Quantitative Ultrasound Techniques Used for Peripheral Nerve Assessment
Abstract
1. Introduction
2. Materials and Methods
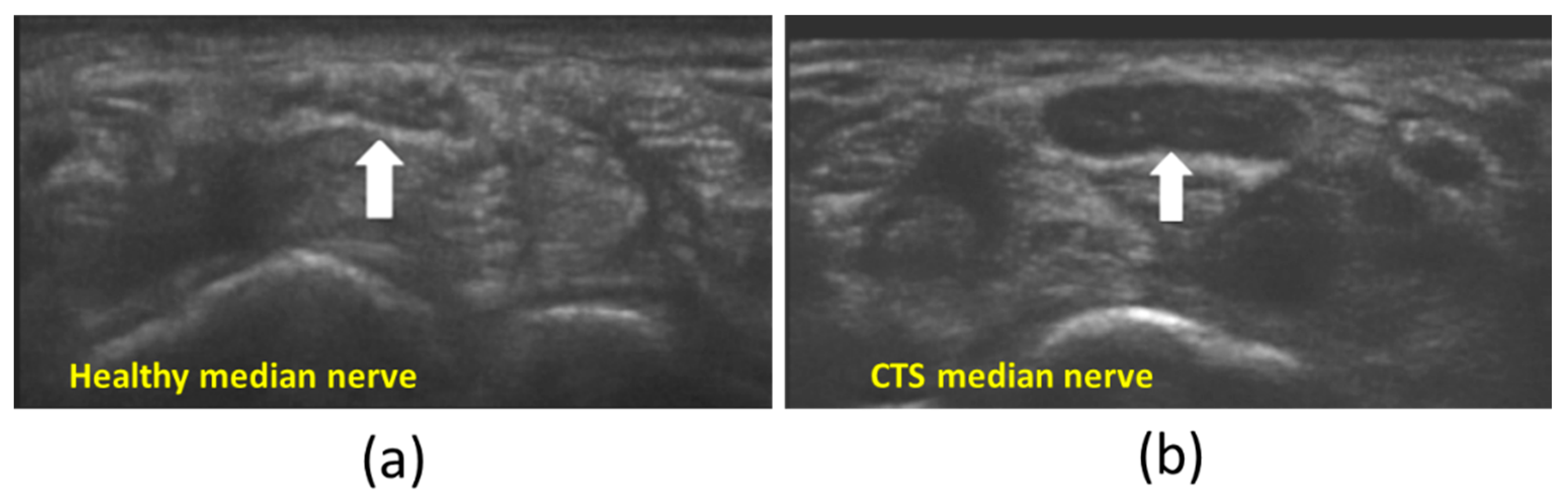
3. B-Mode Echogenicity Measurement
4. US Elastography
4.1. Strain Ultrasonography
Strain Ultrasonography Applied to Peripheral Nerves
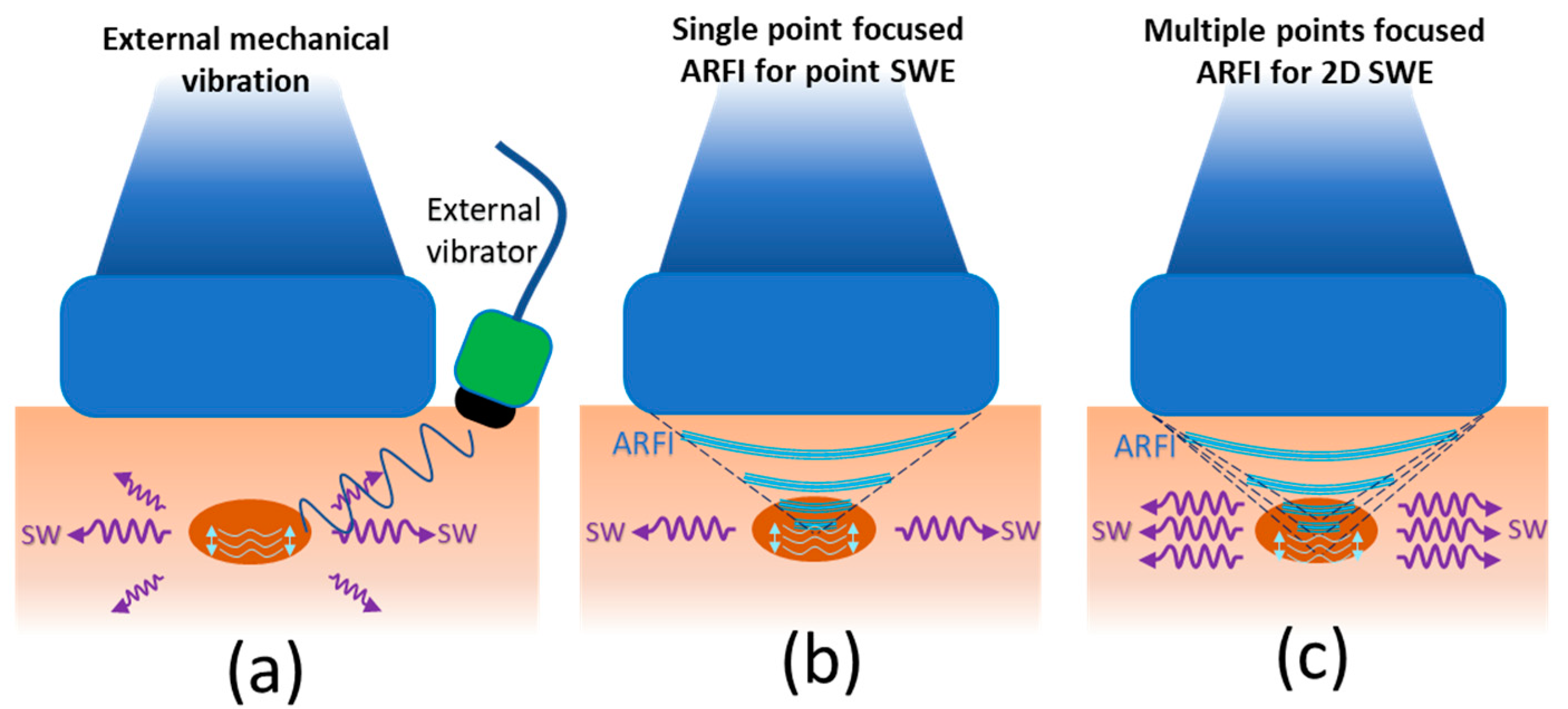
4.2. Shear Wave Elastography (SWE)
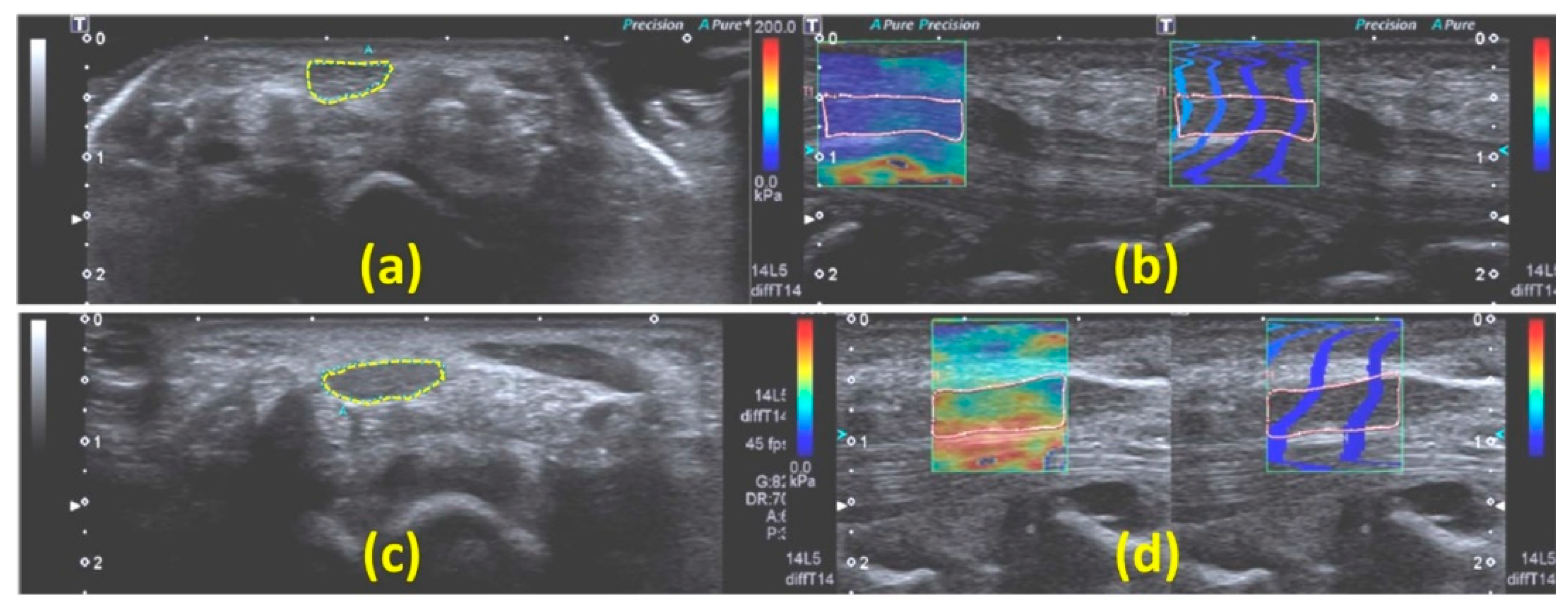
SWE Applied to Peripheral Nerves
Considerations for Evaluating Peripheral Nerves with SWE
5. Backscattered Radiofrequency (RF) Signal Characterization
6. QUS Limitations in Nerve Evaluation
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Wee, T.C.; Simon, N.G. Ultrasound Elastography for the Evaluation of Peripheral Nerves: A Systematic Review. Muscle Nerve 2019, 60, 501–512. [Google Scholar] [CrossRef]
- Shiina, T.; Nightingale, K.R.; Palmeri, M.L.; Hall, T.J.; Bamber, J.C.; Barr, R.G.; Castera, L.; Choi, B.I.; Chou, Y.H.; Cosgrove, D.; et al. WFUMB Guidelines and Recommendations for Clinical Use of Ultrasound Elastography: Part 1: Basic Principles and Terminology. Ultrasound Med. Biol. 2015, 41, 1126–1147. [Google Scholar] [CrossRef]
- Pelosi, L.; Arányi, Z.; Beekman, R.; Bland, J.; Coraci, D.; Hobson-Webb, L.D.; Padua, L.; Podnar, S.; Simon, N.; van Alfen, N.; et al. Expert Consensus on the Combined Investigation of Ulnar Neuropathy at the Elbow Using Electrodiagnostic Tests and Nerve Ultrasound. Clin. Neurophysiol. 2021, 132, 2274–2281. [Google Scholar] [CrossRef]
- Allen, K.W.; Moake, M.M. Ultrasound-Guided Paravenous Saphenous Nerve Block for Lower Extremity Abscess Incision and Drainage in a Male Adolescent. Pediatr. Emerg. Care 2022, 1, 1–10. [Google Scholar] [CrossRef]
- Vlassakov, K.V.; Sala-Blanch, X. Ultrasound of the Peripheral Nerves. Nerves Nerve Inj. 2015, 1, 227–250. [Google Scholar] [CrossRef]
- Jacobson, J.A.; Wilson, T.J.; Yang, L.J.S. Sonography of Common Peripheral Nerve Disorders with Clinical Correlation. J. Ultrasound Med. 2016, 35, 683–693. [Google Scholar] [CrossRef]
- Suk, J.I.; Walker, F.O.; Cartwright, M.S. Ultrasonography of Peripheral Nerves. Curr. Neurol. Neurosci. Rep. 2013, 13, 328. [Google Scholar] [CrossRef] [PubMed]
- VanHorn, T.A.; Cartwright, M.S. Neuromuscular Ultrasound in the Pediatric Population. Diagnostics 2020, 10, 1012. [Google Scholar] [CrossRef]
- Tsai, T.-Y.; Cheong, K.M.; Su, Y.-C.; Shih, M.-C.; Chau, S.W.; Chen, M.-W.; Chen, C.-T.; Lee, Y.-K.; Sun, J.-T.; Chen, K.-F.; et al. Ultrasound-Guided Femoral Nerve Block in Geriatric Patients with Hip Fracture in the Emergency Department. J. Clin. Med. 2022, 11, 2778. [Google Scholar] [CrossRef]
- Can, B.; Kara, M.; Kara, Ö.; Ülger, Z.; Frontera, W.R.; Özçakar, L. The Value of Musculoskeletal Ultrasound in Geriatric Care and Rehabilitation. Int. J. Rehabil. Res. 2017, 40, 285–296. [Google Scholar] [CrossRef]
- Carovac, A.; Smajlovic, F.; Junuzovic, D. Application of Ultrasound in Medicine. Acta Inform. Med. 2011, 19, 168. [Google Scholar] [CrossRef] [PubMed]
- Mamou, J.; Oelze, M.L. Quantitative Ultrasound in Soft Tissues; Springer: Berlin/Heidelberg, Germany, 2013; ISBN 9789400769519. [Google Scholar]
- Levy, J.; Barrett, D.L.; Harris, N.; Jeong, J.J.; Yang, X.; Chen, S.C. High-Frequency Ultrasound in Clinical Dermatology: A Review. Ultrasound J. 2021, 13, 24. [Google Scholar] [CrossRef] [PubMed]
- Moran, C.M.; Thomson, A.J.W. Preclinical Ultrasound Imaging—A Review of Techniques and Imaging Applications. Front. Phys. 2020, 8. [Google Scholar] [CrossRef]
- Oglat, A.; Matjafri, M.; Suardi, N.; Oqlat, M.; Abdelrahman, M.; Oqlat, A. A Review of Medical Doppler Ultrasonography of Blood Flow in General and Especially in Common Carotid Artery. J. Med. Ultrasound 2018, 26, 3. [Google Scholar] [CrossRef]
- Chen, H.-C.; Kadono, T.; Mimura, Y.; Saeki, H.; Tamaki, K. High-Frequency Ultrasound as a Useful Device in the Preliminary Differentiation of Lichen Sclerosus et Atrophicus from Morphea. J. Dermatol. 2004, 31, 556–559. [Google Scholar] [CrossRef]
- Izzetti, R.; Oranges, T.; Janowska, A.; Gabriele, M.; Graziani, F.; Romanelli, M. The Application of Ultra-High-Frequency Ultrasound in Dermatology and Wound Management. Int. J. Low. Extrem. Wounds 2020, 19, 334–340. [Google Scholar] [CrossRef]
- Izzetti, R.; Vitali, S.; Aringhieri, G.; Nisi, M.; Oranges, T.; Dini, V.; Ferro, F.; Baldini, C.; Romanelli, M.; Caramella, D.; et al. Ultra-High Frequency Ultrasound, A Promising Diagnostic Technique: Review of the Literature and Single-Center Experience. Can. Assoc. Radiol. J. 2021, 72, 418–431. [Google Scholar] [CrossRef]
- Shung, K.K. High Frequency Ultrasonic Imaging. J. Med. Ultrasound 2009, 17, 25–30. [Google Scholar] [CrossRef]
- Wortsman, X. Ultrasound in dermatology: Why, how, and when? Semin. Ultrasound CT MRI 2013, 34, 177–195. [Google Scholar] [CrossRef]
- Byra, M.; Wan, L.; Wong, J.H.; Du, J.; Shah, S.B.; Andre, M.P.; Chang, E.Y. Quantitative Ultrasound and B-Mode Image Texture Features Correlate with Collagen and Myelin Content in Human Ulnar Nerve Fascicles. Ultrasound Med. Biol. 2019, 45, 1830–1840. [Google Scholar] [CrossRef]
- Bignotti, B.; Ghio, M.; Panico, N.; Tagliafico, G.; Martinoli, C.; Tagliafico, A. High-Resolution Ultrasound of Peripheral Nerves in Systemic Sclerosis: A Pilot Study of Computer-Aided Quantitative Assessment of Nerve Density. Skelet. Radiol. 2015, 44, 1761–1767. [Google Scholar] [CrossRef]
- Coleman, D.J.; Silverman, R.H.; Rondeau, M.J.; Lloyd, H.O.; Daly, S. Explaining the Current Role of High Frequency Ultrasound in Ophthalmic Diagnosis (Ophthalmic Ultrasound). Expert Rev. Ophthalmol. 2006, 1, 63–76. [Google Scholar] [CrossRef] [PubMed]
- Byra, M.; Hentzen, E.; Du, J.; Andre, M.; Chang, E.Y.; Shah, S. Assessing the Performance of Morphologic and Echogenic Features in Median Nerve Ultrasound for Carpal Tunnel Syndrome Diagnosis. J. Ultrasound Med. 2020, 39, 1165–1174. [Google Scholar] [CrossRef] [PubMed]
- Fu, T.; Cao, M.; Liu, F.; Zhu, J.; Ye, D.; Feng, X.; Xu, Y.; Wang, G.; Bai, Y. Carpal Tunnel Syndrome Assessment with Ultrasonography: Value of Inlet-to-Outlet Median Nerve Area Ratio in Patients versus Healthy Volunteers. PLoS ONE 2015, 10, e0116777. [Google Scholar] [CrossRef] [PubMed]
- Li, G.Y.; Cao, Y. Mechanics of Ultrasound Elastography. Proc. R. Soc. A Math. Phys. Eng. Sci. 2017, 473, 20160841. [Google Scholar] [CrossRef]
- Sigrist, R.M.S.; Liau, J.; Kaffas, A.E.; Chammas, M.C.; Willmann, J.K. Ultrasound Elastography: Review of Techniques and Clinical Applications. Therasonics 2017, 7, 1303–1328. [Google Scholar] [CrossRef] [PubMed]
- Oelze, M.L.; Mamou, J. Review of Quantitative Ultrasound: Envelope Statistics and Backscatter Coefficient Imaging and Contributions to Diagnostic Ultrasound. IEEE Trans. Ultrason. Ferroelectr. Freq. Control. 2016, 63, 336–351. [Google Scholar] [CrossRef]
- Tagliafico, A.; Tagliafico, G.; Martinoli, C. Nerve Density: A New Parameter to Evaluate Peripheral Nerve Pathology on Ultrasound. Preliminary Study. Ultrasound Med. Biol. 2010, 36, 1588–1593. [Google Scholar] [CrossRef]
- Boom, J.; Visser, L.H. Quantitative Assessment of Nerve Echogenicity: Comparison of Methods for Evaluating Nerve Echogenicity in Ulnar Neuropathy at the Elbow. Clin. Neurophysiol. 2012, 123, 1446–1453. [Google Scholar] [CrossRef]
- Simon, N.G.; Ralph, J.W.; Poncelet, A.N.; Engstrom, J.W.; Chin, C.; Kliot, M. A Comparison of Ultrasonographic and Electrophysiologic “inching” in Ulnar Neuropathy at the Elbow. Clin. Neurophysiol. 2015, 126, 391–398. [Google Scholar] [CrossRef]
- Palmeri, M.L.; Dahl, J.J.; Macleod, D.B.; Grant, S.A.; Nightingale, K.R. On the Feasibility of Imaging Peripheral Nerves Using Acoustic Radiation Force Impulse Imaging. Ultrason. Imaging 2009, 31, 172–182. [Google Scholar] [CrossRef]
- Orman, G.; Ozben, S.; Huseyinoglu, N.; Duymus, M.; Orman, K.G. Ultrasound Elastographic Evaluation in the Diagnosis of Carpal Tunnel Syndrome: Initial Findings. Ultrasound Med. Biol. 2013, 39, 1184–1189. [Google Scholar] [CrossRef] [PubMed]
- Miyamoto, H.; Halpern, E.J.; Kastlunger, M.; Gabl, M.; Arora, R.; Bellmann-Weiler, R.; Feuchtner, G.M.; Jaschke, W.R.; Klauser, A.S. Carpal Tunnel Syndrome Diagnosis by Means of Median Nerve Elasticity—Improved Diagnostic Accuracy of US with Sonoelastography. Radiology 2014, 270, 481–486. [Google Scholar] [CrossRef]
- Yoshii, Y.; Ishii, T.; Tanaka, T.; Tung, W.L.; Sakai, S. Detecting Median Nerve Strain Changes with Cyclic Compression Apparatus: A Comparison of Carpal Tunnel Syndrome Patients and Healthy Controls. Ultrasound Med. Biol. 2015, 41, 669–674. [Google Scholar] [CrossRef] [PubMed]
- Ghajarzadeh, M.; Dadgostar, M.; Sarraf, P.; Emami-Razavi, S.Z.; Miri, S.; Malek, M. Application of Ultrasound Elastography for Determining Carpal Tunnel Syndrome Severity. Jpn. J. Radiol. 2015, 33, 273–278. [Google Scholar] [CrossRef]
- Tatar, I.G.; Kurt, A.; Yavasoglu, N.G.; Hekimoglu, B. Carpal Tunnel Syndrome: Elastosonographic Strain Ratio and Crosssectional Area Evaluation for the Diagnosis and Disease Severity. Med. Ultrason. 2016, 18, 305–311. [Google Scholar] [CrossRef]
- Ishibashi, F.; Taniguchi, M.; Kojima, R.; Kawasaki, A.; Kosaka, A.; Uetake, H. Elasticity of the Tibial Nerve Assessed by Sonoelastography Was Reduced before the Development of Neuropathy and Further Deterioration Associated with the Severity of Neuropathy in Patients with Type 2 Diabetes. J. Diabetes Investig. 2016, 7, 404–412. [Google Scholar] [CrossRef] [PubMed]
- Kesikburun, S.; Adigüzel, E.; Kesikburun, B.; Yaşar, E. Sonoelastographic Assessment of the Median Nerve in the Longitudinal Plane for Carpal Tunnel Syndrome. PMR 2016, 8, 183–185. [Google Scholar] [CrossRef]
- Yoshii, Y.; Tung, W.L.; Ishii, T. Measurement of Median Nerve Strain and Applied Pressure for the Diagnosis of Carpal Tunnel Syndrome. Ultrasound Med. Biol. 2017, 43, 1205–1209. [Google Scholar] [CrossRef]
- Nogueira-Barbosa, M.H.; Lugão, H.B.; Gregio-Júnior, E.; Crema, M.D.; Kobayashi, M.T.T.; Frade, M.A.C.; Pavan, T.Z.; Carneiro, A.A.O. Ultrasound Elastography Assessment of the Median Nerve in Leprosy Patients. Muscle Nerve 2017, 56, 393–398. [Google Scholar] [CrossRef]
- Martin, M.J.; Cartwright, M.S. A Pilot Study of Strain Elastography in the Diagnosis of Carpal Tunnel Syndrome. J. Clin. Neurophysiol. 2017, 34, 114–118. [Google Scholar] [CrossRef]
- Tezcan, S.; Ozturk, F.U.; Uslu, N.; Nalbant, M.; Yemisci, O.U. Carpal Tunnel Syndrome Evaluation of the Effects of Low-Level Laser Therapy with Ultrasound Strain Imaging. J. Ultrasound Med. 2019, 38, 113–122. [Google Scholar] [CrossRef] [PubMed]
- Kantarci, F.; Ustabasioglu, F.E.; Delil, S.; Olgun, D.C.; Korkmazer, B.; Dikici, A.S.; Tutar, O.; Nalbantoglu, M.; Uzun, N.; Mihmanli, I. Median Nerve Stiffness Measurement by Shear Wave Elastography: A Potential Sonographic Method in the Diagnosis of Carpal Tunnel Syndrome. Eur. Radiol. 2014, 24, 434–440. [Google Scholar] [CrossRef] [PubMed]
- Andrade, R.J.; Nordez, A.; Hug, F.; Ates, F.; Coppieters, M.W.; Pezarat-Correia, P.; Freitas, S.R. Non-Invasive Assessment of Sciatic Nerve Stiffness during Human Ankle Motion Using Ultrasound Shear Wave Elastography. J. Biomech. 2016, 49, 326–331. [Google Scholar] [CrossRef] [PubMed]
- Inal, M.; Tan, S.; Demirkan, S.; Burulday, V.; Gündüz, Ö.; Örnek, K. Evaluation of Optic Nerve with Strain and Shear Wave Elastography in Patients with Behçet’s Disease and Healthy Subjects. Ultrasound Med. Biol. 2017, 43, 1348–1354. [Google Scholar] [CrossRef] [PubMed]
- Inal, M.; Tan, S.; Yumusak, M.E.; Sahan, M.H.; Alpua, M.; Örnek, K. Evaluation of the Optic Nerve Using Strain and Shear Wave Elastography in Patients with Multiple Sclerosis and Healthy Subjects. Med. Ultrason. 2017, 19, 39–44. [Google Scholar] [CrossRef] [PubMed]
- Dikici, A.S.; Ustabasioglu, F.E.; Delil, S.; Nalbantoglu, M.; Korkmaz, B.; Bakan, S.; Kula, O.; Uzun, N.; Mihmanli, I.; Kantarci, F. Evaluation of the Tibial Nerve with Shear-Wave Elastography: A Potential Sonographic Method for the Diagnosis of Diabetic Peripheral Neuropathy. Radiology 2017, 282, 494–501. [Google Scholar] [CrossRef]
- Bortolotto, C.; Turpini, E.; Felisaz, P.; Fresilli, D.; Fiorina, I.; Raciti, M.V.; Belloni, E.; Bottinelli, O.; Cantisani, V.; Calliada, F. Median Nerve Evaluation by Shear Wave Elastosonography: Impact of “Bone-Proximity” Hardening Artifacts and Inter-Observer Agreement. J. Ultrasound 2017, 20, 293–299. [Google Scholar] [CrossRef]
- Arslan, H.; Yavuz, A.; Ilgen, F.; Aycan, A.; Ozgokce, M.; Akdeniz, H.; Batur, A. The Efficiency of Acoustic Radiation Force Impulse (ARFI) Elastography in the Diagnosis and Staging of Carpal Tunnel Syndrome. J. Med. Ultrason. 2018, 45, 453–459. [Google Scholar] [CrossRef]
- Zhu, B.; Yan, F.; He, Y.; Wang, L.; Xiang, X.; Tang, Y.; Yang, Y.; Qiu, L. Evaluation of the Healthy Median Nerve Elasticity. Medicine 2018, 97, e12956. [Google Scholar] [CrossRef]
- Cingoz, M.; Kandemirli, S.G.; Alis, D.C.; Samanci, C.; Kandemirli, G.C.; Adatepe, N.U. Evaluation of Median Nerve by Shear Wave Elastography and Diffusion Tensor Imaging in Carpal Tunnel Syndrome. Eur. J. Radiol. 2018, 101, 59–64. [Google Scholar] [CrossRef]
- Bedewi, M.A.; Nissman, D.; Aldossary, N.M.; Maetani, T.H.; El Sharkawy, M.S.; Koura, H. Shear Wave Elastography of the Brachial Plexus Roots at the Interscalene Groove. Neurol. Res. 2018, 40, 805–810. [Google Scholar] [CrossRef]
- Aslan, H.; Analan, P.D. Effects of Chronic Flexed Wrist Posture on the Elasticity and Crosssectional Area of the Median Nerve at the Carpal Tunnel among Chronic Stroke Patients. Med. Ultrason. 2018, 20, 71–75. [Google Scholar] [CrossRef] [PubMed]
- Kültür, T.; Okumuş, M.; Inal, M.; Yalçın, S. Evaluation of the Brachial Plexus with Shear Wave Elastography after Radiotherapy for Breast Cancer. J. Ultrasound Med. 2018, 37, 2029–2035. [Google Scholar] [CrossRef] [PubMed]
- Paluch, Ł.; Noszczyk, B.; Nitek, Ż.; Walecki, J.; Osiak, K.; Pietruski, P. Shear-Wave Elastography: A New Potential Method to Diagnose Ulnar Neuropathy at the Elbow. Eur. Radiol. 2018, 28, 4932–4939. [Google Scholar] [CrossRef] [PubMed]
- Burulday, V.; Doğan, A.; Şahan, M.H.; Arıkan, Ş.; Güngüneş, A. Ultrasound Elastography of the Median Nerve in Patients with Acromegaly: A Case-Control Study. J. Ultrasound Med. 2018, 37, 2371–2377. [Google Scholar] [CrossRef] [PubMed]
- Neto, T.; Freitas, S.R.; Andrade, R.J.; Gomes, J.; Mendes, B.; Mendes, T.; Nordez, A.; Oliveira, R. Sciatic Nerve Stiffness Is Not Changed Immediately after a Slump Neurodynamics Technique. Muscle Ligaments Tendons J. 2019, 07, 583. [Google Scholar] [CrossRef]
- Neto, T.; Freitas, S.R.; Andrade, R.J.; Vaz, J.R.; Mendes, B.; Firmino, T.; Bruno, P.M.; Nordez, A.; Oliveira, R. Noninvasive Measurement of Sciatic Nerve Stiffness in Patients with Chronic Low Back Related Leg Pain Using Shear Wave Elastography. J. Ultrasound Med. 2019, 38, 157–164. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.; Huang, S.; Teng, H.; Wang, P.; Wu, M.; Zhou, X.; Xu, W.; Zhang, Q.; Ran, H. Diagnostic Performance of Two-Dimensional Shear Wave Elastography for Evaluating Tibial Nerve Stiffness in Patients with Diabetic Peripheral Neuropathy. Eur. Radiol. 2019, 29, 2167–2174. [Google Scholar] [CrossRef]
- He, Y.; Xiang, X.; Zhu, B.H.; Qiu, L. Shear Wave Elastography Evaluation of the Median and Tibial Nerve in Diabetic Peripheral Neuropathy. Quant. Imaging Med. Surg. 2019, 9, 273–282. [Google Scholar] [CrossRef]
- Aslan, M.; Aslan, A.; Emeksiz, H.C.; Candan, F.; Erdemli, S.; Tombul, T.; Gunaydın, G.D.; Kabaalioğlu, A. Assessment of Peripheral Nerves with Shear Wave Elastography in Type 1 Diabetic Adolescents Without Diabetic Peripheral Neuropathy. J. Ultrasound Med. 2019, 38, 1583–1596. [Google Scholar] [CrossRef]
- Şahan, M.H.; Doǧan, A.; İnal, M.; Alpua, M.; Asal, N. Evaluation of the Optic Nerve by Strain and Shear Wave Elastography in Patients with Migraine. J. Ultrasound Med. 2019, 38, 1153–1161. [Google Scholar] [CrossRef]
- Moran, L.; Royuela, A.; de Vargas, A.P.; Lopez, A.; Cepeda, Y.; Martinelli, G. Carpal Tunnel Syndrome: Diagnostic Usefulness of Ultrasound Measurement of the Median Nerve Area and Quantitative Elastographic Measurement of the Median Nerve Stiffness. J. Ultrasound Med. 2020, 39, 331–339. [Google Scholar] [CrossRef]
- Wei, M.; Ye, X. Feasibility of Point Shear Wave Elastography for Evaluating Diabetic Peripheral Neuropathy. J. Ultrasound Med. 2020, 39, 1135–1141. [Google Scholar] [CrossRef]
- Bedewi, M.A.; Elsifey, A.A.; Kotb, M.A.; Bediwy, A.M.; Ahmed, Y.M.; Swify, S.M.; Abodonya, A.M. Shear Wave Elastography of the Saphenous Nerve. Medicine 2020, 99, e22120. [Google Scholar] [CrossRef]
- Schrier, V.J.M.M.; Lin, J.; Gregory, A.; Thoreson, A.R.; Alizad, A.; Amadio, P.C.; Fatemi, M. Shear Wave Elastography of the Median Nerve: A Mechanical Study. Muscle Nerve 2020, 61, 826–833. [Google Scholar] [CrossRef]
- Rugel, C.L.; Franz, C.K.; Lee, S.S.M. Influence of Limb Position on Assessment of Nerve Mechanical Properties by Using Shear Wave Ultrasound Elastography. Muscle Nerve 2020, 61, 616–622. [Google Scholar] [CrossRef]
- Bruno, F.; Palumbo, P.; Arrigoni, F.; Mariani, S.; Aringhieri, G.; Carotti, M.; Natella, R.; Zappia, M.; Cipriani, P.; Giacomelli, R.; et al. Advanced Diagnostic Imaging and Intervention in Tendon Diseases. Acta Biomed. 2020, 91, 98–106. [Google Scholar] [CrossRef]
- Chianca, V.; Di Pietto, F.; Zappia, M.; Albano, D.; Messina, C.; Sconfienza, L.M. Musculoskeletal Ultrasound in the Emergency Department. Semin. Musculoskelet. Radiol. 2020, 24, 167–174. [Google Scholar] [CrossRef]
- Ophir, J.; Céspedes, I.; Ponnekanti, H.; Yazdi, Y.; Li, X. Elastography: A Quantitative Method for Imaging the Elasticity of Biological Tissues. Ultrason. Imaging 1991, 13, 111–134. [Google Scholar] [CrossRef]
- Eisenscher, A.; Schweg-Toffler, E.; Pelletier, G.; Jacquemard, P. Rhythmic Echographic Palpation. Echosismography. A New Technic of Differentiating Benign and Malignant Tumors by Ultrasonic Study of Tissue Elasticity. J. Radiol. 1983, 64 4, 255–261. [Google Scholar]
- Taljanovic, M.S.; Gimber, L.H.; Giles, W.B.; Latt, L.D.; Melville, D.M.; Gao, L.; Witte, R.S. Shear-Wave Elastography: Basic Physics and Musculoskeletal Applications. Imaging Phys. 2017, 37, 855–870. [Google Scholar] [CrossRef]
- Krouskop, T.A.; Dougherty, D.R.; Vinson, F.S. A Pulsed Doppler Ultrasonic System for Making Noninvasive Measurements of the Mechanical Properties of Soft Tissue. J. Rehabil. Res. Dev. 1987, 24, 1–8. [Google Scholar]
- Sugimoto, T.; Ueha, S.; Itoh, K. Tissue Hardness Measurement Using the Radiation Force of Focused Ultrasound. In Proceedings of the IEEE Symposium on Ultrasonics, Honolulu, HI, USA, 4–7 December 1990; Volume 3, pp. 1377–1380. [Google Scholar]
- Wright, T.W.; Glowczewskie, F.; Wheeler, D.; Miller, G.; Cowin, D. Excursion and Strain of the Median Nerve. J. Bone Jt. Surg. 1996, 78, 1897–1903. [Google Scholar] [CrossRef]
- Kubo, K.; Zhou, B.; Cheng, Y.S.; Yang, T.H.; Qiang, B.; An, K.N.; Moran, S.L.; Amadio, P.C.; Zhang, X.; Zhao, C. Ultrasound Elastography for Carpal Tunnel Pressure Measurement: A Cadaveric Validation Study. J. Orthop. Res. 2018, 36, 477–483. [Google Scholar] [CrossRef]
- Tsui, P.H.; Wan, Y.L. Effects of Fatty Infiltration of the Liver on the Shannon Entropy of Ultrasound Backscattered Signals. Entropy 2016, 18, 341. [Google Scholar] [CrossRef]
- Han, A.; O’Brien, W.D. Structure Function Estimated from Histological Tissue Sections. IEEE Trans. Ultrason. Ferroelectr. Freq. Control. 2016, 63, 1296–1305. [Google Scholar] [CrossRef]
- Shankar, P.M. A General Statistical Model for Ultrasonic Backscattering from Tissues. IEEE Trans. Ultrason. Ferroelectr. Freq. Control. 2000, 47, 727–736. [Google Scholar] [CrossRef]
- Lin, S.C.; Heba, E.; Wolfson, T.; Ang, B.; Gamst, A.; Han, A.; Erdman, J.W.; O’Brien, W.D.; Andre, M.P.; Sirlin, C.B.; et al. Noninvasive Diagnosis of Nonalcoholic Fatty Liver Disease Andquantification of Liver Fat Using a New Quantitative Ultrasound Technique. Clin. Gastroenterol. Hepatol. 2015, 13, 1337–1345.e6. [Google Scholar] [CrossRef]
- Byra, M.; Nowicki, A.; Wróblewska-Piotrzkowska, H.; Dobruch-Sobczak, K. Classification of Breast Lesions Using Segmented Quantitative Ultrasound Maps of Homodyned K Distribution Parameters. Med. Phys. 2016, 43, 5561. [Google Scholar] [CrossRef]
- Weng, W.C.; Tsui, P.H.; Lin, C.Y.C.W.; Lu, C.H.; Lin, C.Y.C.W.; Shieh, J.Y.; Lu, F.L.; Ee, T.W.; Wu, K.W.; Lee, W.T. Evaluation of Muscular Changes by Ultrasound Nakagami Imaging in Duchenne Muscular Dystrophy. Sci. Rep. 2017, 7, 4429. [Google Scholar] [CrossRef]
- Lin, Y.H.; Yang, T.H.; Wang, S.H.; Su, F.C. Quantitative Assessment of First Annular Pulley and Adjacent Tissues Using High-Frequency Ultrasound. Sensors 2017, 17, 107. [Google Scholar] [CrossRef]
- Piotrzkowska-Wroblewska, H.; Litniewski, J.; Szymanska, E.; Nowicki, A. Quantitative Sonography of Basal Cell Carcinoma. Ultrasound Med. Biol. 2015, 41, 748–759. [Google Scholar] [CrossRef]
- Rosso, G.; Guck, J. Mechanical Changes of Peripheral Nerve Tissue Microenvironment and Their Structural Basis during Development. APL Bioeng. 2019, 3, 36107. [Google Scholar] [CrossRef]




| Type of QUS | Study | Goal | Anatomy | Scanner | Probe Frequency | Findings |
|---|---|---|---|---|---|---|
| B-mode echogenicity measurement | Tagliafico et al., 2010 [29] | To investigate the feasibility of using US-based measure of the peripheral nerve density (hypoechoic fraction) in detecting CTS and neurofibromas. |
| iU-22, Philips (Eindhoven, The Netherlands) |
|
|
| Boom and Visser, 2012 [30] | To investigate the feasibility of using hypoechoic fraction obtained with various thresholding methods in detecting neuropathy. |
| Xario XG, Toshiba (Tokyo, Japan) |
|
| |
| Bignotti et al., 2015 [22] | To investigate the feasibility of using US nerve density index in evaluating patients with limited cutaneous systemic sclerosis (lcSSc). |
| iU-22, Philips (Eindhoven, The Netherlands) |
|
| |
| Simon et al., 2015 [31] | To study the relationship between US B-mode hypoechoic fraction and electrophysiologic measures of the peripheral nerve. |
| A Mindray M7 (Shenzen, China) |
|
| |
| Byra et al., 2020 [21] | To investigate correlations between collagen/myelin content (histology) of nerves with a US-based texture feature, gray level co-occurrence matrix (GLCM). |
| Vevo MD, FUJIFILM (Toronto, Canada) |
|
| |
| Byra et al., 2020 [24] | To investigate the feasibility of using the nerve–tissue contrast index (NTI) method in detecting CTS, by considering echogenicity differences in the surrounding tissue. |
| Vevo MD, FUJIFILM (Toronto, Canada) |
|
| |
| Strain ultrasonography | Palmeri et al., 2009 [32] | To investigate nerves’ contrast improvement of strain US vs. B-mode US. |
| SONOLINE Antares, Siemens (Erlangen Germany) |
|
|
| Orman et al., 2013 [33] | To investigate the potential of strain US (pure strain) free-hand compression in detecting CTS. |
| Aplio XG, SSA 790A, Toshiba(Nasushiobara, Japan) |
|
| |
| Miyamoto et al., 2014 [34] | To investigate the capability of free-hand compression strain US technique (strain ratio (SR) with respect to acoustic coupler rubber, ACR) in detecting CTS. |
| HI VISION Preirus Hitachi-Aloka Medical (Tokyo, Japan) |
|
| |
| Yoshii et al., 2015 [35] | To investigate the capability of strain US technique with a cyclic compression apparatus (strain and SR with respect to ACR) in detecting CTS. |
| HI VISION Avius, Hitachi Aloka Medical (Tokyo, Japan) |
|
| |
| Ghajarzadeh et al., 2015 [36] | To investigate the capability of free-hand compression strain US technique (blue and red pixel counts in strain image) in detecting CTS severity. |
| MYLAB 70 XVG, Esaote (Genoa, Italy) |
|
| |
| Tatar et al., 2016 [37] | To investigate the capability of free-hand compression strain ultrasonography (SR and strain difference between two nerve sections) in detecting CTS and its severity. |
| MYLAB 60, Esaote (Genoa, Italy) |
|
| |
| Strain ultrasonography—Continued | Ishibashi et al., 2016 [38] | To investigate the capability of free-hand compression strain US (SR ratio nerve/ACR) in detecting neuropathy in type 2 diabetes patients. |
| HI VISION Ascendus, Hitachi Medical (Tokyo, Japan) |
|
|
| Kesikburun et al., 2016 [39] | To investigate the capability of free-hand strain US technique (using SR) in detecting CTS. |
| GE LOGIQ S7, GE Healthcare (Yizhuang, China) |
|
| |
| Yoshii et al., 2017 [40] | To investigate the capability of strain US technique with a cyclic compression apparatus (strain, AC/nerve SR, strain/applied pressure ratio) in detecting CTS |
| HI VISION Avius, Hitachi Aloka Medical (Tokyo, Japan) |
|
| |
| Nogueira-Barbosa et al., 2017 [41] | To investigate the feasibility of employing the free-hand strain US technique (using SR) in detecting leprosy. |
| SonixRP, Ultrasonix (Richmond, Canada) |
|
| |
| Martin and Cartwright, 2017 [42] | To investigate the feasibility of employing the ambient strain US technique (SR was used) in detecting CTS. |
| iU-22, Philips (Eindhoven, The Netherlands) |
|
| |
| Tezcan et al., 2019 [43] | To investigate the feasibility of employing the strain US technique (SR) in evaluating low-level laser therapy on CTS patients. |
| ACUSON S3000, Siemens (Erlangen, Germany) |
|
| |
| Shear wave elastography (SWE) | Kantarci et al., 2014 [44] | To investigate the potential of SWE (stiffness) with ARFI push pulses in detecting CTS. |
| Aixplorer; SuperSonic Imagine (Les Jardins de la Duranne, France) |
|
|
| Andrade et al., 2016 [45] | To employ SWE (shear wave velocity, SWV) to detect the changes in sciatic nerve stiffness during human ankle motion. |
| Aixplorer; SuperSonic Imagine (Les Jardins de la Duranne, France) |
|
| |
| Inal et al., 2017 [46] | To investigate the feasibility of optic nerve evaluations with free-hand compression strain US and SWE in Behcet’s patients. |
| LOGIQ E9, GE Healthcare (Wauwatosa, USA) |
|
| |
| Inal et al., 2017 [47] | To investigate the feasibility of optic nerve evaluations with free-hand compression strain US and SWE (stiffness) in patients with MS. |
| LOGIQ E9, GE Healthcare (Wauwatosa, USA) |
|
| |
| Dikici et al., 2017 [48] | To investigate the feasibility of using SWE (stiffness) for the diagnosis of diabetic peripheral neuropathy. |
| Aixplorer; SuperSonic Imagine (Les Jardins de la Duranne, France) |
|
| |
| Bortolotto et al., 2017 [49] | To investigate the “bone-proximity” hardening artifacts affecting SWE. |
| Aplio 500, Toshiba (Tokyo, Japan) |
|
| |
| Arslan et al., 2018 [50] | To examine the efficiency of SWE (stiffness) in CTS detection and determining CTS severity level. |
| ACUSON S2000, Siemens (Mountain View, USA) |
|
| |
| Zhu et al., 2018 [51] | To examine the nerve tension impacts on the SWE results (SWS) at different nerve sections. |
| Aixplorer; SuperSonic Imagine (Les Jardins de la Duranne, France) |
|
| |
| Cingoz et al., 2018 [52] | To compare the SWE evaluation of nerves with MRI diffusion. |
| Aplio 500, Toshiba (Tokyo, Japan) |
|
| |
| Bedewi et al., 2018 [53] | To investigate the feasibility of SWE (stiffness) in evaluating the brachial plexus nerves. |
| Aixplorer; SuperSonic Imagine (Les Jardins de la Duranne, France) |
|
| |
| Aslan and Analan, 2018 [54] | To study the effects of chronic flexed wrist posture among chronic stroke patients using SWE (SWS). |
| ACUSON S2000, Siemens (Erlangen, Germany |
|
| |
| Kültür et al., 2018 [55] | To evaluate the brachial plexus after radiotherapy for breast cancer using SWE (stiffness). |
| LOGIQ E9, GE (Waukesha, USA) |
|
| |
| Shear wave elastography (SWE)—Continued | Paluch et al., 2018 [56] | To investigate the feasibility of the ulnar neuropathy diagnosis using SWE (stiffness). |
| iAplio 900, Canon (Tokyo, Japan) |
|
|
| Burulday et al., 2018 [57] | To evaluate the median nerve of patients with acromegaly using SWE. |
| LOGIQ E9, GE (Waukesha, USA) |
|
| |
| Neto et al., 2019 [58] | To investigate the immediate impact of slump neurodynamics technique on sciatic nerve stiffness using SWE. |
| Aixplorer; SuperSonic Imagine (Les Jardins de la Duranne, France) |
|
| |
| Tiago Neto et al., 2019 [59]. | To investigate the impact of chronic lower-back-related pain in legs on sciatic nerve stiffness using SWE. |
| Aixplorer; SuperSonic Imagine (Les Jardins de la Duranne, France) |
|
| |
| Jiang et al., 2019 [60] | To investigate the feasibility of using SWE (stiffness) for the diagnosis of diabetic peripheral neuropathy (DPN). |
| Aixplorer; SuperSonic Imagine (Les Jardins de la Duranne, France) |
|
| |
| He et al., 2019 [61] | To evaluate patients with diabetic peripheral neuropathy using SWE (stiffness). |
| Aixplorer; SuperSonic Imagine (Les Jardins de la Duranne, France). |
|
| |
| Shear wave elastography (SWE)—Continued | Aslan et al., 2019 [62] | To evaluate adolescent patients with type-I diabetic without peripheral neuropathy using SWE (stiffness). |
| Resona 7, Mindray (Shenzhen, China) |
|
|
| Şahan et al., 2019 [63] | To investigate the feasibility of optic nerve evaluations with free-hand compression strain US and SWE (stiffness) in patients with migraine. |
| LOGIQ E9, GE Healthcare (Wauwatosa, USA) |
|
| |
| Moran et al., 2020 [64] | To examine the efficiency of SWE (stiffness) compared with CSA changes in CTS detection and determining CTS severity level. |
| Aplio 500, Toshiba (Tokyo, Japan) |
|
| |
| Wei and Ye, 2020 [65] | To evaluate patients with diabetic peripheral neuropathy using SWE (stiffness). |
| ACUSON S2000, Siemens (Erlangen, Germany) |
|
| |
| Shear wave elastography (SWE)—Continued | Bedewi et al., 2020 [66] | To investigate the feasibility of SWE (stiffness) to evaluate the saphenous nerves. |
| Aixplorer, SuperSonic Imagine (Les Jardins de la Duranne, France) |
|
|
| Schrier et al., 2020 [67] | To examine the SWE sensitivity to increasing tensile loading on cadaver nerves at different nerve sections. |
| LOGIQ E9, GE (Waukesha, USA) |
|
| |
| Rugel et al., 2020 [68] | To study the limb position impact on the SWE (SWS). |
| Aixplorer, SuperSonic Imagine (Les Jardins de la Duranne, France) |
|
| |
| Backscattered radiofrequency (RF) signal characterization | Byra et al., 2019 [21] | To investigate correlations between collagen/myelin content (histology) of nerves with back backscatter coefficient (BSC), attenuation coefficient (AC), Nakagami parameter, and entropy. |
| Vevo MD, FUJIFILM (Toronto, Canada) |
|
|
| Mechanical Parameters | Definition | Formula | Schematics |
|---|---|---|---|
| Normal stress | The magnitude of the force applied to the unit area perpendicular to the force direction | 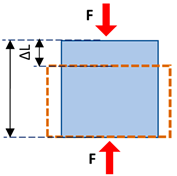 | |
| Normal strain | The change in length of the tissue per its unit length parallel to the force direction | ||
| Young’s modulus (i.e., normal modulus) | Elasticity defined in applied force direction but perpendicular to the unit volume surface | ||
| Shear stress | The magnitude of the force applied to the unit area parallel to the force direction | 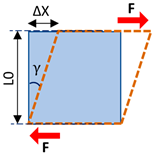 | |
| Shear strain | The angular change in originally right angles of the unit volume after shear stress application | ||
| Shear modulus | Elasticity defined in applied force direction and parallel to the unit volume surface | ||
| Bulk modulus | Elasticity defined over the unit of volume when a uniform volumetric stress is applied, and a uniform strain is induced | 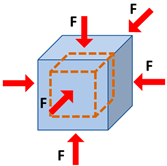 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jerban, S.; Barrère, V.; Andre, M.; Chang, E.Y.; Shah, S.B. Quantitative Ultrasound Techniques Used for Peripheral Nerve Assessment. Diagnostics 2023, 13, 956. https://doi.org/10.3390/diagnostics13050956
Jerban S, Barrère V, Andre M, Chang EY, Shah SB. Quantitative Ultrasound Techniques Used for Peripheral Nerve Assessment. Diagnostics. 2023; 13(5):956. https://doi.org/10.3390/diagnostics13050956
Chicago/Turabian StyleJerban, Saeed, Victor Barrère, Michael Andre, Eric Y. Chang, and Sameer B. Shah. 2023. "Quantitative Ultrasound Techniques Used for Peripheral Nerve Assessment" Diagnostics 13, no. 5: 956. https://doi.org/10.3390/diagnostics13050956
APA StyleJerban, S., Barrère, V., Andre, M., Chang, E. Y., & Shah, S. B. (2023). Quantitative Ultrasound Techniques Used for Peripheral Nerve Assessment. Diagnostics, 13(5), 956. https://doi.org/10.3390/diagnostics13050956





