Lung Ultrasound Artifacts Interpreted as Pathology Footprints
Abstract
1. Introduction
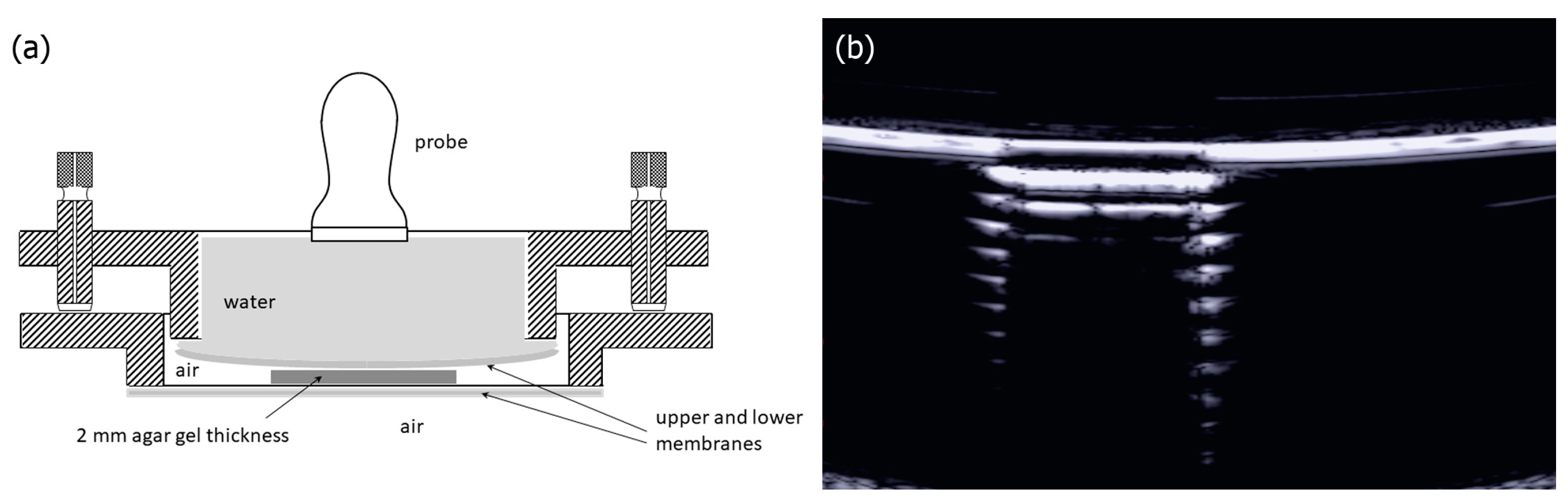
2. Materials and Methods
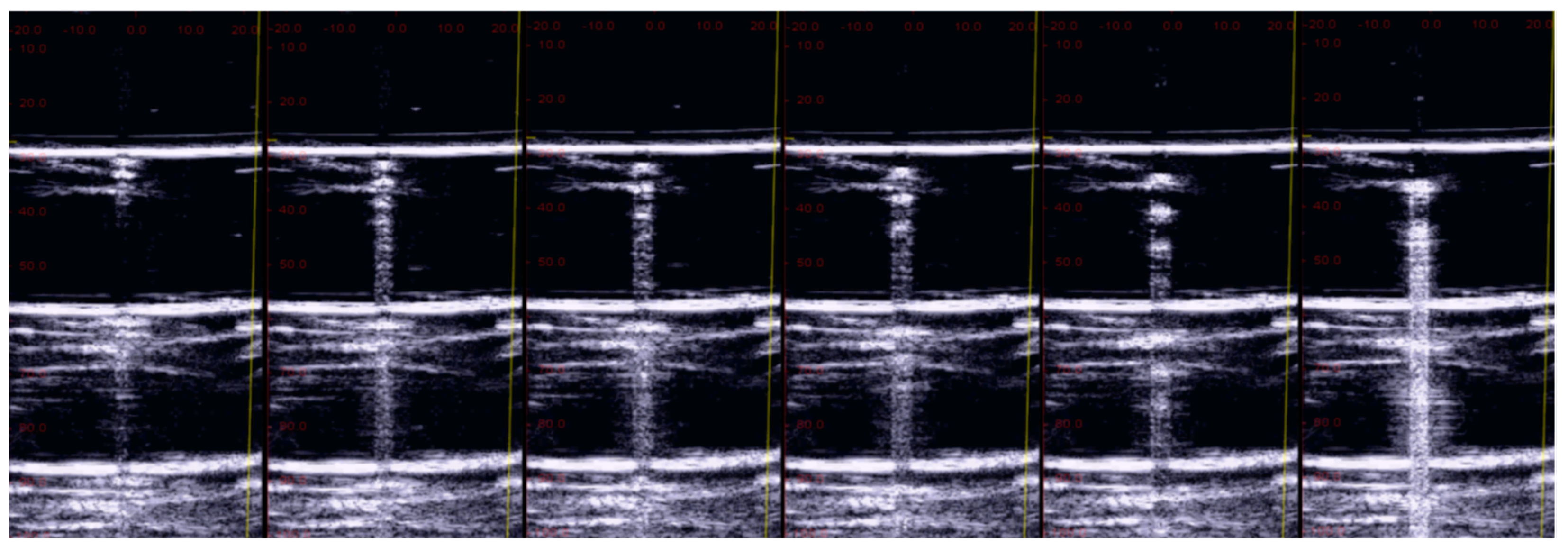
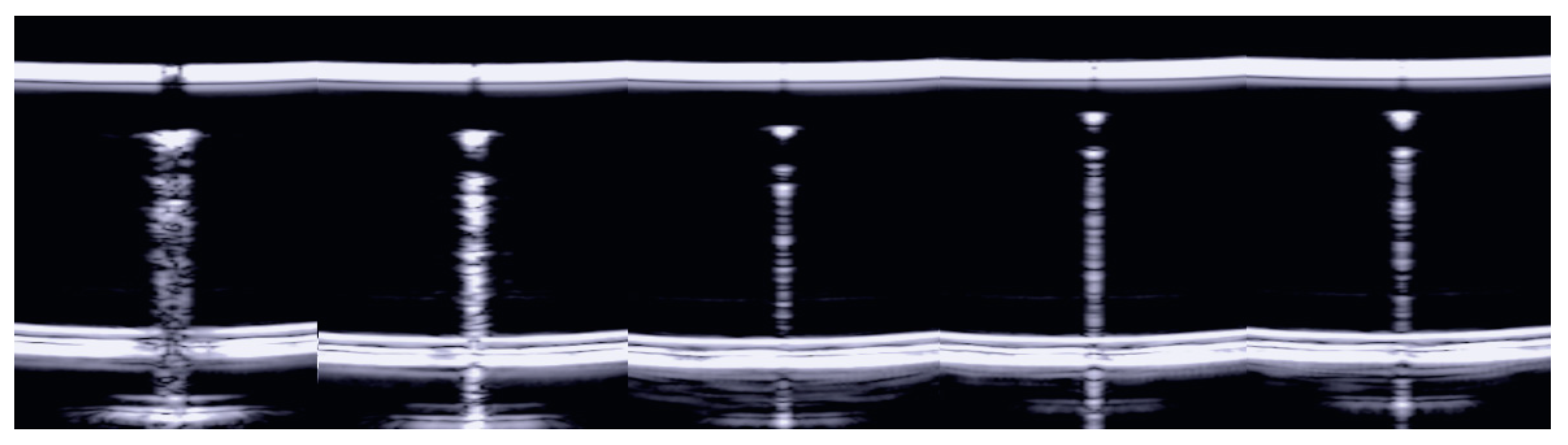
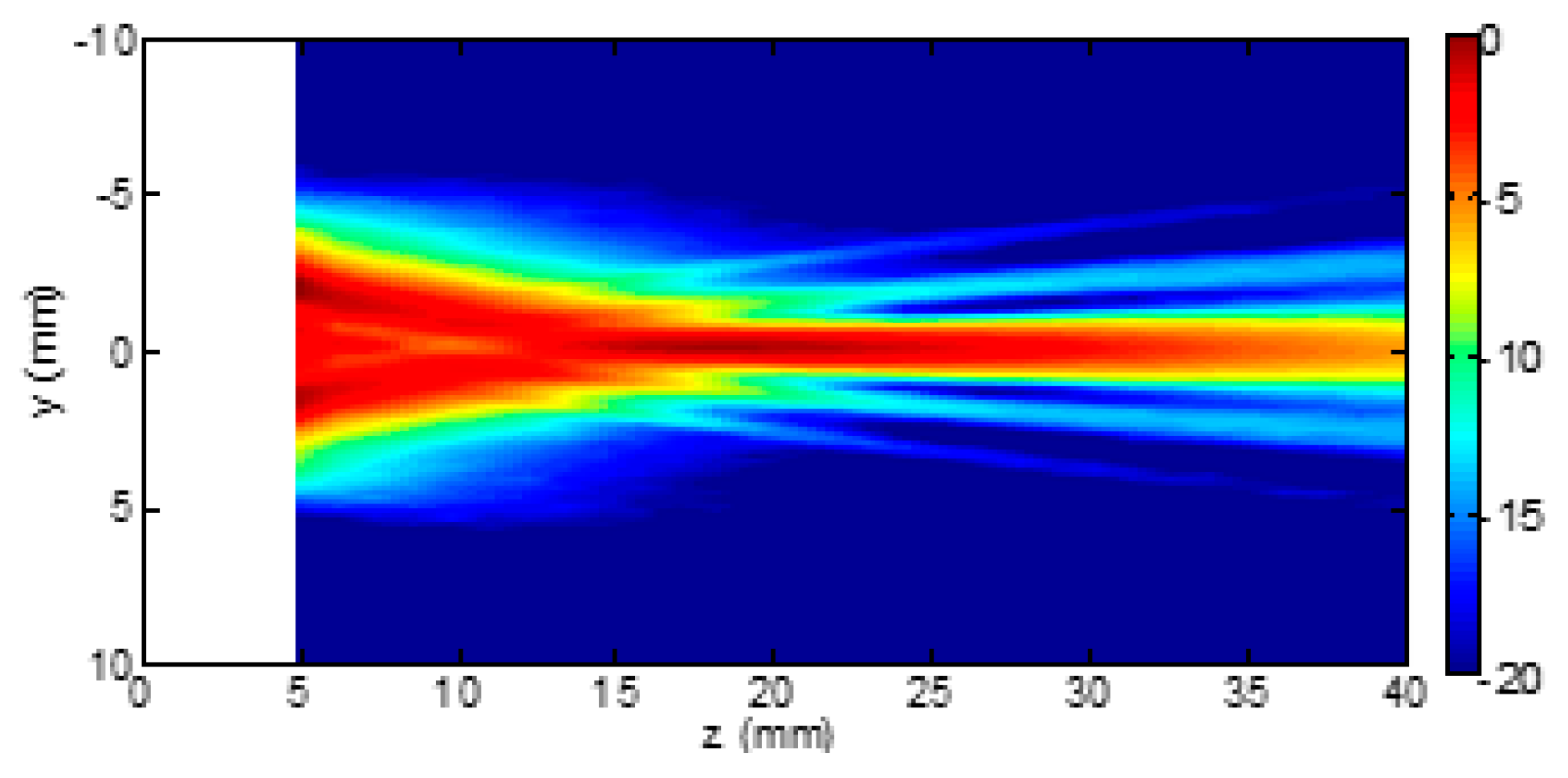
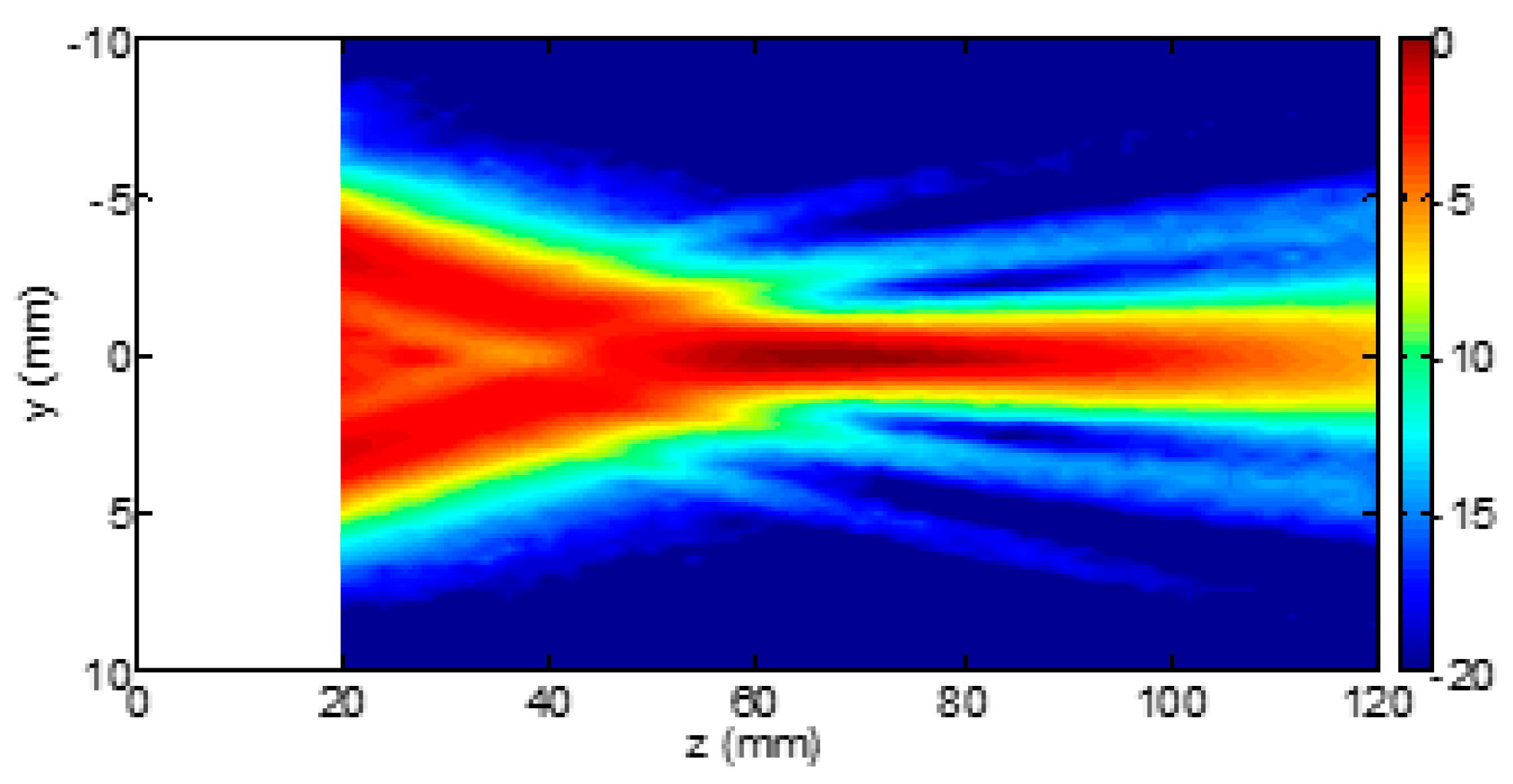
3. Results
3.1. The Four-Step Method
3.2. The Impact of Wrong Utilization of the Ultrasound Equipment
4. Discussion
5. Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lichtenstein, D.; Mezière, G.; Biderman, P.; Gepner, A.; Barré, O. The comet-tail artifact: An ultrasound sign of alveolar interstitial syndrome. Am. J. Respir. Crit. Care Med. 1997, 156, 1640–1646. [Google Scholar] [CrossRef]
- Koegelenberg, C.F.; von Groote-Bidlingmaier, F.; Bolliger, C.T. Transthoracic ultrasonography for the respiratory physician. Respiration 2012, 84, 337–350. [Google Scholar] [CrossRef] [PubMed]
- Vignon, P.; Repessè, X.; Vieillard Baron, A.; Maury, E. Critical care ultrasonography in acute respiratory failure. Crit. Care 2016, 20, 228. [Google Scholar] [CrossRef] [PubMed]
- Rambhia, S.H.; D’Agostino, C.A.; Noor, A.; Villani, R.; Naidich, J.J.; Pellerito, J.S. Thoracic Ultrasound: Technique, Applications, and Interpretation. Curr. Probl. Diagn. Radiol. 2017, 46, 305–316. [Google Scholar] [CrossRef] [PubMed]
- Mayo, P.H.; Copetti, R.; Feller-Kopman, D.; Mathis, G.; Maury, E.; Mongodi, S.; Mojoli, F.; Volpicelli, G.; Zanobetti, M. Thoracic ultrasonography: A narrative review. Intensive Care Med. 2019, 45, 1200–1211. [Google Scholar] [CrossRef]
- Lichtenstein, D.A.; Mezière, G.A. Relevance of lung ultrasound in the diagnosis of acute respiratory failure: The BLUE protocol. Chest 2008, 134, 117–125. [Google Scholar] [CrossRef] [PubMed]
- Volpicelli, G.; Elbarbary, M.; Blaivas, M.; Lichtenstein, D.A.; Mathis, G.; Kirkpatrick, A.W.; Melniker, L.; Gargani, L.; Noble, V.E.; Via, G.; et al. International Liaison Committee on Lung Ultrasound (ILC-LUS) for the International Consensus Conference on Lung Ultrasound (ICC-LUS). International evidence-based recommendations for point-of-care lung ultrasound. Intensive Care Med. 2012, 38, 577–591. [Google Scholar] [CrossRef]
- Soldati, G.; Smargiassi, A.; Inchingolo, R.; Sher, S.; Nenna, R.; Valente, S.; Inchingolo, C.D.; Corbo, G.M. Lung ultrasonography may provide an indirect estimation of lung porosity and airspace geometry. Respiration 2014, 88, 458–468. [Google Scholar] [CrossRef]
- Bushberg, J.T.; Seibert, J.A.; Leidholdt, E.M., Jr.; Boone, J.M. The Essential Physics of Medical Imaging; Williams & Wilkins: Philadelphia, PA, USA, 2002. [Google Scholar]
- Demi, M.; Prediletto, R.; Soldati, G.; Demi, L. Physical mechanisms providing clinical informations from ultrasound lung images: Hypothesis and early confirmations. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2019, 67, 612–623. [Google Scholar] [CrossRef]
- Kameda, T.; Kamiyama, N.; Taniguchi, N. The Mechanisms Underlying Vertical Artifacts in Lung Ultrasound and Their Proper Utilization for the Evaluation of Cardiogenic Pulmonary Edema. Diagnostics 2022, 12, 252. [Google Scholar] [CrossRef]
- Allevato Martins da Silva, F.; Moreno, E.; Coelho de Albuquerque Pereira, W. B-Lines Lung Ultrasonography Simulation Using Finite Element Method. Diagnostics 2022, 12, 2751. [Google Scholar] [CrossRef] [PubMed]
- Demi, M. On the Replica of US Pulmonary Artifacts by Means of Physical Models. Diagnostics 2021, 11, 1666. [Google Scholar] [CrossRef] [PubMed]
- Demi, M. The impact of multiple concurrent factors on the length of the ultrasound pulmonary vertical artifacts as illustrated through the experimental and numerical analysis of simple models. J. Acoust. Soc. Am. 2021, 150, 2106. [Google Scholar] [CrossRef] [PubMed]
- Kameda, T.; Kamiyama, N.; Taniguchi, N. The effect of attenuation inside the acoustic traps on the configuration of vertical artifacts in lung ultrasound: An experimental study with simple models. J. Med. Ultrason. 2022, 49, 545–553. [Google Scholar] [CrossRef] [PubMed]
- Demi, M.; Buda, N.; Soldati, G. Vertical Artifacts in Lung Ultrasonography: Some Common Clinician Questions and the Related Engineer Answers. Diagnostics 2022, 12, 215. [Google Scholar] [CrossRef]
- Mento, F.; Demi, L. On the influence of imaging parameters on lung ultrasound B-line artifacts, in vitro study. J. Acoust. Soc. Am. 2020, 148, 975. [Google Scholar] [CrossRef]
- Boni, E.; Bassi, L.; Dallai, A.; Guidi, F.; Ramalli, A.; Ricci, S.; Housden, R.J.; Tortoli, P. A reconfigurable and programmable FPGA-based system for nonstandard ultrasound methods. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2012, 59, 1378–1385. [Google Scholar] [CrossRef]
- Boni, E.; Yu, A.C.H.; Freear, S.; Jensen, J.A.; Tortoli, P. Ultrasound Open Platforms for Next-Generation Imaging Technique Development. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2018, 65, 1078–1092. [Google Scholar] [CrossRef]
- Lu, J.-Y.; Zou, H.; Greenleaf, J.F. Biomedical ultrasound beam forming. Ultrasound Med. Biol. 1994, 20, 403–428. [Google Scholar] [CrossRef]
- Minnaert, M. On musical air-bubblesand sounds of running water. Phil. Mag. 1933, 16, 235–248. [Google Scholar] [CrossRef]
- Leighton, T.G. The Acoustic Bubble; Academic Press: Cambridge, MA, USA, 1994. [Google Scholar]
- Soldati, G.; Demi, M.; Smargiassi, A.; Inchingolo, R.; Demi, L. The role of ultrasound lung artifacts in the diagnosis of respiratory diseases. Expert Rev. Respir. Med. 2019, 13, 163–172. [Google Scholar] [CrossRef]
- Beranek, L.L. Acoustics; Acoustical Society of America: New York, NY, USA, 1993. [Google Scholar]
- Wikipedia. Available online: https://en.wikipedia.org/wiki/Helmholtz_resonance (accessed on 15 February 2023).
- Bouhemad, B.; Brisson, H.; Le Guen, M.; Arbelot, C.M.B.; Lu, Q.; Rouby, J.J. Bedside ultrasound assessment of positive end-expiratory pressure-induced lung recruitment. Am. J. Respirat. Crit. Care Med. 2011, 183, 341–347. [Google Scholar] [CrossRef] [PubMed]
- Soldati, G.; Giunta, V.; Sher, S.; Melosi, F.; Dini, C. “Synthetic” comets: A new look at lung sonography. Ultrasound Med. Biol. 2011, 37, 1762–1770. [Google Scholar] [CrossRef]
- Vogel, J.; Bom, N.; Ridder, J.; Lancee, C. Transducer design considerations in dynamic focusing. Ultrasound Med. Biol. 1979, 5, 187–193. [Google Scholar] [CrossRef] [PubMed]
- Szabo, T.L. Diagnostic Ultrasound Imaging: Inside Out, 1st ed.; Academic Press: Cambridge, MA, USA, 2004. [Google Scholar]
- Thijssen, J.M.; Mischi, M. Ultrasound Imaging Arrays. In Comprehensive Biomedical Physics; Elsevier: Amsterdam, The Netherlands, 2014; Volume 506, pp. 323–341. [Google Scholar]
- Duck, F.A.; Baker, A.C.; Starritt, H.C. Ultrasound Medicine; Institute of Physics Publishing: Bristol, UK, 1998. [Google Scholar]
- Weibel, E.R. How to make an alveolus. Eur. Respir. J. 2008, 31, 483–485. [Google Scholar] [CrossRef] [PubMed]
- Leslie, K.O. Pathology of interstitial lung disease. Clin. Chest Med. 2004, 25, 657–703. [Google Scholar] [CrossRef]
- Weibel, E.R. Morphometry of the Human Lung; Springer: Berlin/Heidelberg, Germany, 1963. [Google Scholar]
- Leslie, K.; Wick, M. Practical Pulmonary Pathology. A Diagnostic Approach, 1st ed.; Churchill-Livingstone: Phildelphia, PA, USA, 2005. [Google Scholar]
- Hasan, A.A.; Makhlouf, H.A. B-lines: Transthoracic chest ultrasound signs useful in assessment of interstitial lung diseases. Ann. Thorac. Med. 2014, 9, 99–103. [Google Scholar]
- Reissig, A.; Copetti, R.; Kroegel, C. Current role of emergency ultrasound of the chest. Crit. Care Med. 2011, 39, 839–845. [Google Scholar] [CrossRef]
- Scarpelli, E.M. The alveolar surface network: A new anatomy and its physiological significance. Anat. Rec. 1998, 251, 491–527. [Google Scholar] [CrossRef]
- Soldati, G.; Inchingolo, R.; Smargiassi, A.; Sher, S.; Nenna, R.; Inchingolo, C.D.; Valente, S. Ex vivo lung sonography: Morphologic-ultrasound relationship. Ultrasound Med. Biol. 2012, 38, 1169–1179. [Google Scholar] [CrossRef]
- Soldati, G.; Demi, M. The use of lung ultrasound images for the differential diagnosis of pulmonary and cardiac interstitial pathology. J. Ultrasound 2017, 20, 91–96. [Google Scholar] [CrossRef] [PubMed]
- Copetti, R.; Soldati, G.; Copetti, P. Chest sonography: A useful tool to differentiate acute cardiogenic pulmonary edema from acute respiratory distress syndrome. Cardiovasc. Ultrasound 2008, 29, 6–16. [Google Scholar] [CrossRef] [PubMed]
- Soldati, G.; Demi, M. What Is COVID 19 Teaching Us about Pulmonary Ultrasound? Diagnostics 2022, 12, 838. [Google Scholar] [CrossRef]
- Demi, L. Lung Ultrasound: The future ahead and the lessons learned from COVID-19. J. Acoust. Soc. Am. 2020, 148, 2146. [Google Scholar] [CrossRef]
- Spinelli, A.; Vinci, B.; Tirella, A.; Matteucci, M.; Gargani, L.; Ahluwalia, A.; Domenici, C.; Picano, E.; Chiarelli, P. Realization of a poro-elastic ultrasound replica of pulmonary tissue. Biomatter 2012, 2, 37–42. [Google Scholar] [CrossRef]
- Zell, K.; Sperl, J.I.; Vogel, M.W.; Niessner, R.; Haisch, C. Acoustical properties of selected tissue phantom materials for ultrasound imaging. Phys. Med. Biol. 2007, 52, 475–484. [Google Scholar] [CrossRef]
- Ziskin, M.C.; Thickman, D.I.; Goldenberg, N.J.; Lapayowker, M.S.; Becker, J.M. The comet tail artifact. J. Ultrasound Med. 1982, 1, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Avruch, L.; Cooperberg, P.L. The ring-down artifact. J. Ultrasound Med. 1985, 4, 21–28. [Google Scholar] [CrossRef]
- Creditt, A.; Tozer, J.; Vitto, M.; Joyce, M.; Taylor, L. Clinical Ultrasound: A Pocket Manual; Springer: Berlin/Heidelberg, Germany, 2018. [Google Scholar]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Demi, M.; Soldati, G.; Ramalli, A. Lung Ultrasound Artifacts Interpreted as Pathology Footprints. Diagnostics 2023, 13, 1139. https://doi.org/10.3390/diagnostics13061139
Demi M, Soldati G, Ramalli A. Lung Ultrasound Artifacts Interpreted as Pathology Footprints. Diagnostics. 2023; 13(6):1139. https://doi.org/10.3390/diagnostics13061139
Chicago/Turabian StyleDemi, Marcello, Gino Soldati, and Alessandro Ramalli. 2023. "Lung Ultrasound Artifacts Interpreted as Pathology Footprints" Diagnostics 13, no. 6: 1139. https://doi.org/10.3390/diagnostics13061139
APA StyleDemi, M., Soldati, G., & Ramalli, A. (2023). Lung Ultrasound Artifacts Interpreted as Pathology Footprints. Diagnostics, 13(6), 1139. https://doi.org/10.3390/diagnostics13061139







