Overlap of Suspicious and Non-Suspicious Features in the Ultrasound Evaluations of Leiomyosarcoma: A Single-Center Experience
Abstract
:1. Introduction
2. Methods
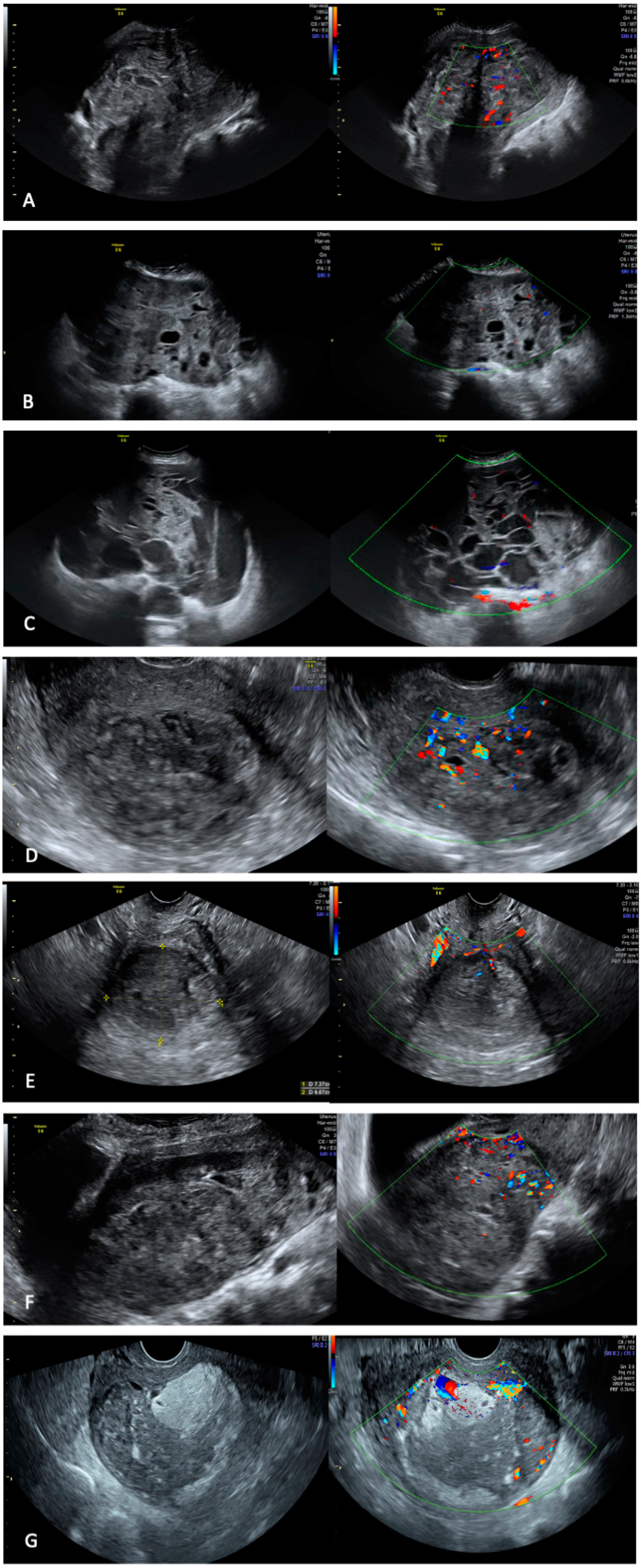
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Raine-Bennett, T.; Tucker, L.-Y.; Zaritsky, E.; Littell, R.D.; Palen, T.; Neugebauer, R.; Axtell, A.; Schultze, P.M.; Kronbach, D.W.; Embry-Schubert, J.; et al. Occult Uterine Sarcoma and Leiomyosarcoma: Incidence of and Survival Associated with Morcellation. Obstet. Gynecol. 2016, 127, 29–39. [Google Scholar] [CrossRef] [PubMed]
- Tropé, C.G.; Abeler, V.M.; Kristensen, G.B. Diagnosis and Treatment of Sarcoma of the Uterus. A Review. Acta Oncol. Stockh. Swed. 2012, 51, 694–705. [Google Scholar] [CrossRef]
- Lim, D.; Alvarez, T.; Nucci, M.R.; Gilks, B.; Longacre, T.; Soslow, R.A.; Oliva, E. Interobserver Variability in the Interpretation of Tumor Cell Necrosis in Uterine Leiomyosarcoma. Am. J. Surg. Pathol. 2013, 37, 650–658. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Wang, Z. Advances in the Preoperative Identification of Uterine Sarcoma. Cancers 2022, 14, 3517. [Google Scholar] [CrossRef] [PubMed]
- Żak, K.; Zaremba, B.; Rajtak, A.; Kotarski, J.; Amant, F.; Bobiński, M. Preoperative Differentiation of Uterine Leiomyomas and Leiomyosarcomas: Current Possibilities and Future Directions. Cancers 2022, 14, 1966. [Google Scholar] [CrossRef]
- Arezzo, F.; Loizzi, V.; La Forgia, D.; Moschetta, M.; Tagliafico, A.S.; Cataldo, V.; Kawosha, A.A.; Venerito, V.; Cazzato, G.; Ingravallo, G.; et al. Radiomics Analysis in Ovarian Cancer: A Narrative Review. Appl. Sci. 2021, 11, 7833. [Google Scholar] [CrossRef]
- Van den Bosch, T.; Dueholm, M.; Leone, F.P.G.; Valentin, L.; Rasmussen, C.K.; Votino, A.; Van Schoubroeck, D.; Landolfo, C.; Installé, A.J.F.; Guerriero, S.; et al. Terms, Definitions and Measurements to Describe Sonographic Features of Myometrium and Uterine Masses: A Consensus Opinion from the Morphological Uterus Sonographic Assessment (MUSA) Group. Ultrasound Obstet. Gynecol. Off. J. Int. Soc. Ultrasound Obstet. Gynecol. 2015, 46, 284–298. [Google Scholar] [CrossRef]
- Testa, A.C.; Di Legge, A.; Bonatti, M.; Manfredi, R.; Scambia, G. Imaging Techniques for Evaluation of Uterine Myomas. Best Pract. Res. Clin. Obstet. Gynaecol. 2016, 34, 37–53. [Google Scholar] [CrossRef]
- Leone, F.P.G.; Timmerman, D.; Bourne, T.; Valentin, L.; Epstein, E.; Goldstein, S.R.; Marret, H.; Parsons, A.K.; Gull, B.; Istre, O.; et al. Terms, Definitions and Measurements to Describe the Sonographic Features of the Endometrium and Intrauterine Lesions: A Consensus Opinion from the International Endometrial Tumor Analysis (IETA) Group. Ultrasound Obstet. Gynecol. Off. J. Int. Soc. Ultrasound Obstet. Gynecol. 2010, 35, 103–112. [Google Scholar] [CrossRef]
- Ludovisi, M.; Moro, F.; Pasciuto, T.; Di Noi, S.; Giunchi, S.; Savelli, L.; Pascual, M.A.; Sladkevicius, P.; Alcazar, J.L.; Franchi, D.; et al. Imaging in Gynecological Disease (15): Clinical and Ultrasound Characteristics of Uterine Sarcoma. Ultrasound Obstet. Gynecol. Off. J. Int. Soc. Ultrasound Obstet. Gynecol. 2019, 54, 676–687. [Google Scholar] [CrossRef]
- Exacoustos, C.; Romanini, M.E.; Amadio, A.; Amoroso, C.; Szabolcs, B.; Zupi, E.; Arduini, D. Can Gray-Scale and Color Doppler Sonography Differentiate between Uterine Leiomyosarcoma and Leiomyoma? J. Clin. Ultrasound JCU 2007, 35, 449–457. [Google Scholar] [CrossRef] [PubMed]
- Bonneau, C.; Thomassin-Naggara, I.; Dechoux, S.; Cortez, A.; Darai, E.; Rouzier, R. Value of Ultrasonography and Magnetic Resonance Imaging for the Characterization of Uterine Mesenchymal Tumors. Acta Obstet. Gynecol. Scand. 2014, 93, 261–268. [Google Scholar] [CrossRef]
- Buttram, V.C.; Reiter, R.C. Uterine Leiomyomata: Etiology, Symptomatology, and Management. Fertil. Steril. 1981, 36, 433–445. [Google Scholar] [CrossRef]
- Cazzato, G.; Colagrande, A.; Cimmino, A.; Caporusso, C.; Candance, P.M.V.; Trabucco, S.M.R.; Zingarelli, M.; Lorusso, A.; Marrone, M.; Stellacci, A.; et al. Urological Melanoma: A Comprehensive Review of a Rare Subclass of Mucosal Melanoma with Emphasis on Differential Diagnosis and Therapeutic Approaches. Cancers 2021, 13, 4424. [Google Scholar] [CrossRef]
- Ghosh, S.; Naftalin, J.; Imrie, R.; Hoo, W.-L. Natural History of Uterine Fibroids: A Radiological Perspective. Curr. Obstet. Gynecol. Rep. 2018, 7, 117–121. [Google Scholar] [CrossRef]
- Lete, I.; González, J.; Ugarte, L.; Barbadillo, N.; Lapuente, O.; Álvarez-Sala, J. Parasitic Leiomyomas: A Systematic Review. Eur. J. Obstet. Gynecol. Reprod. Biol. 2016, 203, 250–259. [Google Scholar] [CrossRef]
- Kadhel, P.; Borja de Mozota, D.; Simon, P.; Toto, T.; Jermidi, C.; Ayhan, G. Leiomyomas in an African Caribbean Hysterectomy Population Considered to Be Ethnically Related to African Americans. J. Gynecol. Obstet. Hum. Reprod. 2020, 49, 101654. [Google Scholar] [CrossRef]
- Moroni, R.M.; Vieira, C.S.; Ferriani, R.A.; dos Reis, R.M.; Nogueira, A.A.; Brito, L.G.O. Presentation and Treatment of Uterine Leiomyoma in Adolescence: A Systematic Review. BMC Womens Health 2015, 15, 4. [Google Scholar] [CrossRef]
- Singh, N.; Al-Ruwaisan, M.; Batra, A.; Itani, D.; Ghatage, P. Factors Affecting Overall Survival in Premenopausal Women with Uterine Leiomyosarcoma: A Retrospective Analysis with Long-Term Follow-Up. J. Obstet. Gynaecol. Can. 2020, 42, 1483–1488. [Google Scholar] [CrossRef]
- Skorstad, M.; Kent, A.; Lieng, M. Preoperative Evaluation in Women with Uterine Leiomyosarcoma. A Nationwide Cohort Study. Acta Obstet. Gynecol. Scand. 2016, 95, 1228–1234. [Google Scholar] [CrossRef]
- Juang, C.M.; Yen, M.S.; Horng, H.C.; Twu, N.F.; Yu, H.C.; Hsu, W.L. Potential Role of Preoperative Serum CA125 for the Differential Diagnosis between Uterine Leiomyoma and Uterine Leiomyosarcoma. Eur. J. Gynaecol. Oncol. 2006, 27, 370–374. [Google Scholar] [PubMed]
- Yilmaz, N.; Sahin, I.; Kilic, S.; Ozgu, E.; Gungor, T.; Bilge, U. Assessment of the Predictivity of Preoperative Serum CA 125 in the Differential Diagnosis of Uterine Leiomyoma and Uterine Sarcoma in the Turkish Female Population. Eur. J. Gynaecol. Oncol. 2009, 30, 412–414. [Google Scholar]
- Seki, K.; Hoshihara, T.; Nagata, I. Leiomyosarcoma of the Uterus: Ultrasonography and Serum Lactate Dehydrogenase Level. Gynecol. Obstet. Investig. 1992, 33, 114–118. [Google Scholar] [CrossRef] [PubMed]
- Nagai, T.; Takai, Y.; Akahori, T.; Ishida, H.; Hanaoka, T.; Uotani, T.; Sato, S.; Matsunaga, S.; Baba, K.; Seki, H. Highly Improved Accuracy of the Revised PREoperative Sarcoma Score (RPRESS) in the Decision of Performing Surgery for Patients Presenting with a Uterine Mass. SpringerPlus 2015, 4, 520. [Google Scholar] [CrossRef]
- Sheng, S.L.; Liu, J.J.; Dai, Y.H.; Sun, X.G.; Xiong, X.P.; Huang, G. Knockdown of Lactate Dehydrogenase A Suppresses Tumor Growth and Metastasis of Human Hepatocellular Carcinoma. FEBS J. 2012, 279, 3898–3910. Available online: https://febs.onlinelibrary.wiley.com/doi/10.1111/j.1742-4658.2012.08748.x (accessed on 18 December 2022). [CrossRef]
- Wang, Z.-Y.; Loo, T.Y.; Shen, J.-G.; Wang, N.; Wang, D.-M.; Yang, D.-P.; Mo, S.-L.; Guan, X.-Y.; Chen, J.-P. LDH-A Silencing Suppresses Breast Cancer Tumorigenicity through Induction of Oxidative Stress Mediated Mitochondrial Pathway Apoptosis. Breast Cancer Res. Treat. 2012, 131, 791–800. [Google Scholar] [CrossRef]
- Cho, H.; Kim, K.; Kim, Y.-B.; No, J.H. Differential Diagnosis between Uterine Sarcoma and Leiomyoma Using Preoperative Clinical Characteristics. J. Obstet. Gynaecol. Res. 2016, 42, 313–318. [Google Scholar] [CrossRef]
- Chen, I.; Firth, B.; Hopkins, L.; Bougie, O.; Xie, R.-H.; Singh, S. Clinical Characteristics Differentiating Uterine Sarcoma and Fibroids. JSLS 2018, 22, e2017.00066. [Google Scholar] [CrossRef]
- Russo, C.; Camilli, S.; Martire, F.G.; Di Giovanni, A.; Lazzeri, L.; Malzoni, M.; Zupi, E.; Exacoustos, C. Ultrasound Features of Highly Vascularized Uterine Myomas (Uterine Smooth Muscle Tumors) and Correlation with Histopathology. Ultrasound Obstet. Gynecol. Off. J. Int. Soc. Ultrasound Obstet. Gynecol. 2022, 60, 269–276. [Google Scholar] [CrossRef]
| Characteristics | Quantification/Measurement |
|---|---|
| Age (mean ± sd) | 50.2 ± 7.0 |
| Ca125 (mean ± sd) | 28.8 ± 15 |
| Number of lesions, n (%) | |
| Single | 5 (71.4%) |
| Multiple | 2 (28.6%) |
| Largest diameter (mean ± sd), mm | 152.2 ± 91 |
| The echogenicity of solid tissue, n (%) | |
| Homogeneous | 0 |
| Inhomogeneous | 7 (100%) |
| Tumor borders, n (%) | |
| Regular | 1 (14.3%) |
| Irregular | 6 (85.7%) |
| Shadowing, n (%) No Yes | 3 (42.9%) 4 (57.1%) |
| Vascularization, n (%) Peripheral Intralesional | 0 7 (100%) |
| Color score, n (%) 1−2 3−4 | 4 (57.1%) 3 (42.9%) |
| Cystic areas, n (%) No Yes | 1 (14.3%) 6 (85.7%) |
| Consistency, n (%) Hard Soft | 0 7 (100%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arezzo, F.; Cormio, G.; Putino, C.; Di Lillo, N.; Silvestris, E.; Kardhashi, A.; Cazzolla, A.; Lombardi, C.; Mongelli, M.; Cazzato, G.; et al. Overlap of Suspicious and Non-Suspicious Features in the Ultrasound Evaluations of Leiomyosarcoma: A Single-Center Experience. Diagnostics 2023, 13, 543. https://doi.org/10.3390/diagnostics13030543
Arezzo F, Cormio G, Putino C, Di Lillo N, Silvestris E, Kardhashi A, Cazzolla A, Lombardi C, Mongelli M, Cazzato G, et al. Overlap of Suspicious and Non-Suspicious Features in the Ultrasound Evaluations of Leiomyosarcoma: A Single-Center Experience. Diagnostics. 2023; 13(3):543. https://doi.org/10.3390/diagnostics13030543
Chicago/Turabian StyleArezzo, Francesca, Gennaro Cormio, Carmela Putino, Nicola Di Lillo, Erica Silvestris, Anila Kardhashi, Ambrogio Cazzolla, Claudio Lombardi, Michele Mongelli, Gerardo Cazzato, and et al. 2023. "Overlap of Suspicious and Non-Suspicious Features in the Ultrasound Evaluations of Leiomyosarcoma: A Single-Center Experience" Diagnostics 13, no. 3: 543. https://doi.org/10.3390/diagnostics13030543
APA StyleArezzo, F., Cormio, G., Putino, C., Di Lillo, N., Silvestris, E., Kardhashi, A., Cazzolla, A., Lombardi, C., Mongelli, M., Cazzato, G., & Loizzi, V. (2023). Overlap of Suspicious and Non-Suspicious Features in the Ultrasound Evaluations of Leiomyosarcoma: A Single-Center Experience. Diagnostics, 13(3), 543. https://doi.org/10.3390/diagnostics13030543










