Left Atrioventricular Transvalvular Pressure Gradients Derived from Intraoperative and Postoperative Echocardiograms following Atrioventricular Septal Defect Repair
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Approval, Study Design and Patient Selection
2.2. Anesthesia, Surgical Technique and Cardiopulmonary Bypass
2.3. Echocardiography
2.4. Clinical and Follow-Up Data
2.5. Statistical Analysis
3. Results
3.1. Clinical and Surgical Characteristics
3.2. Echocardiographic Data
3.3. Outcome
4. Discussion
4.1. The Left Atrioventricular Valve following AVSD Repair
4.2. Echocardiographic Modalities for the Assessment of LAVV Function
4.3. Clinical Implications
4.4. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AVSD | Atrioventricular septal defect |
| BSA | Body surface area |
| CI | Cardiac index |
| CPB | Cardiopulmonary bypass |
| CVP | Central venous pressure |
| HR | Heart rate |
| LAVV | Left atrioventricular valve |
| LVEF | Left ventricular ejection fraction |
| MAP | Mean arterial pressure |
| MPG | Mean pressure gradient |
| PPG | Peak pressure gradient |
| r | Correlation coefficient |
| TEE | Transesophageal echocardiography/echocardiogram |
| TTE | Transthoracic echocardiography/echocardiogram |
| VIS | Vasoactive-Inotropic Score |
| VTI | Velocity-time integral |
References
- Reller, M.D.; Strickland, M.J.; Riehle-Colarusso, T.; Mahle, W.T.; Correa, A. Prevalence of congenital heart defects in metropolitan Atlanta, 1998–2005. J. Pediatr. 2008, 153, 807–813. [Google Scholar] [CrossRef]
- Shuhaiber, J.H.; Ho, S.Y.; Rigby, M.; Sethia, B. Current options and outcomes for the management of atrioventricular septal defect. Eur. J. Cardiothorac. Surg. 2009, 35, 891–900. [Google Scholar] [CrossRef]
- Schleiger, A.; Miera, O.; Peters, B.; Schmitt, K.R.L.; Kramer, P.; Buracionok, J.; Murin, P.; Cho, M.Y.; Photiadis, J.; Berger, F.; et al. Long-term results after surgical repair of atrioventricular septal defect. Interact. Cardiovasc. Thorac. Surg. 2019, 28, 789–796. [Google Scholar] [CrossRef]
- Deri, A.; English, K. Educational Series in Congenital Heart Disease: Echocardiographic assessment of left to right shunts: Atrial septal defect, ventricular septal defect, atrioventricular septal defect, patent arterial duct. Echo Res. Pract. 2018, 5, R1–R16. [Google Scholar] [CrossRef]
- Smallhorn, J.F. Cross-sectional echocardiographic assessment of atrioventricular septal defect: Basic morphology and preoperative risk factors. Echocardiography 2001, 18, 415–432. [Google Scholar] [CrossRef]
- Puchalski, M.D.; Lui, G.K.; Miller-Hance, W.C.; Brook, M.M.; Young, L.T.; Bhat, A.; Roberson, D.A.; Mercer-Rosa, L.; Miller, O.I.; Parra, D.A.; et al. Guidelines for Performing a Comprehensive Transesophageal Echocardiographic: Examination in Children and All Patients with Congenital Heart Disease: Recommendations from the American Society of Echocardiography. J. Am. Soc. Echocardiogr. 2019, 32, 173–215. [Google Scholar] [CrossRef]
- Kamra, K.; Russell, I.; Miller-Hance, W.C. Role of transesophageal echocardiography in the management of pediatric patients with congenital heart disease. Paediatr. Anaesth. 2011, 21, 479–493. [Google Scholar] [CrossRef]
- Welke, K.F.; Morris, C.D.; King, E.; Komanapalli, C.; Reller, M.D.; Ungerleider, R.M. Population-based perspective of long-term outcomes after surgical repair of partial atrioventricular septal defect. Ann. Thorac. Surg. 2007, 84, 624–628; discussion 628–629. [Google Scholar] [CrossRef]
- El-Najdawi, E.K.; Driscoll, D.J.; Puga, F.J.; Dearani, J.A.; Spotts, B.E.; Mahoney, D.W.; Danielson, G.K. Operation for partial atrioventricular septal defect: A forty-year review. J. Thorac. Cardiovasc. Surg. 2000, 119, 880–890. [Google Scholar] [CrossRef]
- Chowdhury, U.K.; Airan, B.; Malhotra, A.; Bisoi, A.K.; Kalaivani, M.; Govindappa, R.M.; Venugopal, P. Specific issues after surgical repair of partial atrioventricular septal defect: Actuarial survival, freedom from reoperation, fate of the left atrioventricular valve, prevalence of left ventricular outflow tract obstruction, and other events. J. Thorac. Cardiovasc. Surg. 2009, 137, 548–555.e2. [Google Scholar] [CrossRef]
- Boening, A.; Scheewe, J.; Heine, K.; Hedderich, J.; Regensburger, D.; Kramer, H.H.; Cremer, J. Long-term results after surgical correction of atrioventricular septal defects. Eur. J. Cardiothorac. Surg. 2002, 22, 167–173. [Google Scholar] [CrossRef]
- Gaies, M.G.; Gurney, J.G.; Yen, A.H.; Napoli, M.L.; Gajarski, R.J.; Ohye, R.G.; Charpie, J.R.; Hirsch, J.C. Vasoactive-inotropic score as a predictor of morbidity and mortality in infants after cardiopulmonary bypass. Pediatr. Crit. Care Med. 2010, 11, 234–238. [Google Scholar] [CrossRef]
- Rigby, M. Atrioventricular Septal Defect: What Is in a Name? J. Cardiovasc. Dev. Dis. 2021, 8, 19. [Google Scholar] [CrossRef]
- Ginde, S.; Lam, J.; Hill, G.D.; Cohen, S.; Woods, R.K.; Mitchell, M.E.; Tweddell, J.S.; Earing, M.G. Long-term outcomes after surgical repair of complete atrioventricular septal defect. J. Thorac. Cardiovasc. Surg. 2015, 150, 369–374. [Google Scholar] [CrossRef]
- Fong, L.S.; Betts, K.; Ayer, J.; Andrews, D.; Nicholson, I.A.; Winlaw, D.S.; Orr, Y. Predictors of reoperation and mortality after complete atrioventricular septal defect repair. Eur. J. Cardiothorac. Surg. 2021, 61, 45–53. [Google Scholar] [CrossRef]
- Vera, F.; Sarria, E.; Ortiz, A.; Garcia, N.; Conejo, L.; Ruiz, E. Mitral valve repair in the atrio-ventricular septal defect. Cir. Cardiovasc. 2022, 29, 138–143. [Google Scholar] [CrossRef]
- Mery, C.M.; Zea-Vera, R.; Chacon-Portillo, M.A.; Zhang, W.; Binder, M.S.; Kyle, W.B.; Adachi, I.; Heinle, J.S.; Fraser, C.D., Jr. Contemporary results after repair of partial and transitional atrioventricular septal defects. J. Thorac. Cardiovasc. Surg. 2019, 157, 1117–1127.e4. [Google Scholar] [CrossRef]
- Newburger, J.W.; Jonas, R.A.; Wernovsky, G.; Wypij, D.; Hickey, P.R.; Kuban, K.C.; Farrell, D.M.; Holmes, G.L.; Helmers, S.L.; Constantinou, J.; et al. A comparison of the perioperative neurologic effects of hypothermic circulatory arrest versus low-flow cardiopulmonary bypass in infant heart surgery. N. Engl. J. Med. 1993, 329, 1057–1064. [Google Scholar] [CrossRef]
- Agarwal, H.S.; Wolfram, K.B.; Saville, B.R.; Donahue, B.S.; Bichell, D.P. Postoperative complications and association with outcomes in pediatric cardiac surgery. J. Thorac. Cardiovasc. Surg. 2014, 148, 609–616.e1. [Google Scholar] [CrossRef]
- Stevenson, J.G.; Sorensen, G.K.; Gartman, D.M.; Hall, D.G.; Rittenhouse, E.A. Transesophageal echocardiography during repair of congenital cardiac defects: Identification of residual problems necessitating reoperation. J. Am. Soc. Echocardiogr. 1993, 6, 356–365. [Google Scholar] [CrossRef]
- Ross, F.J.; Nasr, V.G.; Joffe, D.; Latham, G.J. Perioperative and Anesthetic Considerations in Atrioventricular Septal Defect. Semin. Cardiothorac. Vasc. Anesth. 2017, 21, 221–228. [Google Scholar] [CrossRef]
- Cantinotti, M.; Giordano, R.; Koestenberger, M.; Voges, I.; Santoro, G.; Franchi, E.; Assanta, N.; Valverde, I.; Simpson, J.; Kutty, S. Echocardiographic examination of mitral valve abnormalities in the paediatric population: Current practices. Cardiol. Young 2020, 30, 1–11. [Google Scholar] [CrossRef]
- Cantinotti, M.; Giordano, R.; Scalese, M.; Murzi, B.; Assanta, N.; Spadoni, I.; Crocetti, M.; Marotta, M.; Molinaro, S.; Kutty, S.; et al. Nomograms for mitral inflow Doppler and tissue Doppler velocities in Caucasian children. J. Cardiol. 2016, 68, 288–299. [Google Scholar] [CrossRef]
- Lee, H.R.; Montenegro, L.M.; Nicolson, S.C.; Gaynor, J.W.; Spray, T.L.; Rychik, J. Usefulness of intraoperative transesophageal echocardiography in predicting the degree of mitral regurgitation secondary to atrioventricular defect in children. Am. J. Cardiol. 1999, 83, 750–753. [Google Scholar] [CrossRef]
- Freeman, W.K.; Schaff, H.V.; Khandheria, B.K.; Oh, J.K.; Orszulak, T.A.; Abel, M.D.; Seward, J.B.; Tajik, A.J. Intraoperative evaluation of mitral valve regurgitation and repair by transesophageal echocardiography: Incidence and significance of systolic anterior motion. J. Am. Coll. Cardiol. 1992, 20, 599–609. [Google Scholar] [CrossRef]
- Kim, H.K.; Kim, W.H.; Hwang, S.W.; Lee, J.Y.; Song, J.Y.; Kim, S.J.; Jang, K.Y. Predictive value of intraoperative transesophageal echocardiography in complete atrioventricular septal defect. Ann. Thorac. Surg. 2005, 80, 56–59. [Google Scholar] [CrossRef]
- Degertekin, M.; Basaran, Y.; Gencbay, M.; Yaymaci, B.; Dindar, I.; Turan, F. Validation of flow convergence region method in assessing mitral valve area in the course of transthoracic and transesophageal echocardiographic studies. Am. Heart J. 1998, 135, 207–214. [Google Scholar] [CrossRef]
- Lopez, L.; Colan, S.D.; Frommelt, P.C.; Ensing, G.J.; Kendall, K.; Younoszai, A.K.; Lai, W.W.; Geva, T. Recommendations for quantification methods during the performance of a pediatric echocardiogram: A report from the Pediatric Measurements Writing Group of the American Society of Echocardiography Pediatric and Congenital Heart Disease Council. J. Am. Soc. Echocardiogr. 2010, 23, 465–495; quiz 576-467. [Google Scholar] [CrossRef]
- Banerjee, A.; Kohl, T.; Silverman, N.H. Echocardiographic evaluation of congenital mitral valve anomalies in children. Am. J. Cardiol. 1995, 76, 1284–1291. [Google Scholar] [CrossRef]

| Parameter | Result |
|---|---|
| Demographic data | |
| Age, days | 151 (119–252) |
| Weight, kg | 5.4 (4.7–6.2) |
| Height, cm | 61 (58–64) |
| Body mass index, kg/m² | 14.4 ± 1.5 |
| BSA, m² | 0.31 (0.28–0.33) |
| Morbidity | |
| AVSD | |
| Complete, n | 34 (87) |
| Partial, n | 4 (10) |
| Transitional, n | 1 (3) |
| LAVV regurgitation, n | 32 (82) |
| Right atrioventricular valve regurgitation, n | 28 (72) |
| Additional congenital heart defects | |
| Patent Ductus Arteriosus, n | 8 (21) |
| Pulmonary valve stenosis, n | 4 (10) |
| Right ventricular outflow tract obstruction obstruction, n | 2 (5) |
| Aortic stenosis, n | 2 (5) |
| Coarctation, n | 1 (3) |
| Borderline hypoplastic left heart syndrome, n | 1 (3) |
| * Impaired left ventricular systolic function, n | 4 (10) |
| * Impaired right ventricular systolic function, n | 4 (10) |
| Symptoms of heart failure †, n | 32 (82) |
| Pulmonary hypertension ‡, n | 20 (51) |
| Born preterm, n | 7 (18) |
| Trisomy 21, n | 25 (64) |
| Holt–Oram syndrome, n | 1 (3) |
| Parameter | Result |
|---|---|
| Surgical data | |
| AVSD repair | |
| Primary repair, n | 38 (97) |
| Revision surgery, n | 1 (3) |
| LAVV reconstruction, n | 24 (62) |
| Right atrioventricular valve reconstruction, n | 19 (49) |
| Pulmonary valve intervention, n | 4 (10) |
| Patent Ductus Arteriosus closure, n | 4 (10) |
| Right ventricular outflow tract myectomy, n | 1 (3) |
| Aortic arch reconstruction, n | 1 (3) |
| Duration of surgery, min | 221 ± 56 |
| Clamp time, min | 88 ± 27 |
| Reperfusion time, min | 10 (7–16) |
| Residual LAVV regurgitation, n | 34 (87) |
| Hemodynamics and mechanical ventilation during TEE | |
| MAP, mmHg | 57 ± 11 |
| CVP, mmHg | 11 ± 4 |
| PEEP, mbar | 5 (5-5) |
| Mean airway pressure, mbar | 9 (8–10) |
| Mean driving pressure, mbar | 4 (3–5) |
| Vasopressors/inotropes | |
| Milrinone, mcg/kg/min | 0.5 (0.3–0.5) |
| Norepinephrine, mcg/kg/min | 0 (0–0.05) |
| Epinephrine, mcg/kg/min | 0 (0–0) |
| VIS | 6.0 (3.7–10.7) |
| Parameter | Intraoperative | Discharge | p-Value |
|---|---|---|---|
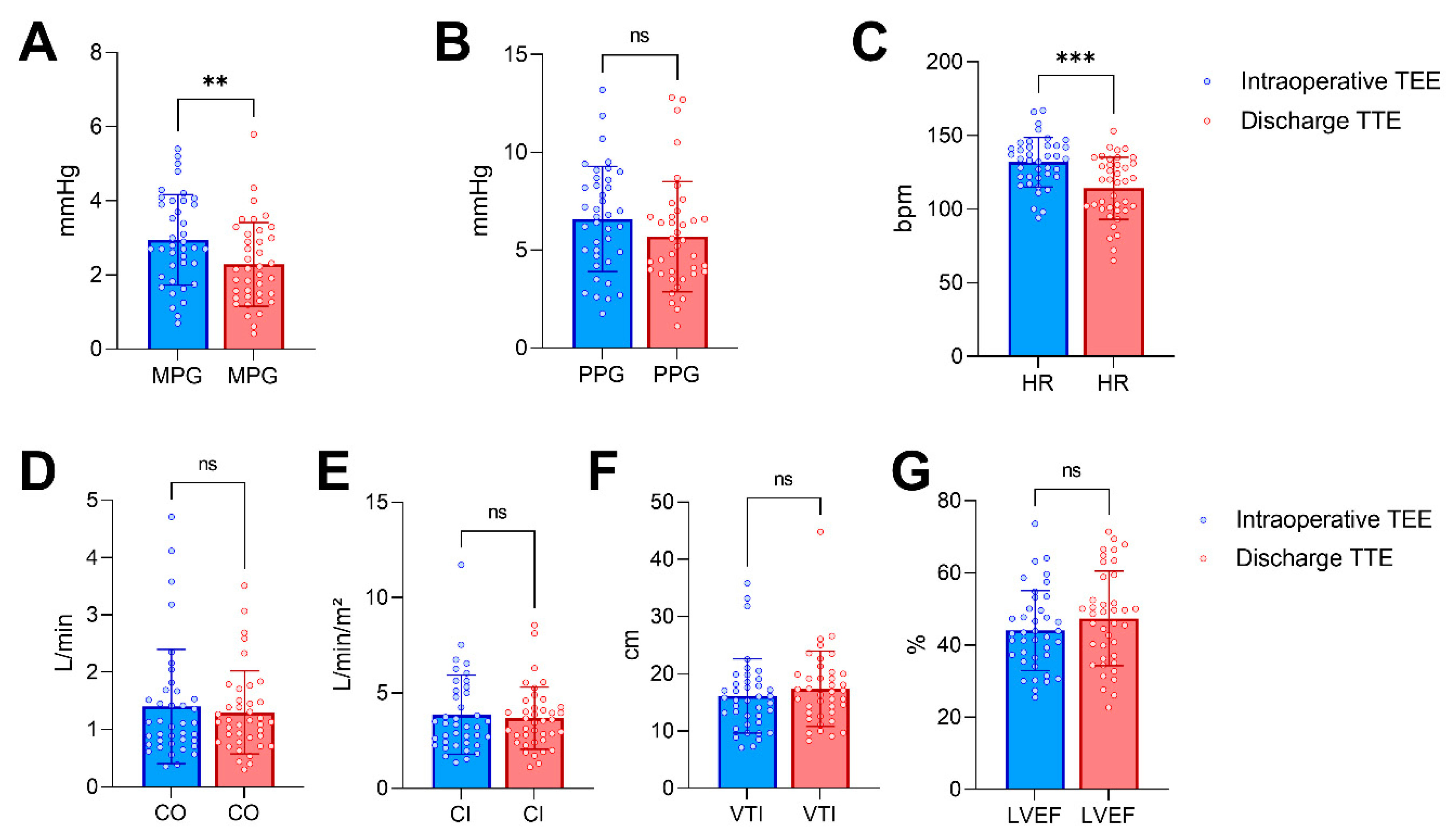
| Heart rate, bpm | 132 ± 17 | 114 ± 21 | <0.001 |
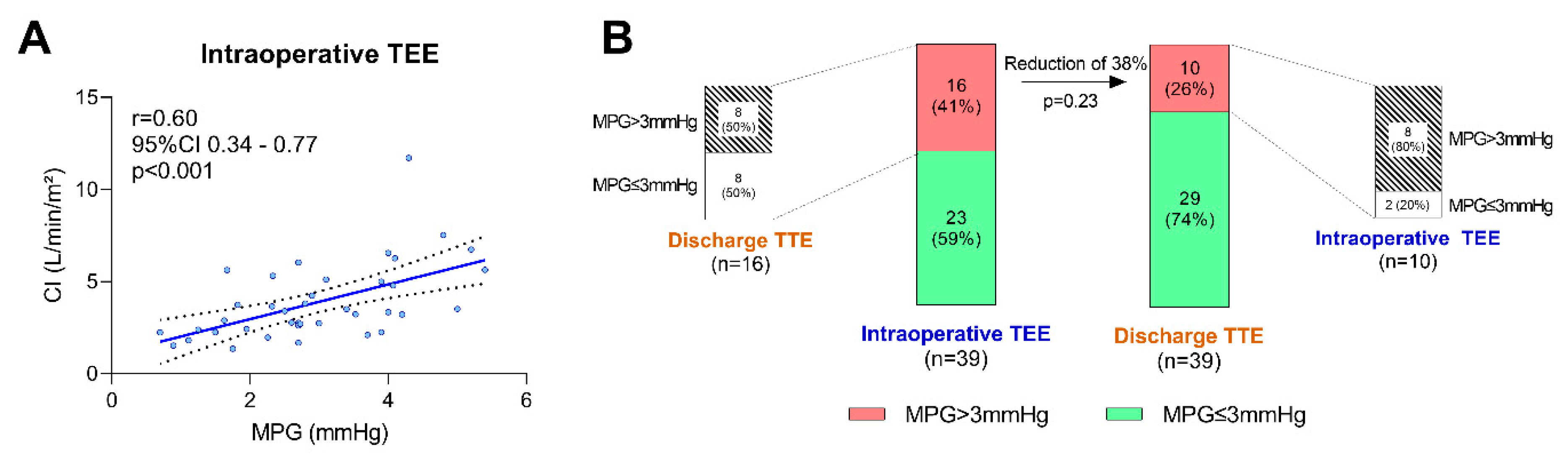
| LAVV MPG, mmHg | 3.0 ± 1.2 | 2.3 ± 1.1 | <0.01 |
| LAVV PPG, mmHg | 6.6 ± 2.7 | 5.7 ± 2.8 | 0.06 |
| LAVV VTI, cm | 16.1 ± 6.5 | 17.4 ± 6.6 | 0.18 |
| Cardiac output, L/min | 1.1 (0.8–1.7) | 1.1 (0.8–1.5) | 0.52 |
| Cardiac index, L/min/m² | 3.3 (2.4–5.1) | 3.5 (2.5–4.3) | 0.54 |
| LVEF, % | 44 ± 11 | 47 ± 13 | 0.16 |
| Parameter | Result |
|---|---|
| In-hospital | |
| Days between intraoperative TEE and discharge TTE | 10 (6–15) |
| Duration of postoperative mechanical ventilation, h | 70 (25–96) |
| Length of stay in intensive care unit, days | 5 (3–9) |
| Length of stay in hospital, days | 13 (10–17) |
| Adverse events | |
| None, n | 21 (54) |
| Asystole / cardiopulmonary resuscitation, n | 1 (3) |
| AVSD revision surgery, n | 1 (3) |
| Intubation due to respiratory failure, n | 1 (3) |
| Necrotizing enterocolitis, n | 1 (3) |
| Persistent low cardiac output syndrome, n | 1 (3) |
| Pulmonary bleeding, n | 2 (5) |
| Pleural/pericardial effusion, n | 5 (13) |
| Arrhythmias, n | 13 (33) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bamberg, M.; Simon, M.; Bandini, A.; Hahn, J.K.; Schlensak, C.; Icheva, V.; Hofbeck, M.; Rosenberger, P.; Magunia, H.; Keller, M. Left Atrioventricular Transvalvular Pressure Gradients Derived from Intraoperative and Postoperative Echocardiograms following Atrioventricular Septal Defect Repair. Diagnostics 2023, 13, 957. https://doi.org/10.3390/diagnostics13050957
Bamberg M, Simon M, Bandini A, Hahn JK, Schlensak C, Icheva V, Hofbeck M, Rosenberger P, Magunia H, Keller M. Left Atrioventricular Transvalvular Pressure Gradients Derived from Intraoperative and Postoperative Echocardiograms following Atrioventricular Septal Defect Repair. Diagnostics. 2023; 13(5):957. https://doi.org/10.3390/diagnostics13050957
Chicago/Turabian StyleBamberg, Maximilian, Mark Simon, Andrea Bandini, Julia Kelley Hahn, Christian Schlensak, Vanya Icheva, Michael Hofbeck, Peter Rosenberger, Harry Magunia, and Marius Keller. 2023. "Left Atrioventricular Transvalvular Pressure Gradients Derived from Intraoperative and Postoperative Echocardiograms following Atrioventricular Septal Defect Repair" Diagnostics 13, no. 5: 957. https://doi.org/10.3390/diagnostics13050957
APA StyleBamberg, M., Simon, M., Bandini, A., Hahn, J. K., Schlensak, C., Icheva, V., Hofbeck, M., Rosenberger, P., Magunia, H., & Keller, M. (2023). Left Atrioventricular Transvalvular Pressure Gradients Derived from Intraoperative and Postoperative Echocardiograms following Atrioventricular Septal Defect Repair. Diagnostics, 13(5), 957. https://doi.org/10.3390/diagnostics13050957







