Quantification of Pulmonary Artery Configuration after the Arterial Switch Operation: A Pilot Study
Abstract
1. Introduction
2. Methods
2.1. Overview
2.2. Surgical Technique
2.3. Patient Cohort
2.4. CMR Measurements
2.5. Parameters (Figure 1)
- (1)
- Neo-pulmonary to neo-aortic angle
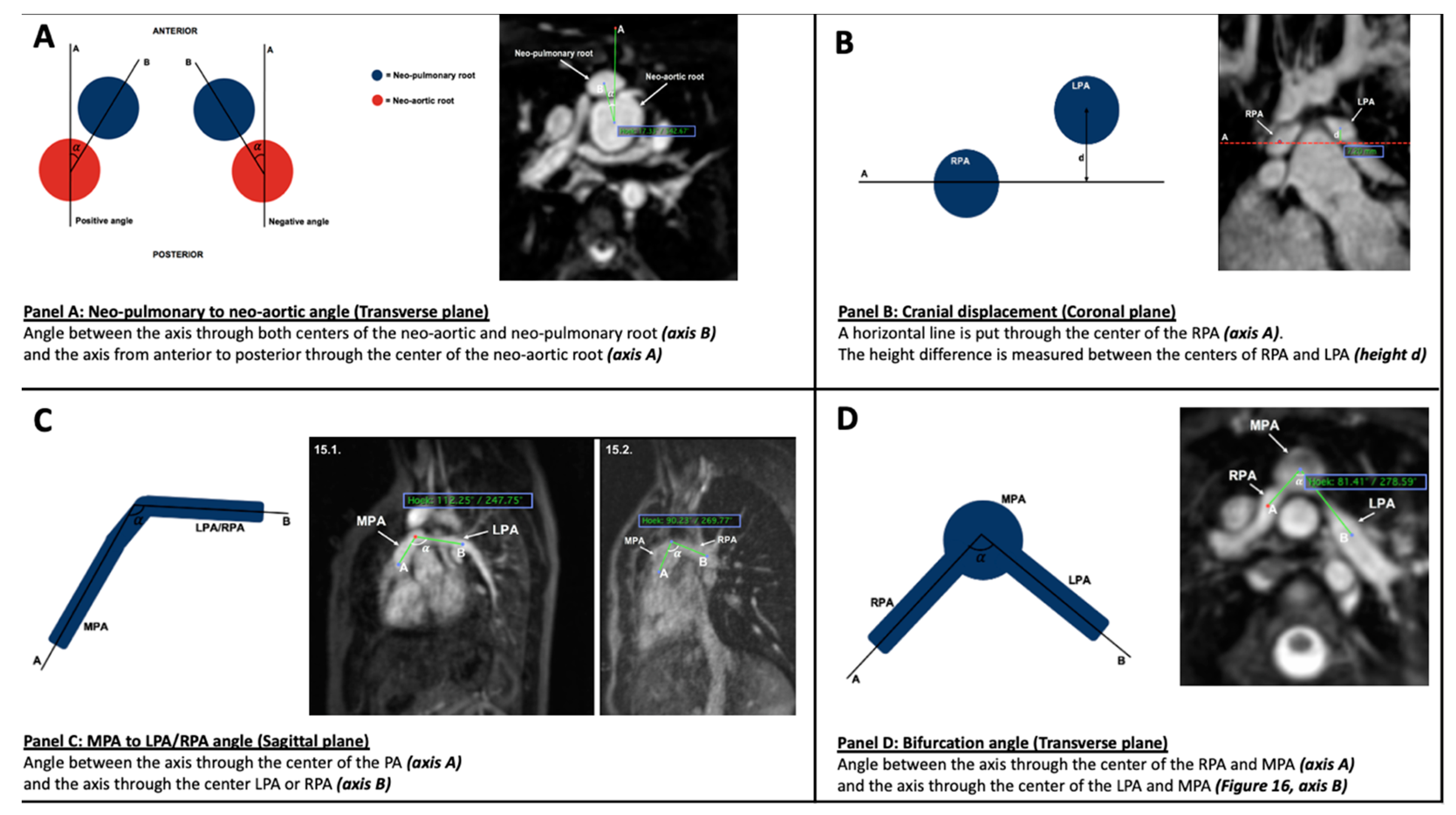
- (2)
- Cranial displacement
- (3)
- MPA to left pulmonary artery (LPA) and right pulmonary artery (RPA) angle
- (4)
- Bifurcation angle
2.6. Statistical Analysis
3. Results
3.1. Patients
CMR
4. Discussion
5. Conclusions
Future Research
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fricke, T.A.; D’Udekem, Y.; Richardson, M.; Thuys, C.; Dronavalli, M.; Ramsay, J.M.; Wheaton, G.; Grigg, L.E.; Brizard, C.P.; Konstantinov, I.E. Outcomes of the arterial switch operation for transposition of the great arteries: 25 years of experience. Ann. Thorac. Surg. 2012, 94, 139–145. [Google Scholar] [CrossRef] [PubMed]
- van der Palen, R.L.F.; van der Bom, T.; Dekker, A.; Tsonaka, R.; van Geloven, N.; Kuipers, I.M.; Konings, T.C.; Rammeloo, L.A.J.; Ten Harkel, A.D.J.; Jongbloed, M.R.M.; et al. Progression of aortic root dilatation and aortic valve regurgitation after the arterial switch operation. Heart 2019, 105, 1732–1740. [Google Scholar] [CrossRef] [PubMed]
- Fricke, T.A.; Buratto, E.; Weintraub, R.G.; Bullock, A.; Wheaton, G.; Grigg, L.; Disney, P.; D’Udekem, Y.; Brizard, C.P.; Konstantinov, I.E. Long-term outcomes of the arterial switch operation. J. Thorac. Cardiovasc. Surg. 2022, 163, 212–219. [Google Scholar] [CrossRef] [PubMed]
- Losay, J.; Touchot, A.; Serraf, A.; Litvinova, A.; Lambert, V.; Piot, J.; Lacour-Gayet, F.; Capderou, A.; Planche, C. Late outcome after arterial switch operation for transposition of the great arteries. Circulation 2001, 104, 121–126. [Google Scholar] [CrossRef]
- Santens, B.; Van De Bruaene, A.; De Meester, P.; Gewillig, M.; Troost, E.; Claus, P.; Bogaert, J.; Budts, W. Outcome of arterial switch operation for transposition of the great arteries. A 35-year follow-up study. Int. J. Cardiol. 2020, 316, 94–100. [Google Scholar] [CrossRef]
- Bex, J.P.; Lecompte, Y.; Baillot, F.; Hazan, E. Anatomical correction of transposition of the great arteries. Ann. Thorac. Surg. 1980, 29, 86–88. [Google Scholar] [CrossRef]
- Lang, S.M.; Crawford, R.L.; Shivaram, P.; Daily, J.A.; Bolin, E.H.; Tang, X.; Collins, R.T. Feasibility of Transthoracic Echocardiography Evaluation of Pulmonary Arteries Following Arterial Switch Operation. Pediatr. Cardiol. 2018, 39, 1523–1529. [Google Scholar] [CrossRef]
- Baumgartner, H.; De Backer, J.; Babu-Narayan, S.V.; Budts, W.; Chessa, M.; Diller, G.-P.; Lung, B.; Kluin, J.; Lang, I.M.; Meijboom, F.; et al. 2020 ESC Guidelines for the management of adult congenital heart disease. Eur. Heart J. 2021, 42, 563–645. [Google Scholar] [CrossRef]
- Stout, K.K.; Daniels, C.J.; Aboulhosn, J.A.; Bozkurt, B.; Broberg, C.S.; Colman, J.M.; Crumb, S.R.; Dearani, J.A.; Fuller, S.; Gurvitz, M.; et al. 2018 AHA/ACC Guideline for the Management of Adults with Congenital Heart Disease: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation 2019, 139, e698–e800. [Google Scholar]
- Morgan, C.T.; Mertens, L.; Grotenhuis, H.; Yoo, S.J.; Seed, M.; Grosse-Wortmann, L. Understanding the mechanism for branch pulmonary artery stenosis after the arterial switch operation for transposition of the great arteries. Eur. Heart J. Cardiovasc. Imaging 2017, 18, 180–185. [Google Scholar] [CrossRef]
- Hutter, P.A.; Kreb, D.L.; Mantel, S.F.; Hitchcock, J.F.; Meijboom, E.J.; Bennink, G.B. Twenty-five years’ experience with the arterial switch operation. J. Thorac. Cardiovasc. 2002, 124, 790–797. [Google Scholar] [CrossRef] [PubMed]
- Koolbergen, D.R.; Manshanden, J.S.J.; Yazdanbakhsh, A.P.; Bouma, B.; Blom, N.A.; De Mol, B.A.J.M.; Mulder, B.J.; Hazekamp, M.G. Reoperation for neoaortic root pathology after the arterial switch operation. Eur. J. Cardiothorac. 2014, 46, 474–479. [Google Scholar] [CrossRef] [PubMed]
- Moe, T.G.; Bardo, D.M.E. Long-term Outcomes of the Arterial Switch Operation for d-Transposition of the Great Arteries. Progr. Cardiovasc. Dis. 2018, 61, 360–364. [Google Scholar] [CrossRef]
- Delmo Walter, E.M.; Miera, O.; Nasseri, B.; Huebler, M.; Alexi-Meskishvili, V.; Berger, F.; Hetzer, R. Onset of pulmonary stenosis after arterial switch operation for transposition of great arteries with intact ventricular septum. HSR Proc. Intens. Care Cardiovasc. Anesth. 2011, 3, 177–187. [Google Scholar]
- AlKattan, H.N.; Diraneyya, O.M.; Elmontaser, H.A.; Jaweed, J.; Alsaiad, A.M.; Arifi, A.A.; Alghamdi, A.A. The behavior of residual pulmonary artery gradient after arterial switch operation: A longitudinal data analysis. J. Card. Surg. 2020, 35, 2927–2933. [Google Scholar] [CrossRef]
- Li, W.; West, C.; McGhie, J.; van den Bosch, A.E.; Babu-Narayan, S.V.; Meijboom, F.; Mongeon, F.P.; Khairy, P.; Kimball, T.R.; Beauchesne, L.M.; et al. Consensus recommendations for echocardiography in adults with congenital heart defects from the International Society of Adult Congenital Heart Disease (ISACHD). Int. J. Cardiol. 2018, 272, 77–83. [Google Scholar] [CrossRef]
- Broda, C.R.; Shugh, S.B.; Parikh, R.B.; Wang, Y.; Schlingmann, T.R.; Noel, C.V. Post-operative Assessment of the Arterial Switch Operation: A Comparison of Magnetic Resonance Imaging and Echocardiography. Pediatr. Cardiol. 2018, 39, 1036–1041. [Google Scholar] [CrossRef] [PubMed]
- Nellis, J.R.; Turek, J.W.; Aldoss, O.T.; Atkins, D.L.; Ng, B.Y. Intervention for Supravalvar Pulmonary Stenosis After the Arterial Switch Operation. Ann. Thorac. Surg. 2016, 102, 154–162. [Google Scholar] [CrossRef]
- Lee, J.; Abdullah Shahbah, D.; El-Said, H.; Rios, R.; Ratnayaka, K.; Moore, J. Pulmonary artery interventions after the arterial switch operation: Unique and significant risks. Congen. Heart Dis. 2019, 14, 288–296. [Google Scholar] [CrossRef]
- Bonello, B.; Kostolny, M.; Marek, J. Enhanced Echocardiography Imaging in Reoperation for Complex Congenital Heart Disease in a Child. CASE 2021, 5, 81–85. [Google Scholar] [CrossRef]
- Milano, E.G.; Pajaziti, E.; Sauvage, E.; Cook, A.; Schievano, S.; Mortensen, K.H.; Taylor, A.M.; Marek, J.; Kostolny, M.; Capelli, C. Taking Surgery Out of Reality. Circ. Cardiovasc. Imaging 2019, 12, e009297. [Google Scholar] [CrossRef] [PubMed]
- Belhadjer, Z.; Soulat, G.; Ladouceur, M.; Pitocco, F.; Legendre, A.; Bonnet, D.; Iserin, L.; Mousseaux, E. Neopulmonary Outflow Tract Obstruction Assessment by 4D Flow MRI in Adults with Transposition of the Great Arteries After Arterial Switch Operation. J. Magn. Reson. Imaging 2020, 51, 1699–1705. [Google Scholar] [CrossRef] [PubMed]

| All n = 38 Median (IQR) or % | All n = 38 Mean (SD) | PAS n = 21 Mean (SD) or % | No PAS n = 17 Mean (SD) or % | p-Value | |
|---|---|---|---|---|---|
| Age ASO (days)—SD | 11 (7–22) | 24 (47.6) | 27.2 (42.4) | 20.1 (18.2) | 0.1 |
| Age CMR (years)—SD | 10 (3.5–13) | 10 (5) | 6.5 (5.4) | 10.7 (3.3) | 0.03 |
| VSD (%) | 42.1 (16) | 43 | 41 | ||
| Septostomy (%) | 73.7 (28) | 67 | 82 |
| Overall Mean (SD) | Overall Median (IQR) | Mean (SD) PAS Group n = 21 | Mean (SD) Non-PAS Group n = 17 | p-Value | |
|---|---|---|---|---|---|
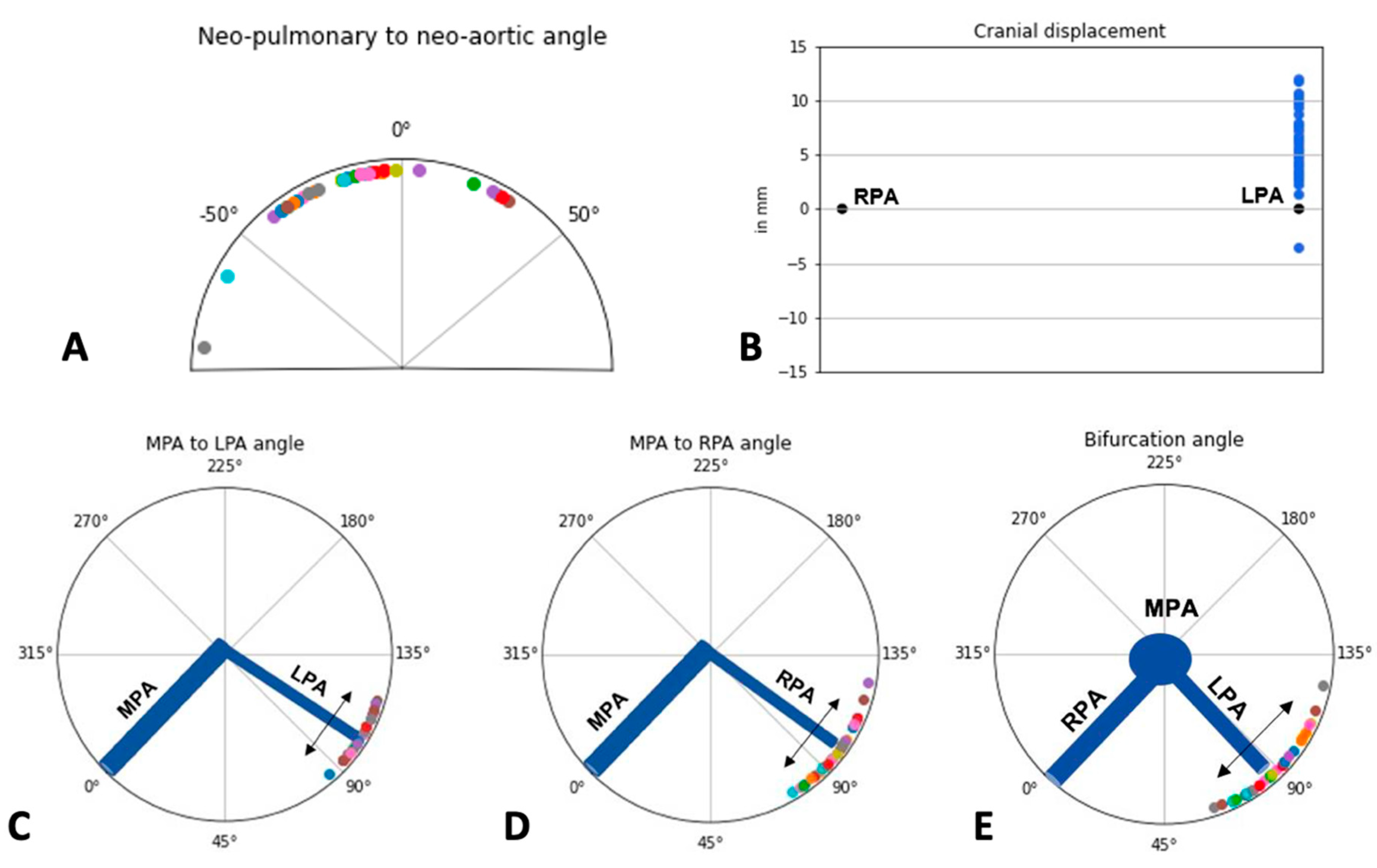
| Neo-pulmonary to Neo-aortic Angle (degrees) | −15.11 (23.77) | −14 (24.8) | −17.05 (22.39) | −12.44 (26.04) | 0.747 |
| Cranial Displacement (mm) | 6.19 (2.84) | 5.7 (4.2) | 5.41 (2.72) | 7.27 (2.72) | 0.048 |
| MPA-LPA (degrees) | 104.03 (8.19) | 103.5 (13.2) | 103.23 (8.57) | 105.13 (7.76) | 0.473 |
| MPA-RPA (degrees) | 97.61 (11.19) | 99.5 (14.5) | 97 (11.71) | 98.44 (10.76) | 0.737 |
| Bifurcation Angle (degrees) | 88.87 (14.21) | 77.8 (22) | 89.45 (15.64) | 88.06 (12.42) | 0.759 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martens, T.; Claeys, G.; De Groote, J.; Kanakis, M.; Kostolny, M.; Tsang, V.; Hughes, M. Quantification of Pulmonary Artery Configuration after the Arterial Switch Operation: A Pilot Study. Diagnostics 2022, 12, 2629. https://doi.org/10.3390/diagnostics12112629
Martens T, Claeys G, De Groote J, Kanakis M, Kostolny M, Tsang V, Hughes M. Quantification of Pulmonary Artery Configuration after the Arterial Switch Operation: A Pilot Study. Diagnostics. 2022; 12(11):2629. https://doi.org/10.3390/diagnostics12112629
Chicago/Turabian StyleMartens, Thomas, Gillian Claeys, Joachim De Groote, Meletios Kanakis, Martin Kostolny, Victor Tsang, and Marina Hughes. 2022. "Quantification of Pulmonary Artery Configuration after the Arterial Switch Operation: A Pilot Study" Diagnostics 12, no. 11: 2629. https://doi.org/10.3390/diagnostics12112629
APA StyleMartens, T., Claeys, G., De Groote, J., Kanakis, M., Kostolny, M., Tsang, V., & Hughes, M. (2022). Quantification of Pulmonary Artery Configuration after the Arterial Switch Operation: A Pilot Study. Diagnostics, 12(11), 2629. https://doi.org/10.3390/diagnostics12112629






