Demystifying the Role of Prognostic Biomarkers in Breast Cancer through Integrated Transcriptome and Pathway Enrichment Analyses
Abstract
1. Introduction
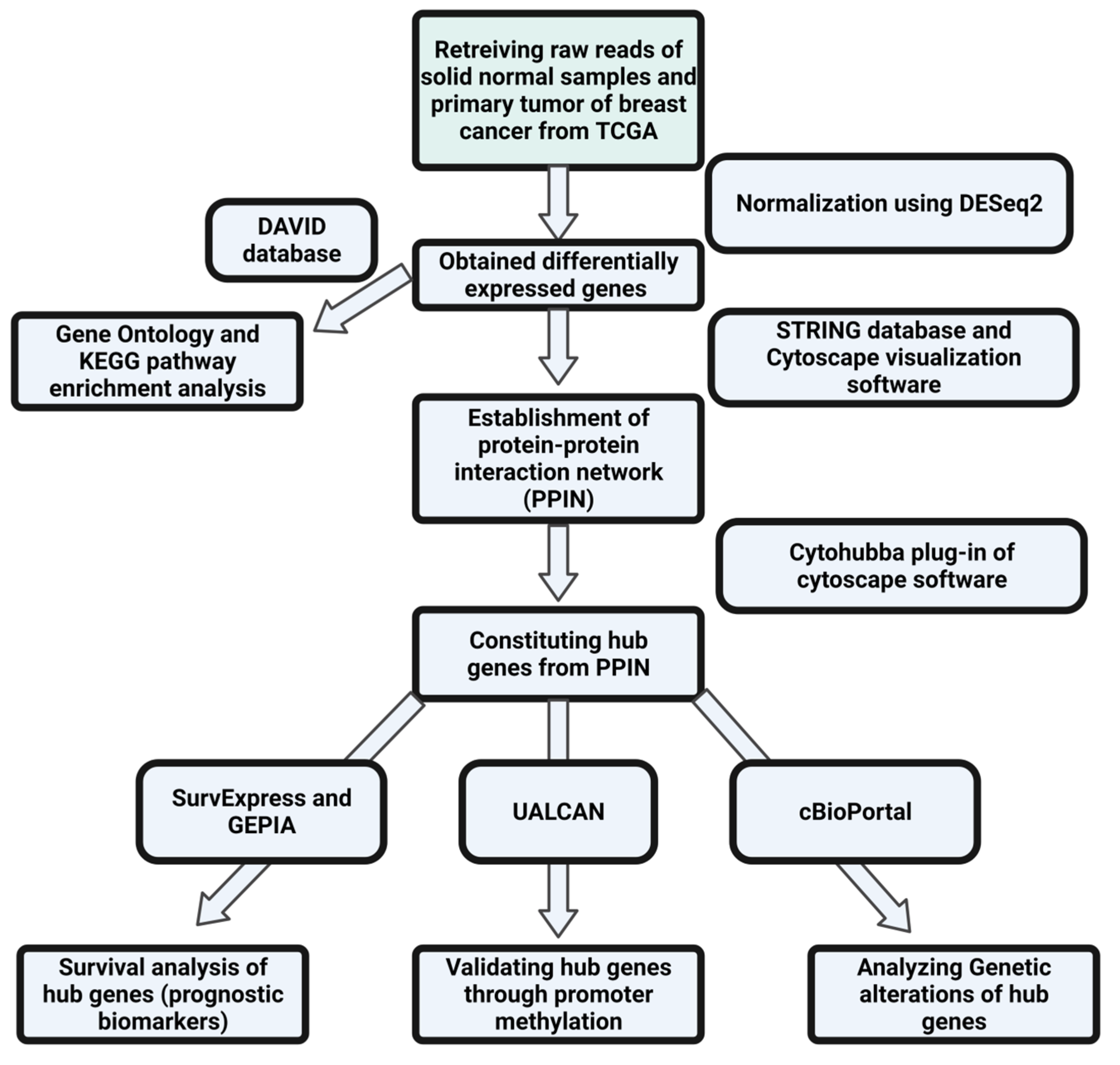
2. Materials and Methods
2.1. Fetching and Preprocessing of Data and Determination of Differentially Expressed Genes through DESeq2 Analysis
2.2. Investigating the Protein–Protein Interaction Network (PPIN) to Establish the Hub Genes as Potential Prognostic Biomarkers
2.3. Analyzing the Gene Ontology (GO) Components and Enriched Pathways Involved in the Progression of Breast Cancer
2.4. Exploring the Epigenetic Regulation of Hub Genes through Promoter Methylation
2.5. Identifying the Genetic Alterations of Hub Genes
2.6. Validating the Differential Expression Pattern and Survival Analysis of Hub Genes
3. Results
3.1. Determination of Differentially Expressed Genes through Statistical Analysis
3.2. Investigation of the Protein–Protein Interaction Network (PPIN) Established the Hub Genes as Potential Prognostic Biomarkers
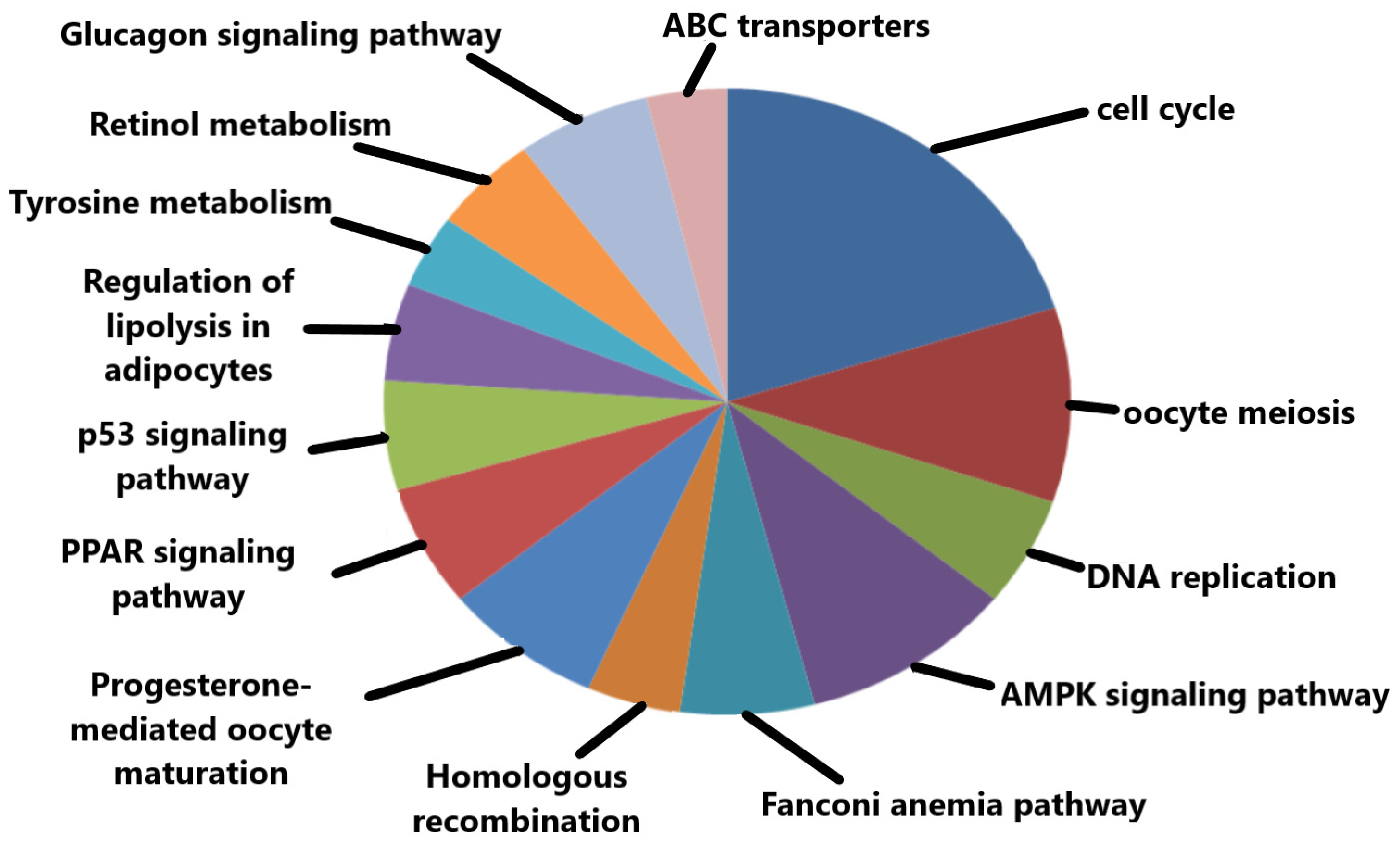
3.3. Gene Oncology (GO) Component and KEGG Pathway Enrichment Analysis
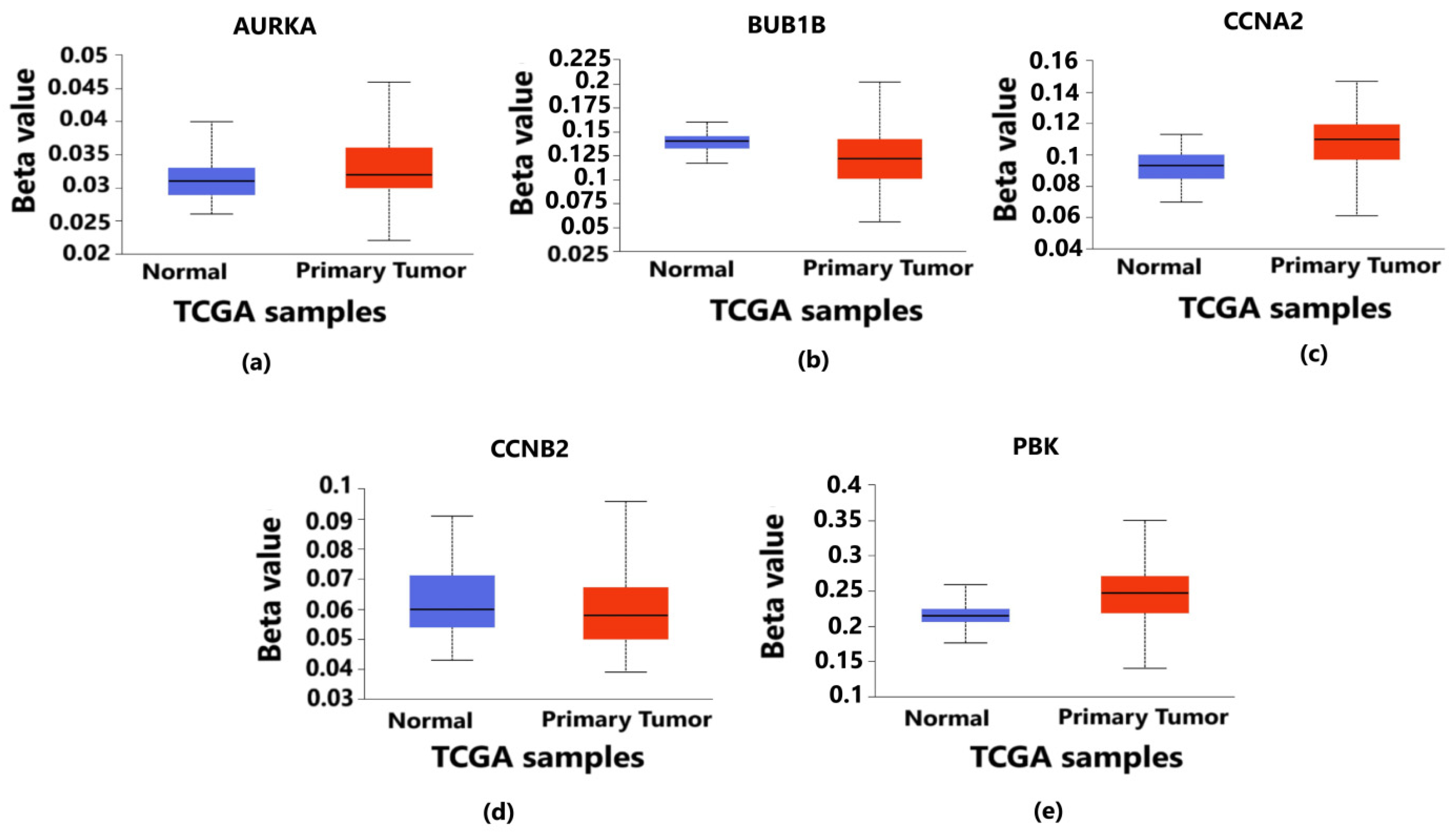
3.4. Exploring the Epigenetic Regulation of Hub Genes through Promoter Methylation
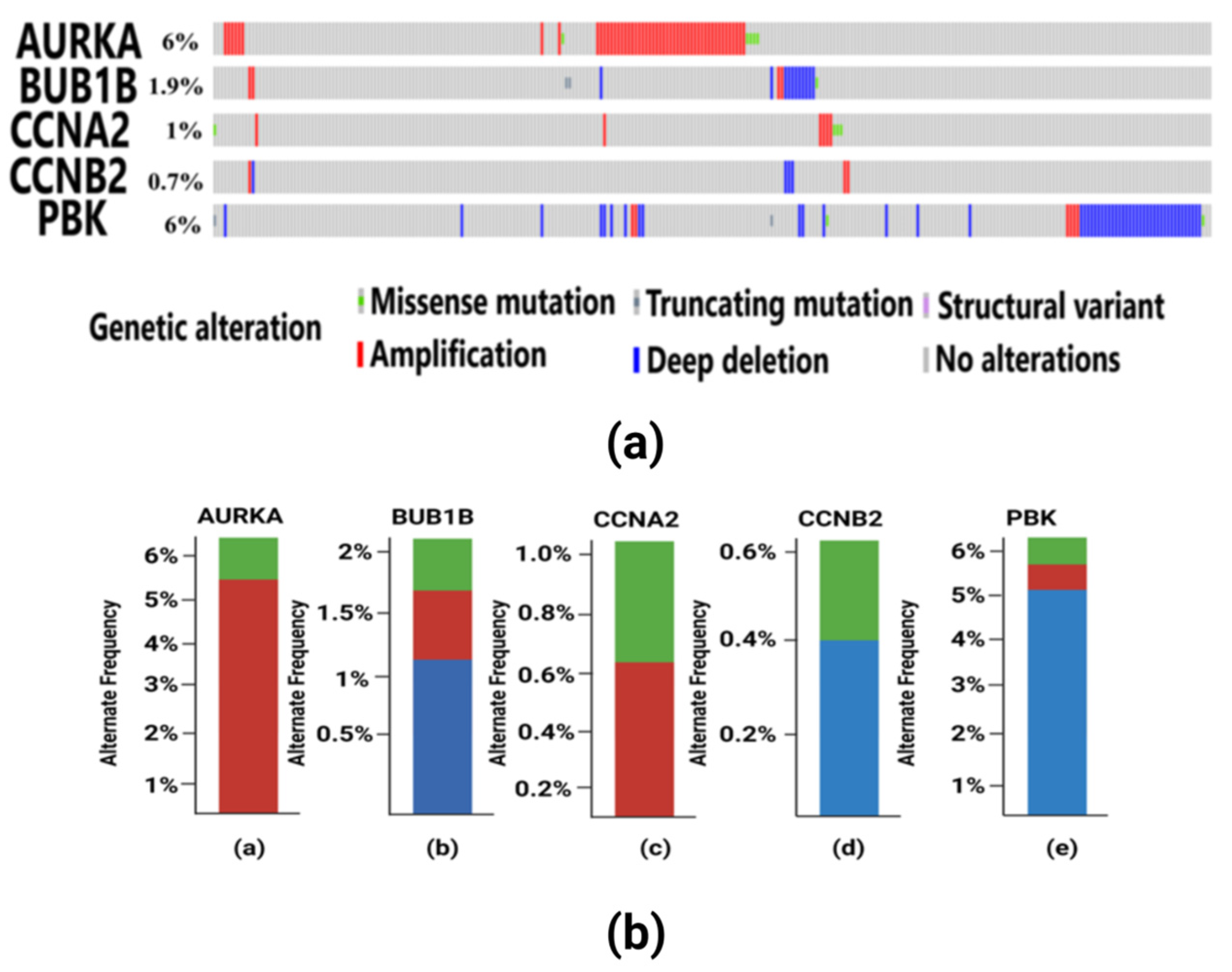
3.5. Findings of Genetic Alterations in Hub Genes
3.6. Survival Analysis Validation of Prognostic Biomarkers
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zeng, X.; Shi, G.; He, Q.; Zhu, P. Screening and Predicted Value of Potential Biomarkers for Breast Cancer Using Bioinformatics Analysis. Sci. Rep. 2021, 11, 20799. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Breast Cancer; World Health Organization: Geneva, Switzerland, 2020. [Google Scholar]
- Pan, Y.; Liu, G.; Yuan, Y.; Zhao, J.; Yang, Y.; Li, Y. Analysis of Differential Gene Expression Profile Identifies Novel Biomarkers for Breast Cancer. Oncotarget 2017, 8, 114613. [Google Scholar] [CrossRef] [PubMed]
- Deng, J.-L.; Xu, Y.-H.; Wang, G. Identification of Potential Crucial Genes and Key Pathways in Breast Cancer Using Bioinformatic Analysis. Front. Genet. 2019, 10, 695. [Google Scholar] [CrossRef] [PubMed]
- Kim, G.-E.; Kim, N.I.; Lee, J.S.; Park, M.H.; Kang, K. Differentially Expressed Genes in Matched Normal, Cancer, and Lymph Node Metastases Predict Clinical Outcomes in Patients with Breast Cancer. Appl. Immunohistochem. Mol. Morphol. 2020, 28, 111. [Google Scholar] [CrossRef] [PubMed]
- Ellsworth, R.E.; Field, L.A.; Love, B.; Kane, J.L.; Hooke, J.A.; Shriver, C.D. Differential Gene Expression in Primary Breast Tumors Associated with Lymph Node Metastasis. Int. J. Breast Cancer 2011, 2011, 142763. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Yan, H.; Yan, X.; Chen, Z.; Zhuo, R. A Novel Prognostic Four-Gene Signature of Breast Cancer Identified by Integrated Bioinformatics Analysis. Dis. Markers. 2022, 2022, 5925982. [Google Scholar] [CrossRef] [PubMed]
- Jin, H.; Huang, X.; Shao, K.; Li, G.; Wang, J.; Yang, H.; Hou, Y. Integrated Bioinformatics Analysis to Identify 15 Hub Genes in Breast Cancer. Oncol. Lett. 2019, 18, 1023–1034. [Google Scholar] [CrossRef]
- Ren, Y.; Deng, R.; Zhang, Q.; Li, J.; Han, B.; Ye, P. Bioinformatics Analysis of Key Genes in Triple Negative Breast Cancer and Validation of Oncogene PLK1. Ann. Transl. Med. 2020, 8, 1637. [Google Scholar] [CrossRef]
- Dobin, A.; Davis, C.A.; Schlesinger, F.; Drenkow, J.; Zaleski, C.; Jha, S.; Batut, P.; Chaisson, M.; Gingeras, T.R. STAR: Ultrafast Universal RNA-seq Aligner. Bioinformatics 2013, 29, 15–21. [Google Scholar] [CrossRef]
- Liao, Y.; Smyth, G.K.; Shi, W. FeatureCounts: An Efficient General Purpose Program for Assigning Sequence Reads to Genomic Features. Bioinformatics 2014, 30, 923–930. [Google Scholar] [CrossRef]
- Love, M.I.; Huber, W.; Anders, S. Moderated Estimation of Fold Change and Dispersion for RNA-seq Data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef] [PubMed]
- Szklarczyk, D.; Gable, A.L.; Nastou, K.C.; Lyon, D.; Kirsch, R.; Pyysalo, S.; Doncheva, N.T.; Legeay, M.; Fang, T.; Bork, P.; et al. The STRING Database in 2021: Customizable Protein-Protein Networks, and Functional Characterization of User-Uploaded Gene/Measurement Sets. Nucleic Acids Res. 2021, 49, D605–D612. [Google Scholar] [CrossRef] [PubMed]
- Shannon, P.; Markiel, A.; Ozier, O.; Baliga, N.S.; Wang, J.T.; Ramage, D.; Amin, N.; Schwikowski, B.; Ideker, T. Cytoscape: A Software Environment for Integrated Models of Biomolecular Interaction Networks. Genome Res. 2003, 13, 2498–2504. [Google Scholar] [CrossRef] [PubMed]
- Bardou, P.; Mariette, J.; Escudié, F.; Djemiel, C.; Klopp, C. Jvenn: An Interactive Venn Diagram Viewer. BMC Bioinform. 2014, 15, 293. [Google Scholar] [CrossRef]
- Dennis, G.; Sherman, B.T.; Hosack, D.A.; Yang, J.; Gao, W.; Lane, H.C.; Lempicki, R.A. DAVID: Database for Annotation, Visualization, and Integrated Discovery. Genome Biol. 2003, 4, R60. [Google Scholar] [CrossRef]
- Supek, F.; Bošnjak, M.; Škunca, N.; Smuc, T. REVIGO Summarizes and Visualizes Long Lists of Gene Ontology Terms. PLoS ONE 2011, 6, e21800. [Google Scholar] [CrossRef]
- Chandrashekar, D.S.; Bashel, B.; Balasubramanya, S.A.H.; Creighton, C.J.; Ponce-Rodriguez, I.; Chakravarthi, B.V.; Varambally, S. UALCAN: A Portal for Facilitating Tumor Subgroup Gene Expression and Survival Analyses. Neoplasia 2017, 19, 649–658. [Google Scholar] [CrossRef]
- Gao, J.; Aksoy, B.A.; Dogrusoz, U.; Dresdner, G.; Gross, B.; Sumer, S.O.; Sun, Y.; Jacobsen, A.; Sinha, R.; Larsson, E.; et al. Integrative analysis of complex cancer genomics and clinical profiles using the cBioPortal. Sci. Signal. 2013, 6, 269. [Google Scholar] [CrossRef] [PubMed]
- Tang, Z.; Li, C.; Kang, B.; Gao, G.; Li, C.; Zhang, Z. GEPIA: A web server for cancer and normal gene expression profiling and interactive analyses. Nucleic Acids Res. 2017, 45, W98–W102. [Google Scholar] [CrossRef]
- Aguirre-Gamboa, R.; Gomez-Rueda, H.; Martínez-Ledesma, E.; Martínez-Torteya, A.; Chacolla-Huaringa, R.; Rodriguez-Barrientos, A.; Tamez-Pena, J.G.; Treviño, V. SurvExpress: An Online Biomarker Validation Tool and Database for Cancer Gene Expression Data Using Survival Analysis. PLoS ONE 2013, 8, e74250. [Google Scholar] [CrossRef] [PubMed]
- Vishnubalaji, R.; Nair, V.S.; Ouararhni, K.; Elkord, E.; Alajez, N.M. Integrated Transcriptome and Pathway Analyses Revealed Multiple Activated Pathways in Breast Cancer. Front. Oncol. 2019, 9, 910. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.K.; Chng, W.J.; Jha, S. Prognostic Biomarkers for Breast Cancer Metastasis. In Cancer Metastasis; IntechOpen: London, UK, 2018. [Google Scholar] [CrossRef]
- Hoffmann, M.J.; Schulz, W. Causes and Consequences of DNA Hypomethylation in Human Cancer. Biochem. Cell Biol. 2005, 83, 296–321. [Google Scholar] [CrossRef]
- Herceg, Z.; Hainaut, P. Genetic and Epigenetic Alterations as Biomarkers for Cancer Detection, Diagnosis and Prognosis. Mol. Oncol. 2007, 1, 26–41. [Google Scholar] [CrossRef] [PubMed]
- Bakhoum, S.F.; Cantley, L.C. The Multifaceted Role of Chromosomal Instability in Cancer and Its Microenvironment. Cell 2018, 174, 1347–1360. [Google Scholar] [CrossRef] [PubMed]
- Thompson, S.L.; Compton, D.A. Chromosomes and Cancer Cells. Chromosom. Res. 2011, 19, 433–444. [Google Scholar] [CrossRef]
- Novitasari, D.; Jenie, R.I.; Kato, J.-Y.; Meiyanto, E. The Integrative Bioinformatic Analysis Deciphers the Predicted Molecular Target Gene and Pathway from Curcumin Derivative CCA-1.1 against Triple-Negative Breast Cancer (TNBC). J. Egypt. Natl. Cancer Inst. 2021, 33, 19. [Google Scholar] [CrossRef]
- Mehlmann, L.M. Signaling for Meiotic Resumption in Granulosa Cells, Cumulus Cells, and Oocyte. In Oogenesis; Springer: London, UK, 2012; pp. 171–182. [Google Scholar] [CrossRef]
- Mahrous, E.; Yang, Q.; Clarke, H.J. Regulation of Mitochondrial DNA Accumulation during Oocyte Growth and Meiotic Maturation in the Mouse. Reproduction 2012, 144, 177. [Google Scholar] [CrossRef]
- Shao, H.; Li, R.; Ma, C.; Chen, E.; Liu, X.J. Xenopus Oocyte Meiosis Lacks Spindle Assembly Checkpoint Control. J. Cell Biol. 2013, 201, 191–200. [Google Scholar] [CrossRef]
- Kokuryo, T.; Yokoyama, Y.; Yamaguchi, J.; Tsunoda, N.; Ebata, T.; Nagino, M. NEK2 Is an Effective Target for Cancer Therapy with Potential to Induce Regression of Multiple Human Malignancies. Anticancer Res. 2019, 39, 2251–2258. [Google Scholar] [CrossRef]
- Xue, D.; Cheng, P.; Han, M.; Liu, X.; Xue, L.; Ye, C.; Wang, K.; Huang, J. An Integrated Bioinformatical Analysis to Evaluate the Role of KIF4A as a Prognostic Biomarker for Breast Cancer. OncoTargets Ther. 2018, 11, 4755–4768. [Google Scholar] [CrossRef]
- Du, R.; Huang, C.; Liu, K.; Li, X.; Dong, Z. Targeting AURKA in Cancer: Molecular Mechanisms and Opportunities for Cancer Therapy. Mol. Cancer. 2021, 20, 15. [Google Scholar] [CrossRef] [PubMed]
- Siggelkow, W.; Boehm, D.; Gebhard, S.; Battista, M.; Sicking, I.; Lebrecht, A.; Solbach, C.; Hellwig, B.; Rahnenführer, J.; Koelbl, H.; et al. Expression of Aurora Kinase A is Associated with Metastasis-Free Survival in Node-Negative Breast Cancer Patients. BMC Cancer 2012, 12, 562. [Google Scholar] [CrossRef] [PubMed]
- Wheater, M.J.; Johnson, P.W.; Blaydes, J.P. The Role of MNK Proteins and eIF4E Phosphorylation in Breast Cancer Cell Proliferation and Survival. Cancer Biol. Ther. 2010, 10, 728–735. [Google Scholar] [CrossRef]
- Baslan, T.; Kendall, J.; Volyanskyy, K.; McNamara, K.; Cox, H.; D’Italia, S.; Ambrosio, F.; Riggs, M.; Rodgers, L.; Leotta, A.; et al. Novel Insights into Breast Cancer Copy Number Genetic Heterogeneity Revealed by Single-Cell Genome Sequencing. Elife 2020, 9, 51480. [Google Scholar] [CrossRef]
- Yuan, B.; Xu, Y.; Woo, J.-H.; Wang, Y.; Bae, Y.K.; Yoon, D.-S.; Wersto, R.P.; Tully, E.; Wilsbach, K.; Gabrielson, E. Increased Expression of Mitotic Checkpoint Genes in Breast Cancer Cells with Chromosomal Instability. Clin. Cancer Res. 2006, 12, 405–410. [Google Scholar] [CrossRef] [PubMed]
- Koyuncu, D.; Sharma, U.; Goka, E.T.; Lippman, M.E. Spindle Assembly Checkpoint Gene BUB1B Is Essential in Breast Cancer Cell Survival. Breast Cancer Res. Treat. 2021, 185, 331–341. [Google Scholar] [CrossRef]
- Gan, Y.; Li, Y.; Li, T.; Shu, G.; Yin, G. CCNA2 Acts as a Novel Biomarker in Regulating the Growth and Apoptosis of Colorectal Cancer. Cancer Manag. Res. 2018, 10, 5113. [Google Scholar] [CrossRef]
- Xu, M.; Xu, S. PBK/TOPK Overexpression and Survival in Solid Tumors: A PRISMA-Compliant Meta-Analysis. Medicine 2019, 98, e14766. [Google Scholar] [CrossRef]
- Qiao, L.; Ba, J.; Xie, J.; Zhu, R.; Wan, Y.; Zhang, M.; Jin, Z.; Guo, Z.; Yu, J.; Chen, S.; et al. Overexpression of PBK/TOPK Relates to Poor Prognosis of Patients with Breast Cancer: A Retrospective Analysis. World J. Surg. Oncol. 2022, 20, 316. [Google Scholar] [CrossRef]
- Sauter, E.R.; Yeo, U.C.; von Stemm, A.; Zhu, W.; Litwin, S.; Tichansky, D.S.; Pistritto, G.; Nesbit, M.; Pinkel, D.; Herlyn, M.; et al. Cyclin D1 Is a Candidate Oncogene in Cutaneous Melanoma. Cancer Res. 2002, 62, 3200–3206. [Google Scholar] [CrossRef]
- Pollock, P.; Harper, U.L.; Hansen, K.S.; Yudt, L.M.; Stark, M.; Robbins, C.M.; Moses, T.Y.; Hostetter, G.; Wagner, U.; Kakareka, J.; et al. High Frequency of BRAF Mutations in Nevi. Nat. Genet. 2003, 33, 19–20. [Google Scholar] [CrossRef] [PubMed]
- Tarighati, E.; Keivan, H.; Mahani, H. A Review of Prognostic and Predictive Biomarkers in Breast Cancer. Clin. Exp. Med. 2023, 23, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Swain, S.M.; Shastry, M.; Hamilton, E. Targeting HER2-positive breast cancer: Advances and future directions. Nat. Rev. Drug Discov. 2023, 22, 101–126. [Google Scholar] [CrossRef] [PubMed]
- Moore, J.; Wang, F.; Pal, T.; Reid, S.; Cai, H.; Bailey, C.E.; Zheng, W.; Lipworth, L.; Shu, X.O. Oncotype DX risk recurrence score and total mortality for early-stage breast cancer by race/ethnicity. Cancer Epidemiol. Biomark. Prev. 2022, 31, 821–830. [Google Scholar] [CrossRef]
- Furrer, D.; Sanschagrin, F.; Jacob, S.; Diorio, C. Advantages and disadvantages of technologies for HER2 testing in breast cancer specimens. Am. J. Clin. Pathol. 2015, 144, 686–703. [Google Scholar] [CrossRef] [PubMed]
- Sagini, K.; Urbanelli, L.; Buratta, S.; Leonardi, L.; Emiliani, C. Nanovesicles from plants as edible carriers of bioactive compounds. AgroLife Sci. J. 2017, 6, 167–171. [Google Scholar]

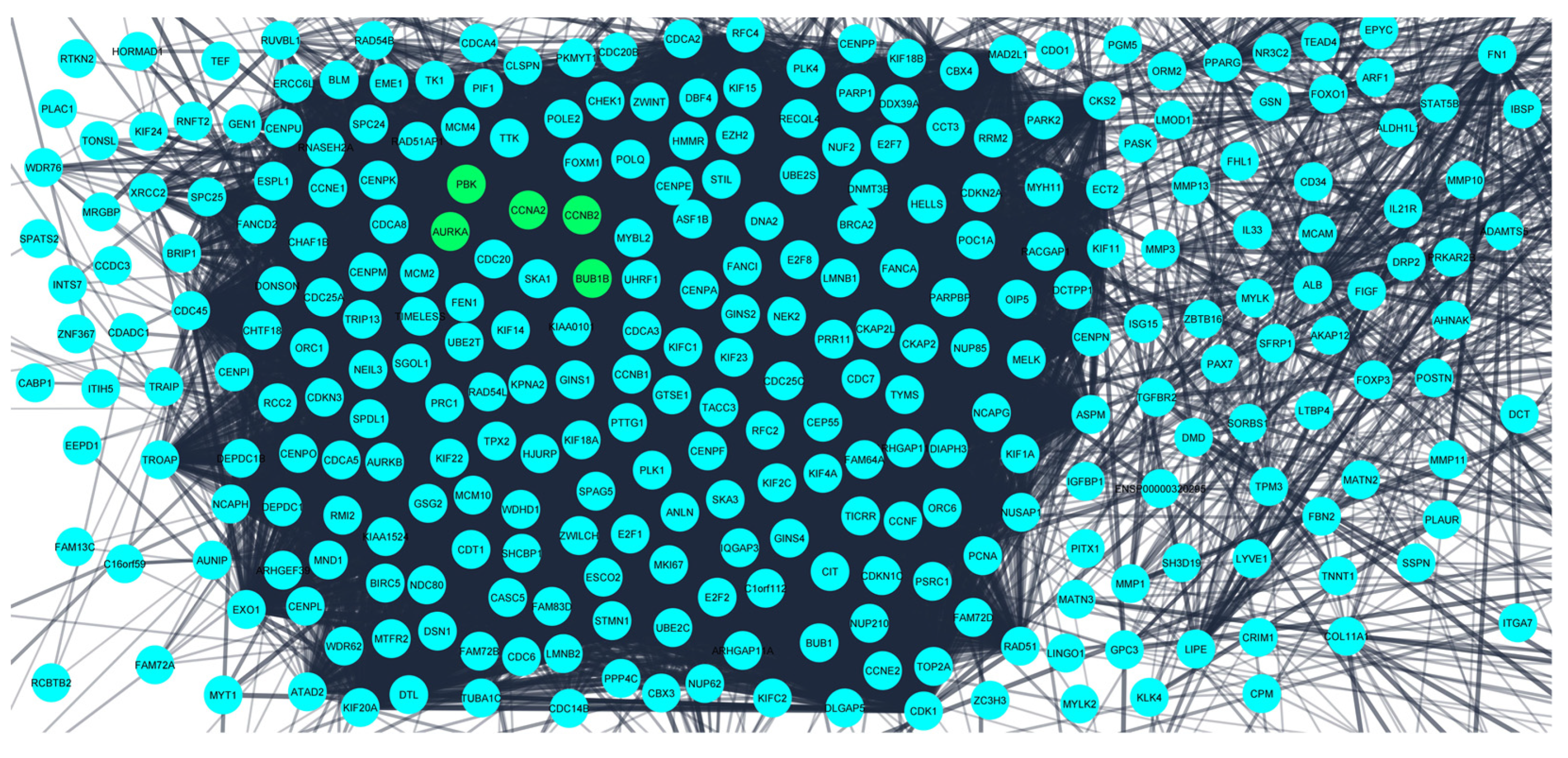




| Hub Genes | Closeness | Degree | EPC | MCC | MNC | Radiality |
|---|---|---|---|---|---|---|
| AURKA | 192.6 | 106 | 38.26 | 1.65 × 10⁵⁷ | 106 | 6.67 |
| BUB1B | 194.7 | 112 | 43.76 | 1.65 × 10⁵⁷ | 112 | 6.62 |
| CCNA2 | 200.4 | 109 | 38.26 | 1.65 × 10⁵⁷ | 109 | 6.73 |
| CCNB2 | 190.6 | 110 | 41.25 | 1.65 × 10⁵⁷ | 110 | 6.52 |
| PBK | 199.5 | 115 | 42.23 | 1.65 × 10⁵⁷ | 115 | 6.61 |
| Biological Process | p-Value |
|---|---|
| GO:0000278~Mitotic Cell Cycle | 1.13 × 10−43 |
| GO:1903047~Mitotic Cell Cycle Process | 2.43 × 10−43 |
| GO:0022402~Cell Cycle Process | 7.17 × 10−40 |
| GO:0007049~Cell Cycle | 1.97 × 10−35 |
| GO:0000280~Nuclear Division | 1.99 × 10−33 |
| GO:0051301~Cell Division | 3.12 × 10−33 |
| GO:0007059~Chromosome Segregation | 4.28 × 10−33 |
| GO:0000819~Sister Chromatid Segregation | 5.08 × 10−33 |
| GO:0007067~Mitotic Nuclear Division | 3.20 × 10−32 |
| GO:0048285~Organelle Fission | 1.05 × 10−31 |
| KEGG Pathways | p-Value |
|---|---|
| hsa04110:Cell Cycle | 1.71 × 10⁻¹⁵ |
| hsa04114:Oocyte Meiosis | 1.27 × 10⁻⁴ |
| hsa03030:DNA Replication | 1.59 × 10⁻⁴ |
| hsa04152:AMPK Signaling Pathway | 4.01 × 10⁻⁴⁰ |
| hsa03460:Fanconi Anemia Pathway | 5.36 × 10⁻⁴ |
| hsa03440:Homologous Recombination | 0.001599 |
| hsa04914:Progesterone-Mediated Oocyte Maturation | 0.001748 |
| hsa03320:PPAR Signaling Pathway | 0.010183 |
| hsa04115:p53 Signaling Pathway | 0.012470 |
| hsa04923:Reguation of Lipolysis in Adipocytes | 0.011456 |
| S. No. | Gene Name | Types of Genetic Alterations (%) | Post Translational Modifications (PTMs) | Mutation Type | Mutation Site | Copy Number Alteration |
|---|---|---|---|---|---|---|
| 1 | AURKA | Mutation (0.24%) Amplification (5.29%) | Phosphorylation | Missense | S98N | Diploid |
| Phosphorylation | Missense | S4Y | Diploid | |||
| Phosphorylation | Missense | S89C | Gain | |||
| NA | Missense | A81V | Gain | |||
| NA | Missense | L26V | Gain | |||
| 2 | BUB1B | Mutation (0.39%) Amplification (0.32%) Deep Deletion (1.12%) | NA | Missense | Q460E | Gain |
| NA | Missense | L669P | Gain | |||
| NA | Nonsense | S564* | Diploid | |||
| NA | FS del | D989Mfs*13 | Diploid | |||
| 3 | CCNA2 | Mutation (0.49%) Amplification (0.62%) | NA | Missense | R112C | Diploid |
| NA | Missense | L315P | Shallow Deletion | |||
| NA | Missense | M189I | Shallow Deletion | |||
| NA | Missense | V85F | Diploid | |||
| 4 | CCNB2 | Amplification (0.29%) Deep Deletion (0.41%) | NA | NA | NA | NA |
| 5 | PBK | Mutation (0.40%) Deep Deletion (5.1%) Amplification (0.8%) | Phosphorylation | Missense | E203K | Shallow Deletion |
| Missense | F40L | Shallow Deletion | ||||
| Nonsense | E295* | Diploid | ||||
| FS del | K18Efs*50 | Gain |
| S. No. | Gene | Hazard Ratio (CI) |
|---|---|---|
| 1 | AURKA | 1.32 (0.91–2.42) |
| 2 | BUB1B | 1.85 (1.32–2.92) |
| 3 | CCNA2 | 0.49 (0.40–0.95) |
| 4 | CCNB2 | 0.62 (0.41–1.01) |
| 5 | PBK | 1.26 (0.90–2.13) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mishra, D.; Mishra, A.; Nand Rai, S.; Vamanu, E.; Singh, M.P. Demystifying the Role of Prognostic Biomarkers in Breast Cancer through Integrated Transcriptome and Pathway Enrichment Analyses. Diagnostics 2023, 13, 1142. https://doi.org/10.3390/diagnostics13061142
Mishra D, Mishra A, Nand Rai S, Vamanu E, Singh MP. Demystifying the Role of Prognostic Biomarkers in Breast Cancer through Integrated Transcriptome and Pathway Enrichment Analyses. Diagnostics. 2023; 13(6):1142. https://doi.org/10.3390/diagnostics13061142
Chicago/Turabian StyleMishra, Divya, Ashish Mishra, Sachchida Nand Rai, Emanuel Vamanu, and Mohan P. Singh. 2023. "Demystifying the Role of Prognostic Biomarkers in Breast Cancer through Integrated Transcriptome and Pathway Enrichment Analyses" Diagnostics 13, no. 6: 1142. https://doi.org/10.3390/diagnostics13061142
APA StyleMishra, D., Mishra, A., Nand Rai, S., Vamanu, E., & Singh, M. P. (2023). Demystifying the Role of Prognostic Biomarkers in Breast Cancer through Integrated Transcriptome and Pathway Enrichment Analyses. Diagnostics, 13(6), 1142. https://doi.org/10.3390/diagnostics13061142








