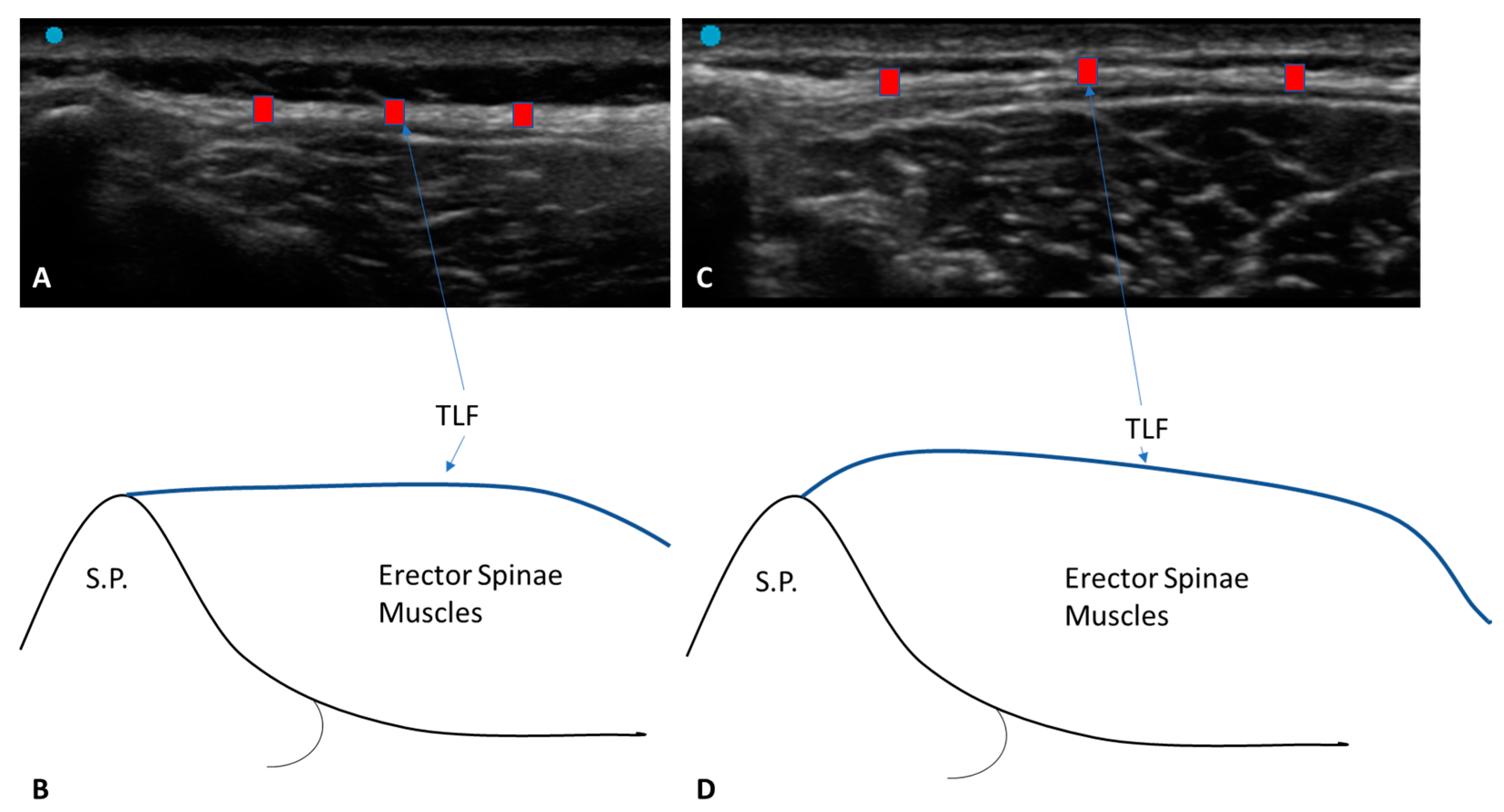
3.1. Ultrasound Measurements of the Thoracolumbar Fascia
3.1.1. Group 1 (Chronic Non-Specific LBP Patients)
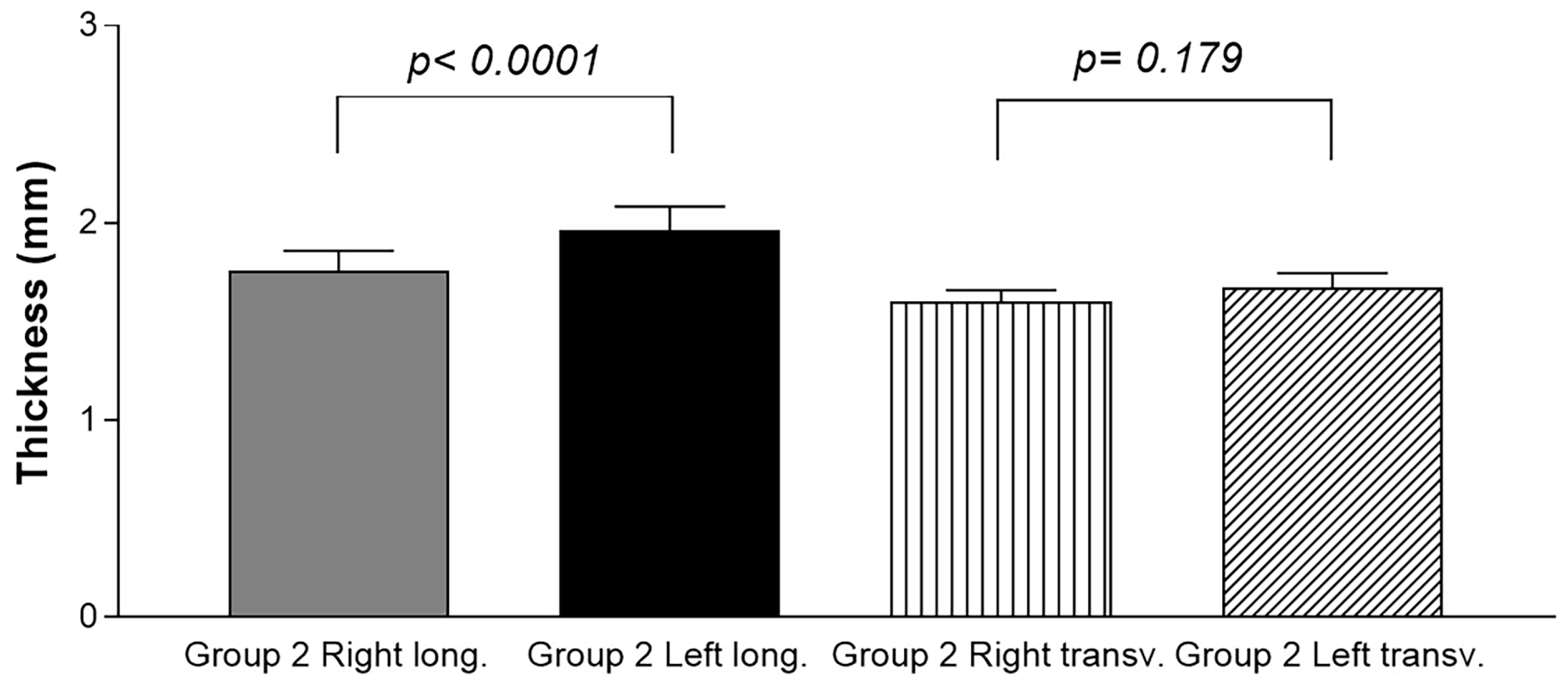
3.1.2. Group 2 (Healthy Volunteers)
3.2. Ultrasound Measurements of the Thoracolumbar Fascia: Comparison between Group 1 and Group 2
3.3. Ultrasound Measurements of the Thoracolumbar Fascia Thickness: Comparison between Group 1 and Group 2 for Both the Longitudinal Axis and the Transverse Axis
3.4. Correlation Ultrasound Measurements and Descriptive Data
3.5. Intra-Rater Reliability
4. Discussion
Limitation of Study
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Knezevic, N.N.; Candido, K.D.; Vlaeyen, J.W.S.; Van Zundert, J.; Cohen, S.P. Low back pain. Lancet 2021, 398, 78–92. [Google Scholar] [CrossRef] [PubMed]
- GBD 2017 Disease and Injury Incidence and Prevalence Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2018, 392, 1789–1858. [Google Scholar] [CrossRef] [PubMed]
- Loney, P.L.; Stratford, P.W. The prevalence of low back pain in adults: A methodological review of the literature. Phys. Ther. 1999, 79, 384–396. [Google Scholar] [CrossRef] [PubMed]
- Minghelli, B. Musculoskeletal spine pain in adolescents: Epidemiology of non-specific neck and low back pain and risk factors. J. Orthop. Sci. 2020, 25, 776–780. [Google Scholar] [CrossRef] [PubMed]
- Andersson, G.B. Epidemiological features of chronic low-back pain. Lancet 1999, 354, 581–585. [Google Scholar] [CrossRef] [PubMed]
- Will, J.S.; Bury, D.C.; Miller, J.A. Mechanical low back pain. Am. Fam. Physician 2018, 98, 421–428. [Google Scholar]
- Herlin, C.; Kjaer, P.; Espeland, A.; Skouen, J.S.; Leboeuf-Yde, C.; Karppinen, J.; Niinimäki, J.; Sørensen, J.S.; Storheim, K.; Jensen, T.S. Modic changes their associations with low back pain and activity limitation: A systematic literature review and meta-analysis. PLoS ONE 2018, 13, e0200677. [Google Scholar] [CrossRef]
- Manchikanti, L.; Benyamin, R.M.; Singh, V.; Falco, F.J.; Hameed, H.; Derby, R.; Wolfer, L.R.; Helm, S.; Calodney, A.K.; Datta, S.; et al. An update of the systematic appraisal of the accuracy and utility of lumbar discography in chronic low back pain. Pain Phys. 2013, 16 (Suppl. S2), SE55-95. [Google Scholar] [CrossRef]
- Simopoulos, T.; Manchikanti, L.; Singh, V.; Gupta, S.; Hameed, H.; Diwan, S.; Cohen, S.P. A systematic evaluation of prevalence and diagnostic accuracy of sacroiliac joint interventions. Pain Phys. 2012, 15, E305–E344. [Google Scholar] [CrossRef]
- Vining, R.D.; Shannon, Z.K.; Minkalis, A.L.; Twist, E.J. Current evidence for diagnosis of common conditions causing low back pain: Systematic review and standardized terminology recommendations. J. Manip. Physiol. Ther. 2019, 42, 651–664. [Google Scholar] [CrossRef]
- Nijs, J.; Apeldoorn, A.; Hallegraeff, H.; Clark, J.; Smeets, R.; Malfliet, A.; Girbes, E.L.; De Kooning, M.; Ickmans, K. Low back pain: Guidelines for the clinical classification of predominant neuropathic, nociceptive, or central sensitization pain. Pain Phys. 2015, 18, E333–E346. [Google Scholar] [CrossRef]
- Hodges, P.W.; Danneels, L. Changes in structure and function of the back muscles in low back pain: Different time points, observations, and mechanisms. J. Orthop. Sports Phys. Ther. 2019, 49, 464–476. [Google Scholar] [CrossRef] [PubMed]
- Langevin, H.M.; Sherman, K.J. Pathophysiological model for chronic low back pain integrating connective tissue and nervous system mechanisms. Med. Hypotheses 2007, 68, 74–80. [Google Scholar] [CrossRef]
- Langevin, H.M.; Stevens-Tuttle, D.; Fox, J.R.; Badger, G.J.; Bouffard, N.A.; Krag, M.H.; Wu, J.; Henry, S.M. Ultrasound evidence of altered lumbar connective tissue structure in human subjects with chronic low back pain. BMC Musculoskelet. Disord. 2009, 10, 151. [Google Scholar] [CrossRef] [PubMed]
- Langevin, H.M.; Fox, J.R.; Koptiuch, C.; Badger, G.J.; GreenanNaumann, A.C.; Bouffard, N.A.; Konofagou, E.E.; Lee, W.-N.; Triano, J.J.; Henry, S.M. Reduced thoracolumbar fascia shear strain in human chronic low back pain. BMC Musculoskelet. Disord. 2011, 12, 203. [Google Scholar] [CrossRef] [PubMed]
- Schilder, A.; Hoheisel, U.; Magerl, W.; Benrath, J.; Klein, T.; Treede, R.D. Sensory findings after stimulation of the thoracolumbar fascia with hypertonic saline suggest its contribution to low back pain. Pain 2014, 155, 222–231. [Google Scholar] [CrossRef] [PubMed]
- Suarez-Rodriguez, V.; Fede, C.; Pirri, C.; Petrelli, L.; Loro-Ferrer, J.F.; Rodriguez-Ruiz, D.; De Caro, R.; Stecco, C. Fascial Innervation: A Systematic Review of the Literature. Int. J. Mol. Sci. 2022, 23, 5674. [Google Scholar] [CrossRef] [PubMed]
- Mense, S. Innervation of the thoracolumbar fascia. Eur. J. Transl. Myol. 2019, 29, 8297. [Google Scholar] [CrossRef]
- Willard, F.H.; Vleeming, A.; Schuenke, M.D.; Danneels, L.; Schleip, R. The thoracolumbar fascia: Anatomy, function and clinical considerations. J. Anat. 2012, 221, 507–536. [Google Scholar] [CrossRef]
- Casato, G.; Stecco, C.; Busin, R. Role of fasciae in nonspecific low back pain. Eur. J. Transl. Myol. 2019, 29, 8330. [Google Scholar] [CrossRef]
- Pirri, C.; Stecco, C.; Fede, C.; Macchi, V.; Özçakar, L. Ultrasound Imaging of the Fascial Layers: You See (Only) What You Know. J. Ultrasound Med. 2020, 39, 827–828. [Google Scholar] [CrossRef]
- Pirri, C.; Ricci, V.; Stecco, C.; Özçakar, L. Clinical and Ultrasound Examination of the Thoracolumbar Fascia: The Hands and the Probe Together. Am. J. Phys. Med. Rehabil. 2021, 100, e157–e158. [Google Scholar] [CrossRef] [PubMed]
- Almazán-Polo, J.; López-López, D.; Romero-Morales, C.; Rodríguez-Sanz, D.; Becerro-de-Bengoa-Vallejo, R.; Losa-Iglesias, M.E.; Bravo-Aguilar, M.; Calvo-Lobo, C. Quantitative Ultrasound Imaging Differences in Multifidus and Thoracolumbar Fasciae between Athletes with and without Chronic Lumbopelvic Pain: A Case-Control Study. J. Clin. Med. 2020, 9, 2647. [Google Scholar] [CrossRef] [PubMed]
- Von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gøtzsche, P.C.; Vandenbroucke, J.P. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: Guidelines for reporting observational studies. J. Clin. Epidemiol. 2008, 61, 344–349. [Google Scholar] [CrossRef] [PubMed]
- World Medical Association declaration of Helsinki: Ethical principles for medical research involving human subjects. JAMA 2013, 310, 2191–2194. [CrossRef]
- Schnapp, W.; Martiatu, K.; Delcroix, G.J. Basivertebral nerve ablation for the treatment of chronic low back pain in a community practice setting: 6 Months follow-up. N. Am. Spine Soc. J. 2023, 14, 100201. [Google Scholar] [CrossRef]
- Bi, M.; Ding, W.; Zheng, M.; Peng, Z.; Li, J.; Ding, S. Arthroscopic Superior Capsule Reconstruction with Combined Fascia Lata Autograft and Synthetic Scaffold Patch Graft for the Treatment of Irreparable Rotator Cuff Tears Yields Favorable Clinical and Radiographic Outcomes at Minimum Two-Year Follow-Up. Arthroscopy 2023. ahead of print. [Google Scholar] [CrossRef]
- Pirri, C.; Pirri, N.; Porzionato, A.; Boscolo-Berto, R.; De Caro, R.; Stecco, C. Inter- and Intra-Rater Reliability of Ultrasound Measurements of Superficial and Deep Fasciae Thickness in Upper Limb. Diagnostics 2022, 12, 2195. [Google Scholar] [CrossRef]
- Cohen, J. Things I have learned (so far). Am. Psychol. 1990, 45, 1304–1312. [Google Scholar] [CrossRef]
- Koo, T.K.; Li, M.Y. A Guideline of Selecting and Reporting Intraclass Correlation Coefficients for Reliability Research. J. Chiropr. Med. 2016, 15, 155–163. [Google Scholar] [CrossRef]
- Brandl, A.; Egner, C.; Reer, R.; Schmidt, T.; Schleip, R. Associations between Deformation of the Thoracolumbar Fascia and Activation of the Erector Spinae and Multifidus Muscle in Patients with Acute Low Back Pain and Healthy Controls: A Matched Pair Case-Control Study. Life 2022, 12, 1735. [Google Scholar] [CrossRef] [PubMed]
- Tamartash, H.; Bahrpeyma, F.; Mokhtari Dizaji, M. Ultrasound evidence of altered lumbar fascia in patients with low back pain. Clin. Anat. 2023, 36, 36–41. [Google Scholar] [CrossRef] [PubMed]
- Wilke, J.; Schleip, R.; Klingler, W.; Stecco, C. The lumbodorsal fascia as a potential source of low back pain: A narrative review. BioMed Res. Int. 2017, 2017, 5349620. [Google Scholar] [CrossRef]
- Bonaldi, L.; Berardo, A.; Pirri, C.; Stecco, C.; Carniel, E.L.; Fontanella, C.G. Mechanical Characterization of Human Fascia Lata: Uniaxial Tensile Tests from Fresh-Frozen Cadaver Samples and Constitutive Modelling. Bioengineering 2023, 10, 226. [Google Scholar] [CrossRef] [PubMed]
- Pavan, P.G.; Pachera, P.; Stecco, C.; Natali, A.N. Biomechanical behavior of human crural fascia in anterior and posterior regions of the lower limb. Med. Biol. Eng. Comput. 2015, 53, 951–959. [Google Scholar] [CrossRef]
- Huijing, P.A. Muscle as a collagen fiber reinforced composite: A review of force transmission in muscle and whole limb. J. Biomech. 1999, 32, 329–345. [Google Scholar] [CrossRef]
- Sinha, U.; Malis, V.; Chen, J.S.; Csapo, R.; Kinugasa, R.; Narici, M.V.; Sinha, S. Role of the Extracellular Matrix in Loss of Muscle Force With Age and Unloading Using Magnetic Resonance Imaging, Biochemical Analysis, and Computational Models. Front Physiol. 2020, 11, 626. [Google Scholar] [CrossRef]
- Schleip, R.; Vleeming, A.; Lehmann-Horn, F.; Klingler, W. Letter to the Editor concerning “A hypothesis of chronic back pain: Ligament subfailure injuries lead to muscle control dysfunction” (M. Panjabi). Eur. Spine J. 2007, 16, 1733–1735. [Google Scholar] [CrossRef]

| Data | Group 1 | Group 2 | p-Value Group 1 vs. Group 2 |
|---|---|---|---|
| Age, y Weight, kg Height, cm BMI, Kg/cm2 | 28.96 ± 10.54 66.22 ± 6.1 168.3 ± 4.83 23.37 ± 5.22 | 27.09 ± 12.38 70.60 ± 12.20 171.30 ± 6.76 24.03 ± 6.1 | p = 0.14 p = 0.45 p = 0.55 p = 0.6 |
| Descriptive Statistics | Right (long.) | Right (trans.) | Left (long.) | Left (trans.) |
|---|---|---|---|---|
| Number of values | 46 | 46 | 46 | 46 |
| Minimum | 0.9 | 1.2 | 1.1 | 1.33 |
| Maximum | 5.32 | 4 | 3.9 | 3.8 |
| Mean | 2.088 | 1.948 | 2.268 | 2.129 |
| Std. deviation | 0.7704 | 0.6273 | 0.631 | 0.6188 |
| Std. error of the mean | 0.1136 | 0.09249 | 0.09304 | 0.09124 |
| Coefficient of variation | 36.89% | 32.2% | 27.82% | 29.06% |
| Descriptive Statistics | Right (long.) | Right (transv.) | Left (long.) | Left (transv.) |
|---|---|---|---|---|
| Number of values | 46 | 46 | 46 | 46 |
| Minimum | 1.01 | 1.1 | 1.1 | 1.1 |
| Maximum | 3.9 | 2.52 | 4.6 | 2.84 |
| Mean | 1.75 | 1.6 | 1.96 | 1.7 |
| Std. deviation | 0.71 | 0.4 | 0.81 | 0.51 |
| Std. error of the mean | 0.11 | 0.1 | 0.12 | 0.1 |
| Coefficient of variation | 40.43% | 24.27% | 41.28% | 30.28% |
| Type of Comparison | Mean Diff. | t | p-Value |
|---|---|---|---|
| Group 1 Right (long.) vs. Group 2 Right (long.) Group 1 Right (transv.) vs. Group 2 Right (transv.) Group 1 Left (long.) vs. Group 2 Left (long.) Group 1 Left (transv.) vs. Group 2 Left (transv.) | 0.3333 0.3450 0.3059 0.4587 | 2.019 3.085 2.117 3.887 | p < 0.05 p = 0.03 p = 0.03 p = 0.0003 |
| Type of Comparison | Mean Diff. | t | p-Value |
|---|---|---|---|
| Group 1 Left (long.) vs. Group 1 Left (transv.) Group 1 Right (long.) vs. Group 1 Right (transv.) Group 2 Left (long.) vs. Group 2 Left (transv.) Group 2 Right (long.) vs. Group 2 Right (transv.) | −0.1391 −0.1497 0.2920 0.1524 | 1.922 1.941 3.306 2.347 | p = 0.06 p = 0.08 p = 0.001 p = 0.02 |
| Type of Axis | ICC |
|---|---|
| Group 1 Left (long.) Group 1 Left (transv.) Group 1 Right (long.) Group 1 Right (transv.) Group 2 Left (long.) Group 2 Left (transv.) Group 2 Right (long.) Group 2 Right (transv.) | 0.91 (0.88–0.94) 0.88 (0.85–0.90) 0.92 (0.88–0.96) 0.88 (0.85–0.90) 0.92 (0.88–0.96) 0.88 (0.85–0.90) 0.92 (0.88–0.96) 0.88 (0.85–0.90) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pirri, C.; Pirri, N.; Guidolin, D.; Macchi, V.; Porzionato, A.; De Caro, R.; Stecco, C. Ultrasound Imaging of Thoracolumbar Fascia Thickness: Chronic Non-Specific Lower Back Pain versus Healthy Subjects; A Sign of a “Frozen Back”? Diagnostics 2023, 13, 1436. https://doi.org/10.3390/diagnostics13081436
Pirri C, Pirri N, Guidolin D, Macchi V, Porzionato A, De Caro R, Stecco C. Ultrasound Imaging of Thoracolumbar Fascia Thickness: Chronic Non-Specific Lower Back Pain versus Healthy Subjects; A Sign of a “Frozen Back”? Diagnostics. 2023; 13(8):1436. https://doi.org/10.3390/diagnostics13081436
Chicago/Turabian StylePirri, Carmelo, Nina Pirri, Diego Guidolin, Veronica Macchi, Andrea Porzionato, Raffaele De Caro, and Carla Stecco. 2023. "Ultrasound Imaging of Thoracolumbar Fascia Thickness: Chronic Non-Specific Lower Back Pain versus Healthy Subjects; A Sign of a “Frozen Back”?" Diagnostics 13, no. 8: 1436. https://doi.org/10.3390/diagnostics13081436
APA StylePirri, C., Pirri, N., Guidolin, D., Macchi, V., Porzionato, A., De Caro, R., & Stecco, C. (2023). Ultrasound Imaging of Thoracolumbar Fascia Thickness: Chronic Non-Specific Lower Back Pain versus Healthy Subjects; A Sign of a “Frozen Back”? Diagnostics, 13(8), 1436. https://doi.org/10.3390/diagnostics13081436












