Transvaginal Ultrasound Accuracy in the Hydrosalpinx Diagnosis: A Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Protocol and Registration
2.2. Data Sources and Search Process
2.3. Study Selection and Data Collection
- (1)
- Prospective and retrospective cohort studies, which include patients with a transvaginal ultrasound assessment and a suspect diagnosis of hydrosalpinx or other adnexal masses.
- (2)
- Definitive histologic diagnosis of the adnexal mass after surgical removal, including confirmative diagnosis of hydrosalpinx.
- (3)
- Reported data that would allow constructing a 2 × 2 table to estimate true positive, true negative, false positive, and false negative values for the sonographic diagnosis of hydrosalpinx.
2.4. Risk of Bias in Individual Studies
2.5. Statistical Analysis
3. Results
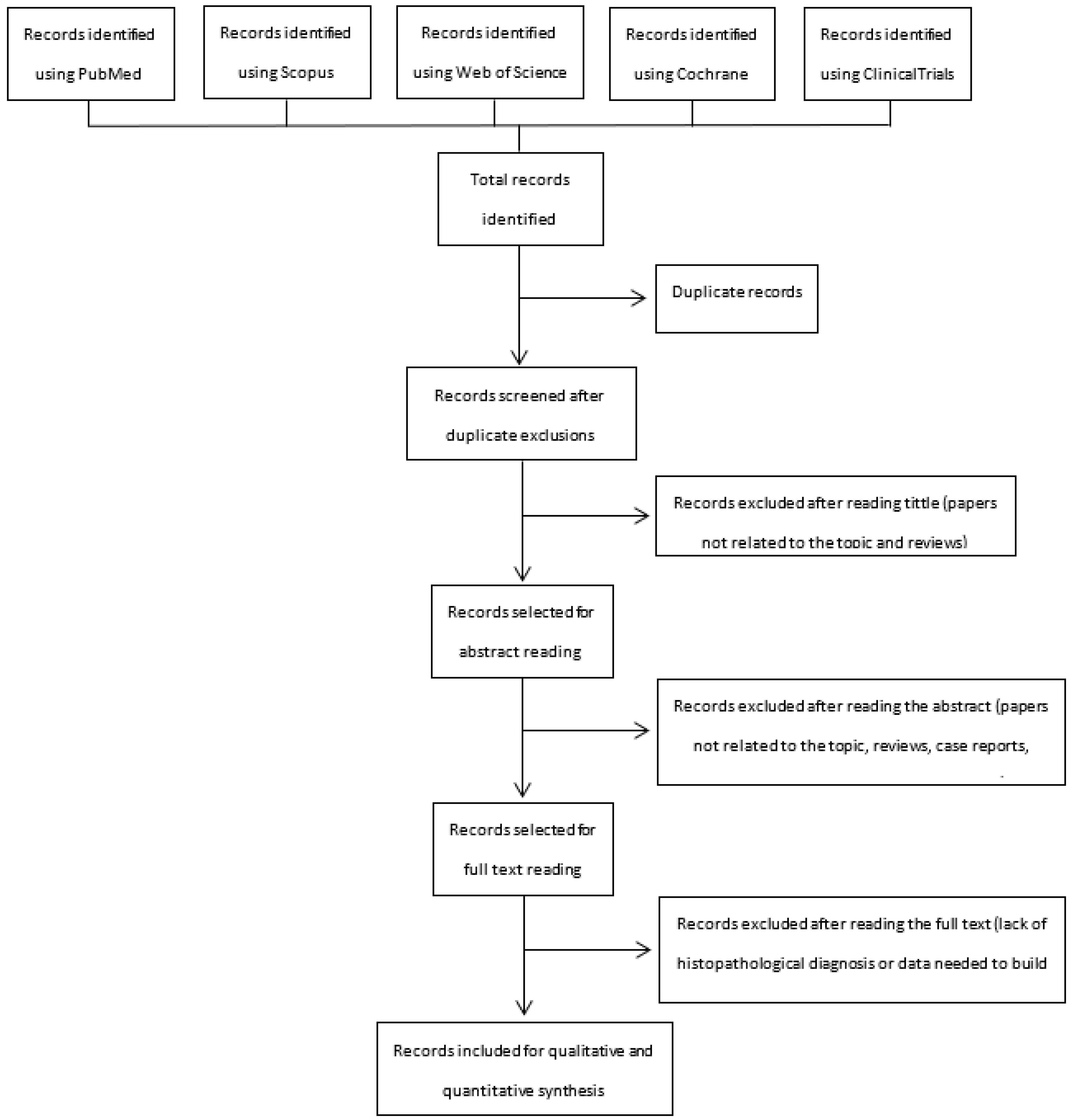
3.1. Search Results
3.2. Characteristics of Included Studies
3.3. Methodological Quality of Included Studies
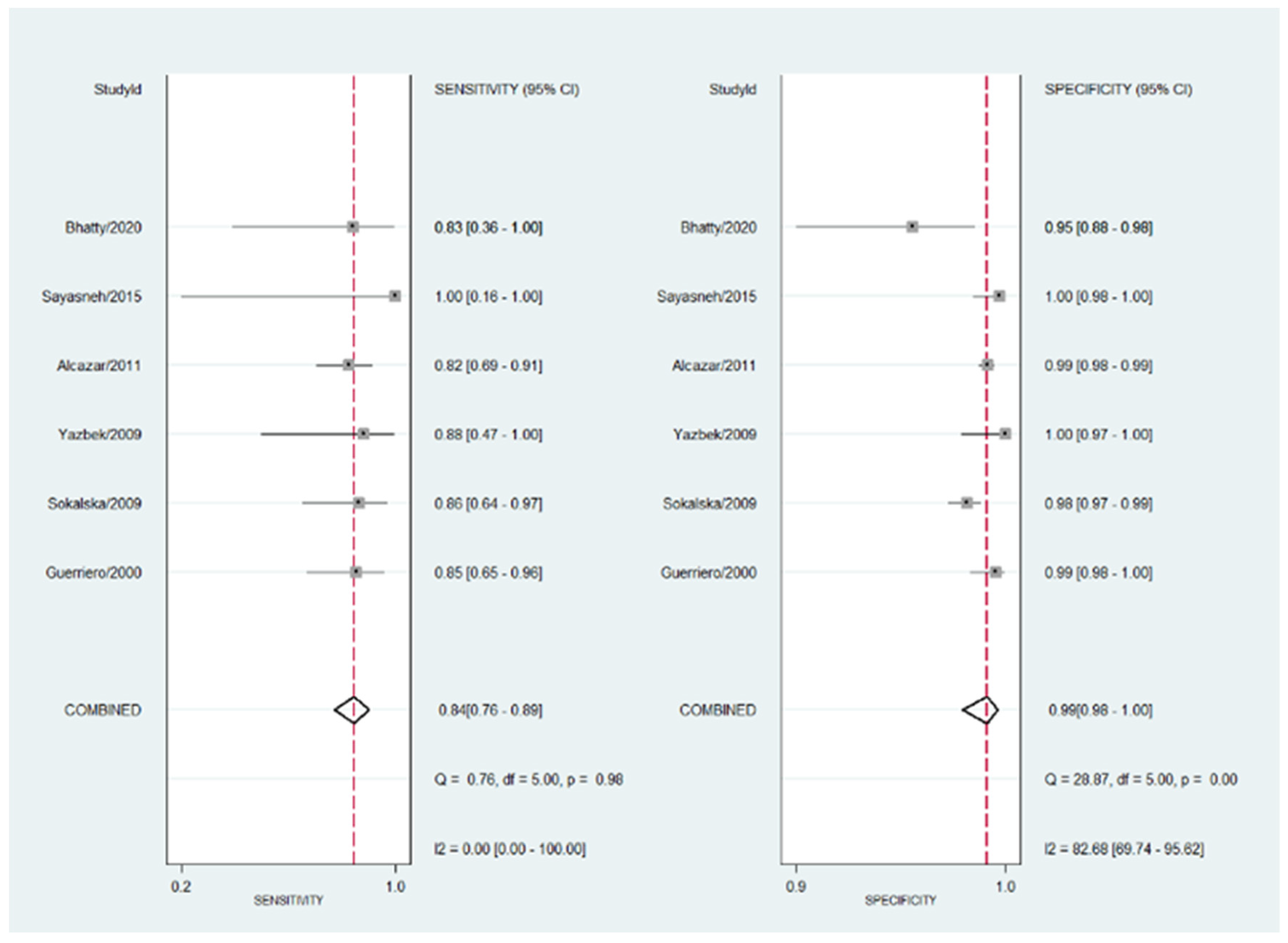
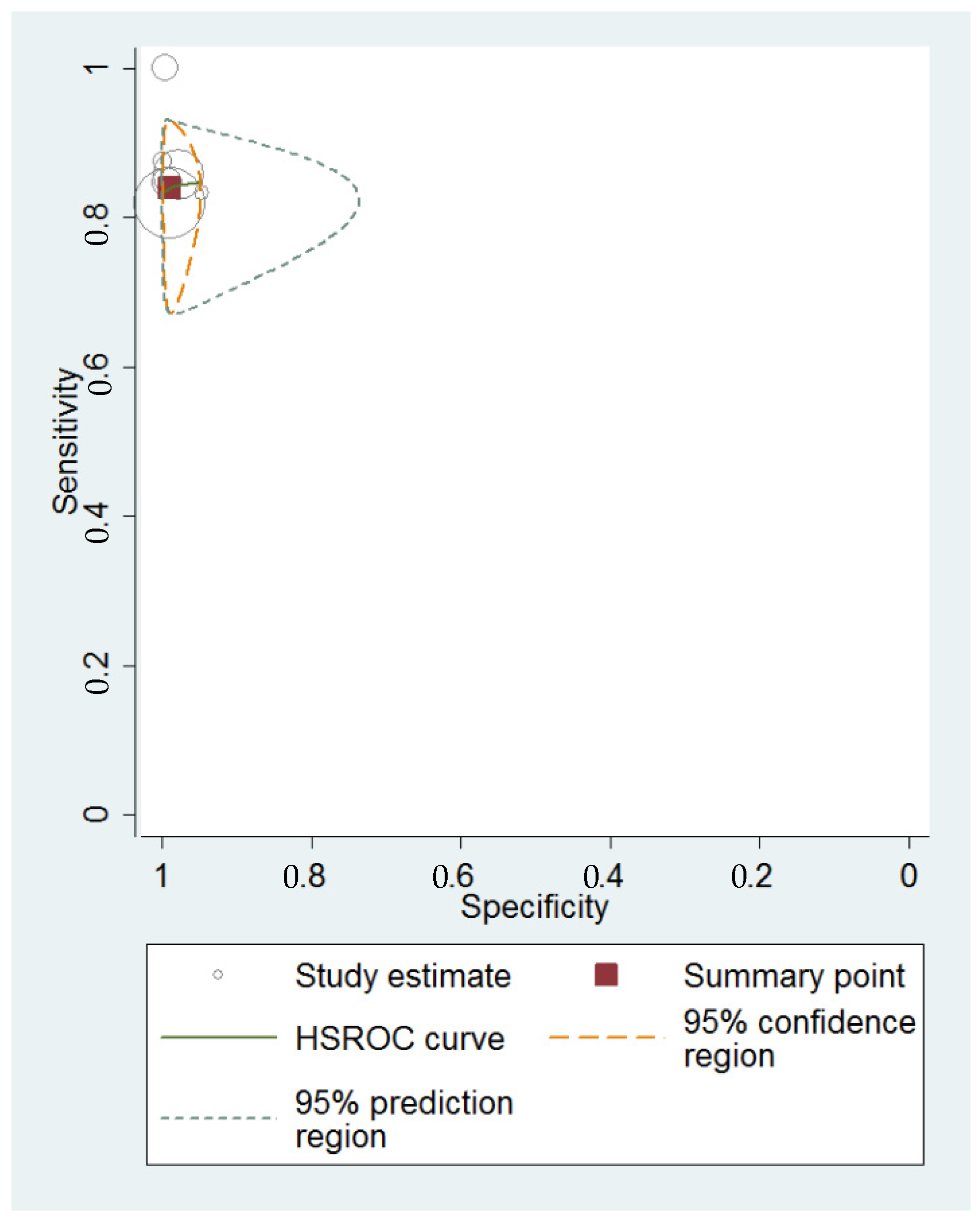
3.4. Diagnostic Performance of Transvaginal Ultrasonography for Hydrosalpinx
4. Discussion
4.1. Summary of Evidence
4.2. Interpretation of the Results and Relevance of the Topic
4.3. Strengths and Limitations
4.4. Future Research Agenda
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Honoré, G.M.; Holden, A.E.; Schenken, R.S. Pathophysiology and management of proximal tubal blockage. Fertil. Steril. 1999, 71, 785–795. [Google Scholar] [CrossRef] [PubMed]
- Capmas, P.; Suarthana, E.; Tulandi, T. Management of hydrosalpinx in the era of assisted reproductive technology: A systematic review and meta-analysis. J. Minim. Invasive Gynecol. 2021, 28, 418–441. [Google Scholar] [CrossRef] [PubMed]
- Romosan, G.; Valentin, L. The sensitivity and specificity of transvaginal ultrasound with regard to acute pelvic inflammatory disease: A review of the literature. Arch. Gynecol. Obstet. 2014, 289, 705–714. [Google Scholar] [CrossRef] [PubMed]
- Taipale, P.; Tarjanne, H.; Ylöstalo, P. Transvaginal sonography in suspected pelvic inflammatory disease. Ultrasound Obstet. Gynecol. 1995, 6, 430–434. [Google Scholar] [CrossRef]
- Guerriero, S.; Ajossa, S.; Gerada, M.; Virgilio, B.; Pilloni, M.; Galvan, R.; Laparte, M.C.; Alcázar, J.L.; Melis, G.B. Transvaginal ultrasonography in the diagnosis of extrauterine pelvic diseases. Expert Rev. Obstet Gynecol 2008, 3, 731–752. [Google Scholar] [CrossRef]
- Timor-Tritsch, I.E.; Lerner, J.P.; Monteagudo, A.; Murphy, K.E.; Heller, D.S. Transvaginal sonographic markers of tubal inflammatory disease. Ultrasound Obstet. Gynecol. 1998, 12, 56–66. [Google Scholar] [CrossRef]
- Molander, P.; Sjöberg, J.; Paavonen, J.; Cacciatore, B. Transvaginal power Doppler findings in laparoscopically proven acute pelvic inflammatory disease. Ultrasound Obstet. Gynecol. 2001, 17, 233–238. [Google Scholar] [CrossRef]
- Sayasneh, A.; Ekechi, C.; Ferrara, L.; Kaijser, J.; Stalder, C.; Sur, S.; Timmerman, D.; Bourne, T. The characteristic ultrasound features of specific types of ovarian pathology. Int. J. Oncol. 2015, 46, 445–458. [Google Scholar] [CrossRef]
- Tessler, F.N.; Perrella, R.R.; Fleischer, A.C.; Grant, E.G. Endovaginal sonographic diagnosis of dilated fallopian tubes. AJR Am. J. Roentgenol. 1989, 153, 523–525. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. PRISMA 2020 explanation and elaboration: Updated guidance and exemplars for reporting systematic reviews. BMJ 2021, 372, n160. [Google Scholar] [CrossRef]
- Sotiriadis, A.; Papatheodorou, S.I.; Martins, W.P. Synthesizing Evidence from Diagnostic Accuracy TEsts: The SEDATE guideline. Ultrasound Obstet. Gynecol. 2016, 47, 386–395. [Google Scholar] [CrossRef] [PubMed]
- Whiting, P.F.; Rutjes, A.W.; Westwood, M.E.; Mallett, S.; Deeks, J.J.; Reitsma, J.B.; Leeflang, M.M.G.; Sterne, J.A.C.; Bossuyt, P.M.M.; QUADAS-2 Group. QUADAS-2: A revised tool for the quality assessment of diagnostic accuracy studies. Ann. Intern. Med. 2011, 155, 529–536. [Google Scholar] [CrossRef]
- Deeks, J.J.; Altman, D.G. Diagnostic tests 4: Likelihood ratios. BMJ 2004, 329, 168–169. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.P.; Thompson, S.G.; Deeks, J.J.; Altman, D.G. Measuring inconsistency in meta-analyses. BMJ 2003, 327, 557–560. [Google Scholar] [CrossRef]
- Deeks, J.J.; Macaskill, P.; Irwig, L. The performance of tests of publication bias and other sample size effects in systematic reviews of diagnostic test accuracy was assessed. J. Clin. Epidemiol. 2005, 58, 882–893. [Google Scholar] [CrossRef] [PubMed]
- Alcázar, J.L.; Guerriero, S.; Laparte, C.; Ajossa, S.; Ruiz-Zambrana, Á.; Melis, G.B. Diagnostic performance of transvaginal gray-scale ultrasound for specific diagnosis of benign ovarian cysts in relation to menopausal status. Maturitas 2011, 68, 182–188. [Google Scholar] [CrossRef] [PubMed]
- Bhatty, S.; Bilal, A.; Abideen, Z.; Ahmad, A.; Laique, T. Role of Ultrasonography in Diagnosing Adnexal Masses: Cross-Sectional Study. Pak. J. Med. Sci. 2020, 14, 917–918. [Google Scholar]
- Guerriero, S.; Ajossa, S.; Lai, M.P.; Mais, V.; Paoletti, A.M.; Melis, G.B. Transvaginal ultrasonography associated with colour Doppler energy in the diagnosis of hydrosalpinx. Hum. Reprod. 2000, 15, 1568–1572. [Google Scholar] [CrossRef] [PubMed]
- Sayasneh, A.; Kaijser, J.; Preisler, J.; Smith, A.A.; Raslan, F.; Johnson, S.; Husicka, R.; Ferrara, L.; Stalder, C.; Ghaem-Maghami, S.; et al. Accuracy of ultrasonography performed by examiners with varied training and experience in predicting specific pathology of adnexal masses. Ultrasound Obstet. Gynecol. 2015, 45, 605–612. [Google Scholar] [CrossRef]
- Sokalska, A.; Timmerman, D.; Testa, A.C.; Van Holsbeke, C.; Lissoni, A.A.; Leone, F.P.G.; Jurkovic, D.; Valentin, L. Diagnostic accuracy of transvaginal ultrasound examination for assigning a specific diagnosis to adnexal masses. Ultrasound Obstet. Gynecol. 2009, 34, 462–470. [Google Scholar] [CrossRef]
- Yazbek, J.; Helmy, S.; Ben-Nagi, J.; Holland, T.; Sawyer, E.; Jurkovic, D. Value of preoperative ultrasound examination in the selection of women with adnexal masses for laparoscopic surgery. Ultrasound Obstet. Gynecol. 2007, 30, 883–888. [Google Scholar] [CrossRef] [PubMed]
- Savaris, R.F.; Giudice, L.C. The influence of hydrosalpinx on markers of endometrial receptivity. Semin. Reprod. Med. 2007, 25, 476–482. [Google Scholar] [CrossRef]
- Mukherjee, T.; Copperman, A.B.; McCaffrey, C.; Cook, C.A.; Bustillo, M.; Obasaju, M.F. Hydrosalpinx fluid has embryotoxic effects on murine embryogenesis: A case for prophylactic salpingectomy. Fertil. Steril. 1996, 66, 851–853. [Google Scholar] [CrossRef] [PubMed]
- Nackley, A.C.; Muasher, S.J. The significance of hydrosalpinx in in vitro fertilization. Fertil. Steril. 1998, 69, 373–384. [Google Scholar] [CrossRef] [PubMed]
- Granot, I.; Dekel, N.; Segal, I.; Fieldust, S.; Shoham, Z.; Barash, A. Is hydrosalpinx fluid cytotoxic? Hum. Reprod. 1998, 13, 1620–1624. [Google Scholar] [CrossRef] [PubMed]
- Mansour, R.T.; Aboulghar, M.A.; Serour, G.I.; Riad, R. Fluid accumulation of the uterine cavity before embryo transfer: A possible hindrance for implantation. J. Vitr. Fertil. Embryo. Transf. 1991, 8, 157–159. [Google Scholar] [CrossRef] [PubMed]
- Johnson, N.; Van Voorst, S.; Sowter, M.C.; Strandell, A.; Mol, B.W.J. Surgical treatment for tubal disease in women due to undergo in vitro fertilisation. Cochrane Database Syst. Rev. 2010, 1, CD002125. [Google Scholar] [CrossRef]
- Harb, H.; Al-Rshoud, F.; Karunakaran, B.; Gallos, I.D.; Coomarasamy, A. Hydrosalpinx and pregnancy loss: A systematic review and meta-analysis. Reprod. Biomed. Online 2019, 38, 427–441. [Google Scholar] [CrossRef]
- Valentin, L. Use of morphology to characterize and manage common adnexal masses. Best Pract Res. Clin. Obstet. Gynaecol. 2004, 18, 71–89. [Google Scholar] [CrossRef]



| Author | Year | Country | Study’s Design | N of Patients | Patients’ Mean Age | Menopausal Status | N of Masses | N of Suspected Hydrosalpinxes | N of Confirmed Hydrosalpinxes | N of Examiners | Experience of the Examiner | Blinded Examiner | Index Test | Reference Test | Time Elapsed from TVS to Surgery |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Alcázar | 2011 | Spain | Prospective ^ | 1980 | 42.6 | Pre- and postmenopausal | 2146 | 66 | 55 | 2 | Expert | yes | TVS | Histology | ≤1 week |
| Bhatty | 2020 | Pakistan | Prospective | 100 | NA | NA | 100 | 10 | 6 | NA | NA | yes | TVS | Histology | NA |
| Guerriero | 2000 | Italy | Prospective | 378 | 32.3 | Premenopausal | 378 | 24 | 26 | NA | NA | yes | TVS | Histology | ≤2 days |
| Sayasneh | 2015 | UK | Prospective | 313 | 47 | Pre- and postmenopausal | 301 | 3 | 2 | 37 | Trained | yes | TVS | Histology | ≤120 days |
| Sokalska | 2009 | Poland | Prospective ^ | 1066 | 47.4 | Pre- and postmenopausal | 1066 | 41 | 21 | NA | Expert * | yes | TVS | Histology | ≤120 days |
| Yazbek | 2007 | UK | Prospective | 137 | 35 | Pre- and postmenopausal | 153 | 7 | 8 | NA | NA | yes | TVS | Histology | NA |
| Study | Risk of Bias | Concerns of Applicability | |||||
|---|---|---|---|---|---|---|---|
| Patient Selection | Index Test | Reference Standard | Flow and Timing | Patient Selection | Index Test | Reference Standard | |
| Alcázar | Low risk | Low risk | High risk | Low risk | Low concern | Low concern | Low concern |
| Bhatty | Unclear | Unclear | Unclear | Unclear | Low concern | Low concern | Low concern |
| Guerriero | Low risk | Low risk | Unclear | Low risk | Low concern | Low concern | Low concern |
| Sayasneh | Low risk | Low risk | Unclear | Low risk | Low concern | Low concern | Low concern |
| Sokalska | Unclear | Low risk | Unclear | Low risk | Low concern | Low concern | Low concern |
| Yazbek | High risk | Low risk | Unclear | Unclear | Low concern | Low concern | Low concern |
| Author | Sensitivity | Specificity | PPV | NPV |
|---|---|---|---|---|
| Alcázar | 81.82% | 99.00% | 68.18% | 99.52% |
| Bhatty | 83.33% | 94.68% | 50.00% | 98.89% |
| Guerriero | 84.62% | 99.43% | 91.67% | 98.87% |
| Sayasneh | 100.00% | 99.68% | 66.67% | 100.00% |
| Sokalska | 85.71% | 97.80% | 43.90% | 99.71% |
| Yazbek | 87.50% | 100.00% | 100.00% | 99.32% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Delgado-Morell, A.; Nieto-Tous, M.; Andrada-Ripollés, C.; Pascual, M.Á.; Ajossa, S.; Guerriero, S.; Alcázar, J.L. Transvaginal Ultrasound Accuracy in the Hydrosalpinx Diagnosis: A Systematic Review and Meta-Analysis. Diagnostics 2023, 13, 948. https://doi.org/10.3390/diagnostics13050948
Delgado-Morell A, Nieto-Tous M, Andrada-Ripollés C, Pascual MÁ, Ajossa S, Guerriero S, Alcázar JL. Transvaginal Ultrasound Accuracy in the Hydrosalpinx Diagnosis: A Systematic Review and Meta-Analysis. Diagnostics. 2023; 13(5):948. https://doi.org/10.3390/diagnostics13050948
Chicago/Turabian StyleDelgado-Morell, Aina, Mar Nieto-Tous, Cristina Andrada-Ripollés, Maria Ángela Pascual, Silvia Ajossa, Stefano Guerriero, and Juan Luis Alcázar. 2023. "Transvaginal Ultrasound Accuracy in the Hydrosalpinx Diagnosis: A Systematic Review and Meta-Analysis" Diagnostics 13, no. 5: 948. https://doi.org/10.3390/diagnostics13050948
APA StyleDelgado-Morell, A., Nieto-Tous, M., Andrada-Ripollés, C., Pascual, M. Á., Ajossa, S., Guerriero, S., & Alcázar, J. L. (2023). Transvaginal Ultrasound Accuracy in the Hydrosalpinx Diagnosis: A Systematic Review and Meta-Analysis. Diagnostics, 13(5), 948. https://doi.org/10.3390/diagnostics13050948






