Refined Automatic Brain Tumor Classification Using Hybrid Convolutional Neural Networks for MRI Scans
Abstract
1. Introduction
2. Related Work
3. Materials and Methods
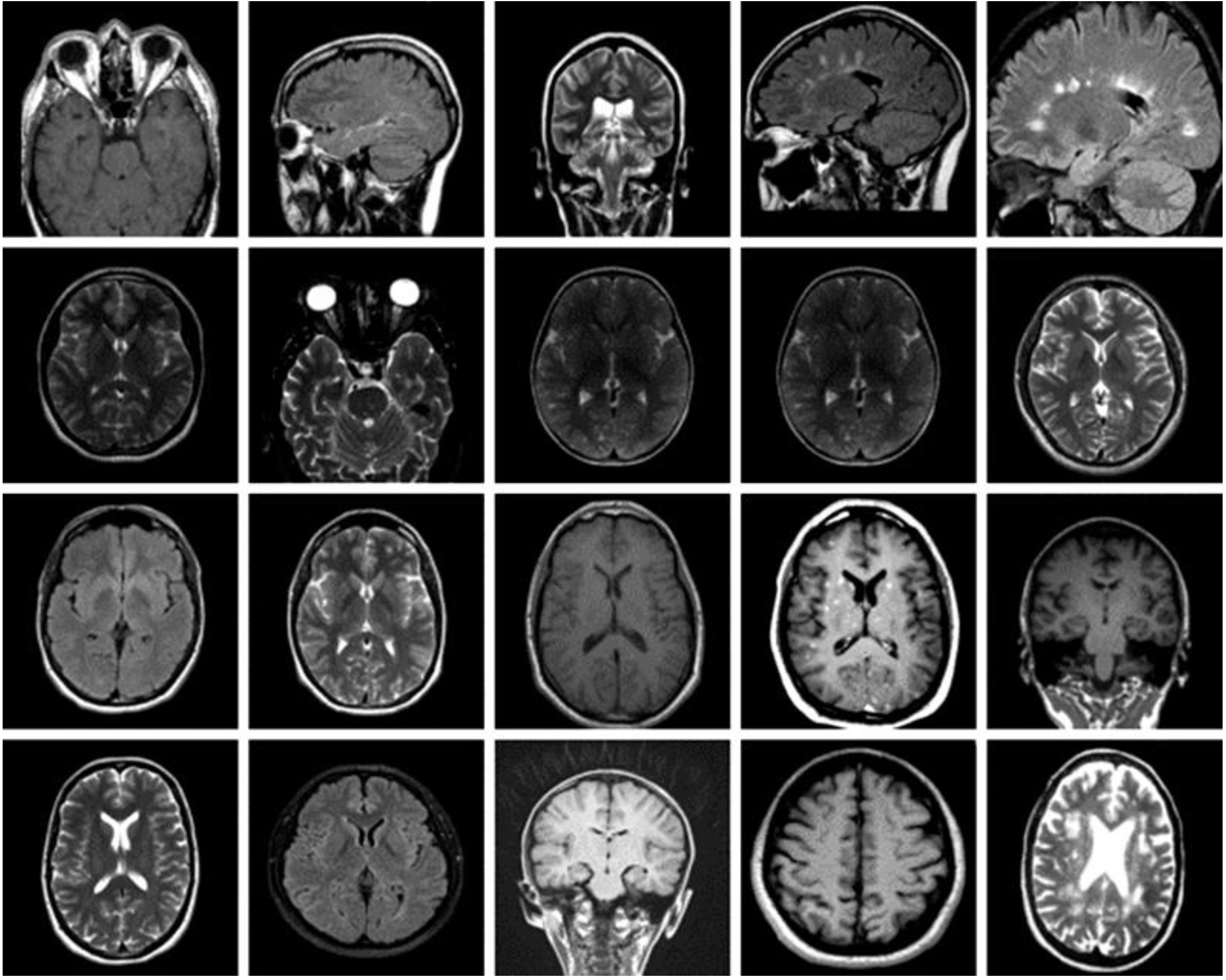
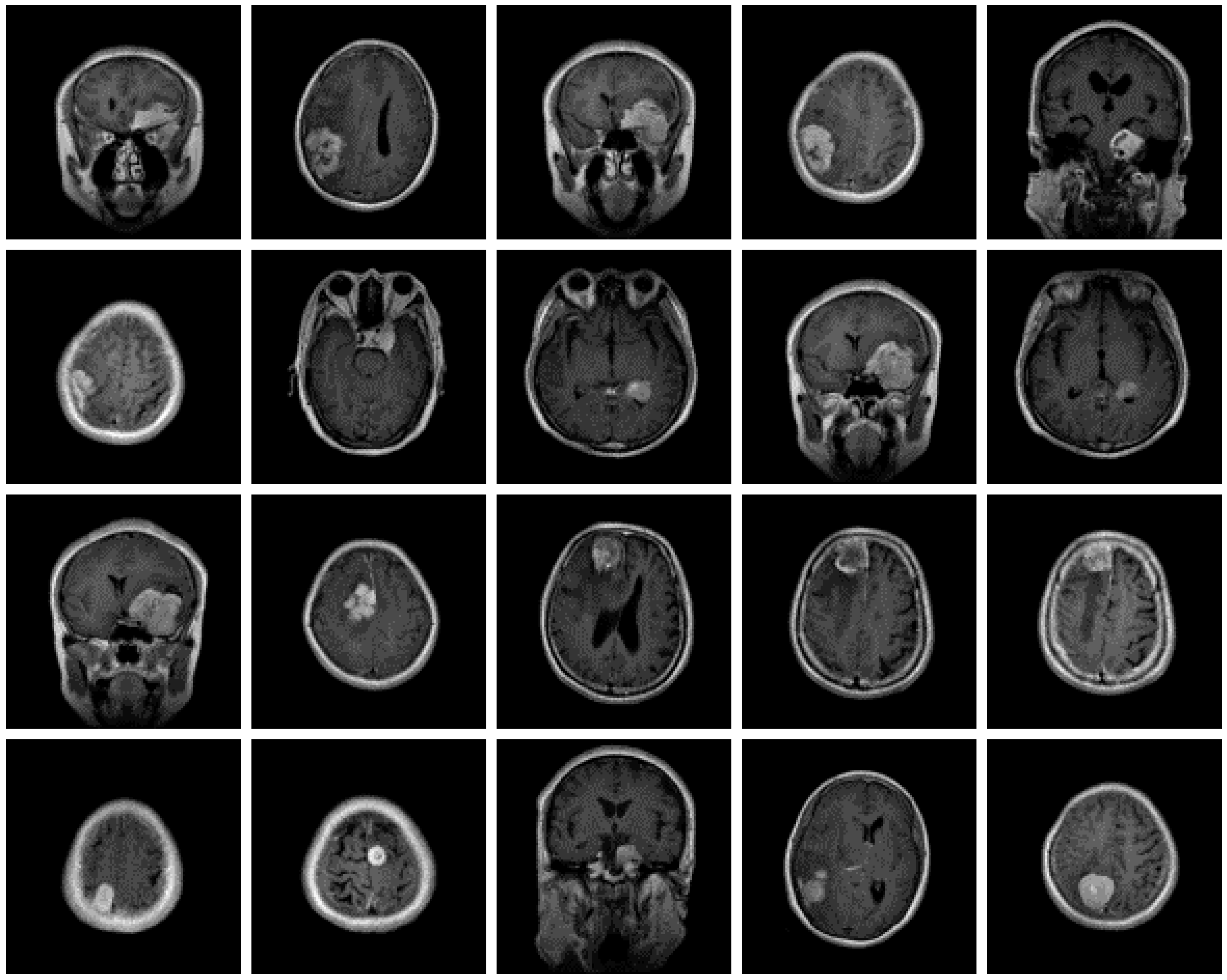
3.1. Dataset of the Brain MRI Scans
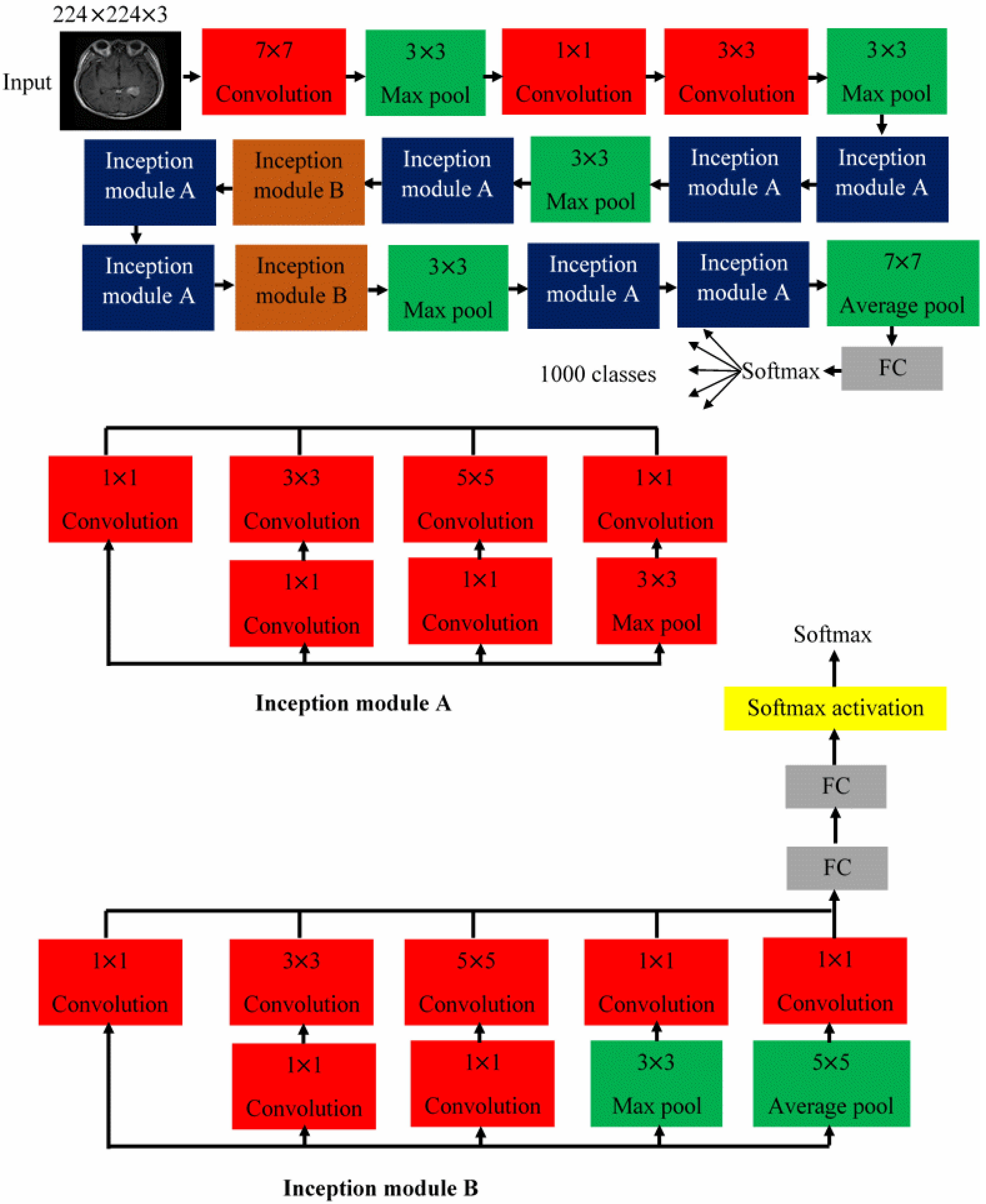
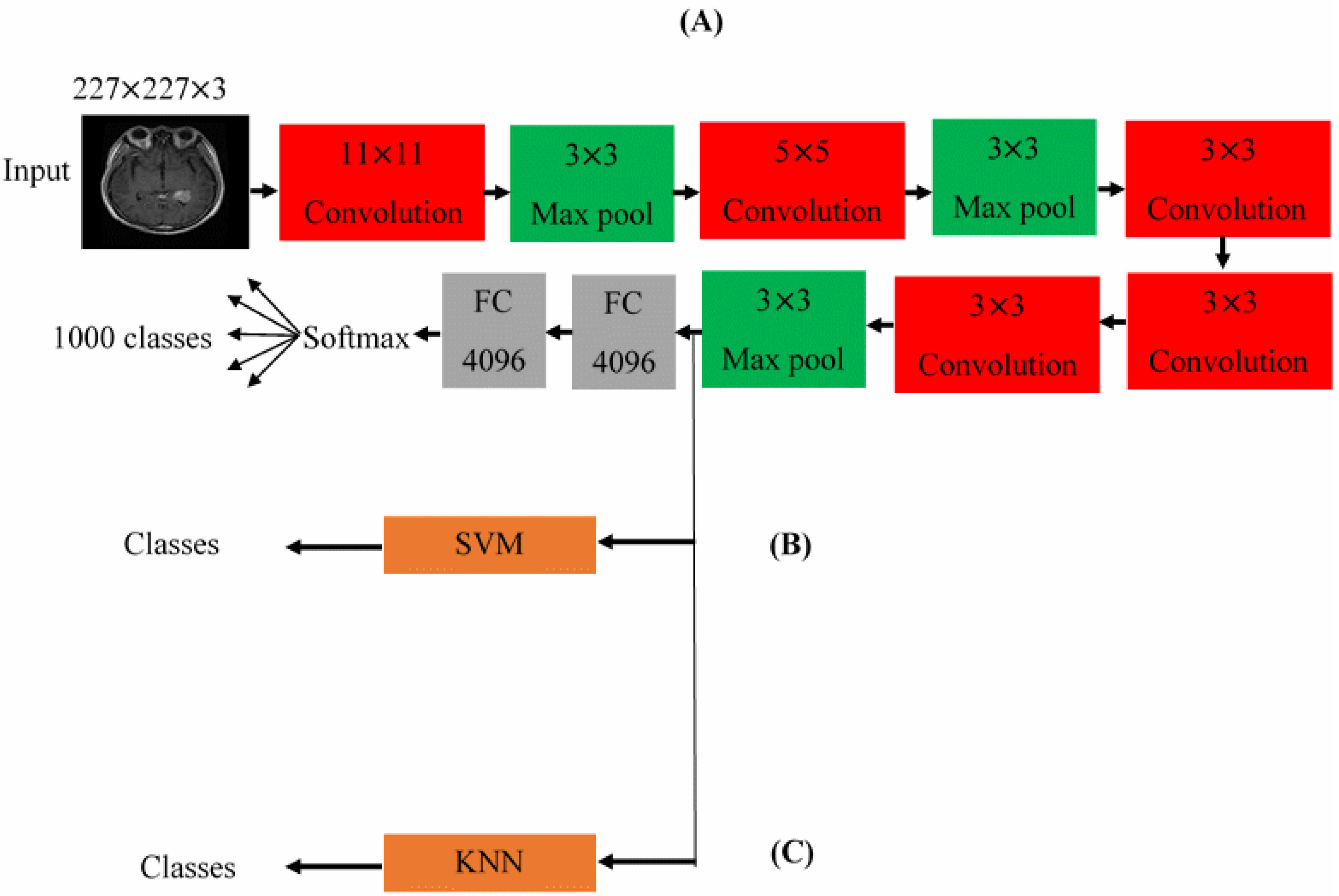
3.2. The Architectures of the Proposed CNNs
4. Experimental Results and Analysis
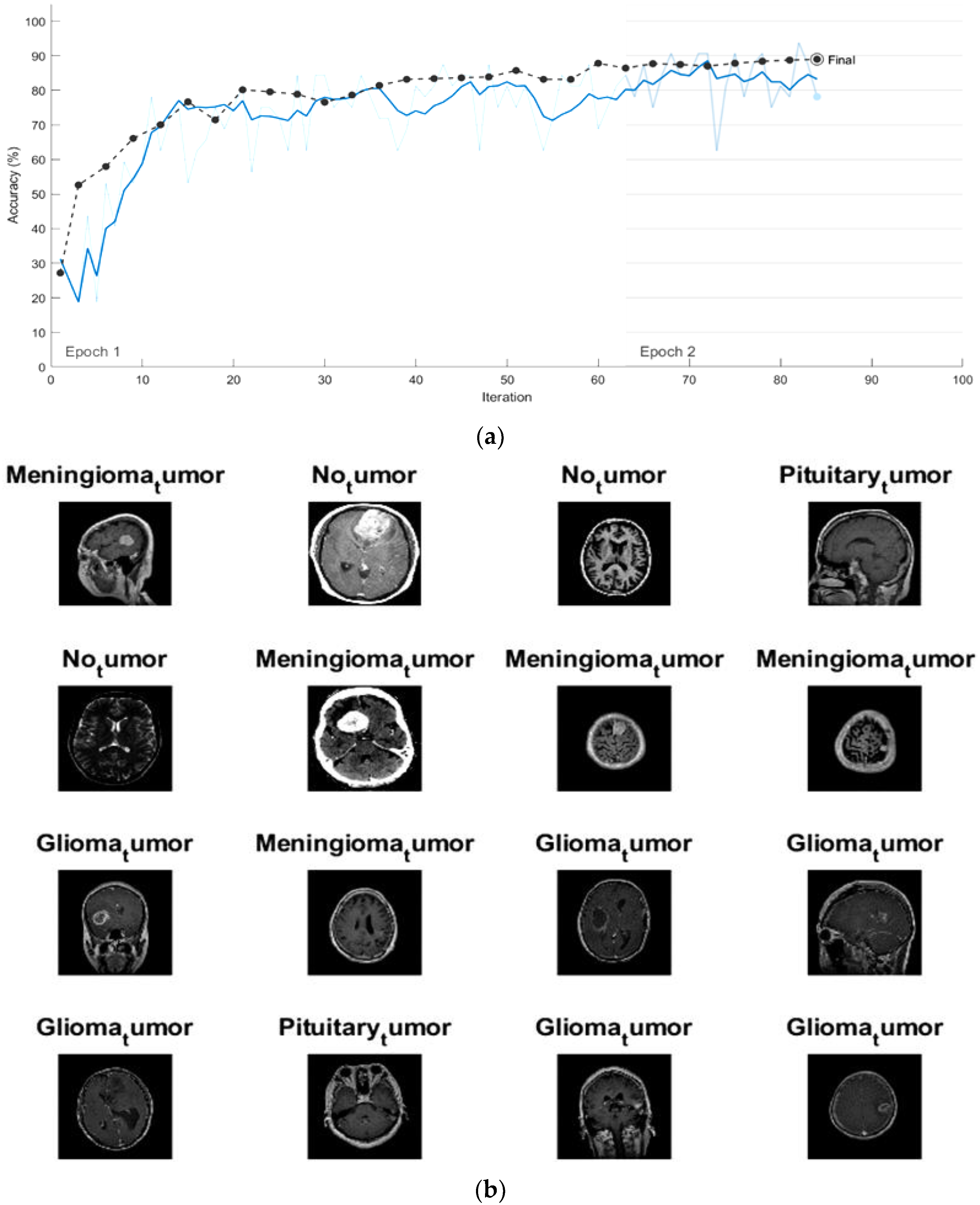
4.1. Classification the Brain Tumor Using CNNs
4.2. Enhancing the Classification Performance of the Fine-Tuning AlexNet for the Brain Tumor Classes Using Hybrid Networks
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Neugut, A.I.; Sackstein, P.; Hillyer, G.C.; Jacobson, J.; Bruce, J.; Lassman, A.B.; Stieg, P.A. Magnetic Resonance Imaging-Based Screening for Asymptomatic Brain Tumors: A Review. Oncologist 2019, 24, 375–384. [Google Scholar] [CrossRef] [PubMed]
- Van Maele-Fabry, G.; Gamet-Payrastre, L.; Lison, D. Residential exposure to pesticides as risk factor for childhood and young adult brain tumors: A systematic review and meta-analysis. Environ. Int. 2017, 106, 69–90. [Google Scholar] [CrossRef] [PubMed]
- Jones, D.T.W.; Banito, A.; Grünewald, T.G.P.; Haber, M.; Jäger, N.; Kool, M.; Milde, T.; Molenaar, J.J.; Nabbi, A.; Pugh, T.J.; et al. Molecular characteristics and therapeutic vulnerabilities across paediatric solid tumours. Nat. Rev. Cancer 2019, 19, 420–438. [Google Scholar] [CrossRef] [PubMed]
- Lapointe, S.; Perry, A.; Butowski, N.A. Primary brain tumours in adults. Lancet 2018, 392, 432–446. [Google Scholar] [CrossRef] [PubMed]
- Rehman, A.; Naz, S.; Razzak, M.I.; Akram, F.; Imran, M. A Deep Learning-Based Framework for Automatic Brain Tumors Classification Using Transfer Learning. Circuits Syst. Signal Process. 2020, 39, 757–775. [Google Scholar] [CrossRef]
- Miller, K.D.; Ostrom, Q.T.; Kruchko, C.; Patil, N.; Tihan, T.; Cioffi, G.; Fuchs, H.E.; Waite, K.A.; Jemal, A.; Siegel, R.L.; et al. Brain and other central nervous system tumor statistics. CA Cancer J. Clin. 2021, 71, 381–406. [Google Scholar] [CrossRef] [PubMed]
- Abdelaziz, H.A.; Sallah, M.; Elgarayhi, A.; AlTahhan, F.E. Accurate automatic classification system for 3D CT images of some vertebrate remains from Egypt. J. Taibah Univ. Sci. 2022, 16, 632–645. [Google Scholar] [CrossRef]
- Rawat, W.; Wang, Z. Deep Convolutional Neural Networks for Image Classification: A Comprehensive Review. Neural Comput. 2017, 29, 2352–2449. [Google Scholar] [CrossRef]
- Abo-Lila, G.M.; Sokkar, T.Z.N.; Seisa, E.A.; Omar, E.Z. Adaptive investigation of the optical properties of polymer fibers from mixing noisy phase shifting microinterferograms using deep learning algorithms. Microsc. Res. Tech. 2022, 85, 667–684. [Google Scholar] [CrossRef]
- AlTahhan, F.E.; Fares, M.E.; Sakr, A.A.; Aladle, D.A. Accurate automatic detection of acute lymphatic leukemia using a refined simple classification. Microsc. Res. Tech. 2020, 83, 1178–1189. [Google Scholar]
- Omar, E.Z.; Sokkar, T.Z.N.; Hamza, A.A. In situ investigation and detection of opto-mechanical properties of polymeric fibres from their digital distorted microinterferograms using machine learning algorithms. Opt. Laser Technol. 2020, 129, 106295–106311. [Google Scholar] [CrossRef]
- Pedraza, A.; Bueno, G.; Deniz, O.; Cristóbal, G.; Blanco, S.; Borrego-Ramos, M. Automated diatom classification (part B): A deep learning approach. Appl. Sci. 2017, 7, 460. [Google Scholar] [CrossRef]
- Paluszek, M.; Thomas, S. MATLAB Machine Learning; Apress: Princeton, NJ, USA, 2017. [Google Scholar]
- Cheng, J.; Yang, W.; Huang, M.; Huang, W.; Jiang, J.; Zhou, Y.; Yang, R.; Zhao, J.; Feng, Y.; Feng, Q. Retrieval of brain tumors by adaptive spatial pooling and fisher vector representation. PLoS ONE 2016, 11, e0157112. [Google Scholar] [CrossRef] [PubMed]
- Ismael, M.R.; Abdel-Qader, I. Brain tumor classification via statistical features and back-propagation neural network. In Proceedings of the 2018 IEEE International Conference on Electro/Information Technology (EIT), Rochester, MI, USA, 3–5 May 2018; pp. 252–257. [Google Scholar]
- Afshar, P.; Mohammadi, A.; Plataniotis, K.N. Brain tumor type classification via capsule networks. arXiv 2018, arXiv:1802.10200. [Google Scholar]
- Abir, T.A.; Siraji, J.A.; Ahmed, E.; Khulna, B. Analysis of a novel MRI based brain tumour classification using probabilistic neural network (PNN). Int. J. Sci. Res. Sci. Eng. Technol. 2018, 4, 65–79. [Google Scholar]
- Abiwinanda, N.; Hanif, M.; Hesaputra, S.T.; Handayani, A.; Mengko, T.R. Brain tumor classification using convolutional neural network. In World Congress on Medical Physics and Biomedical Engineering; Lhotska, L., Sukupova, L., Lacković, I., Ibbott, G.S., Eds.; Springer: Singapore, 2019; pp. 183–189. [Google Scholar]
- Chattopadhyay, A.; Maitra, M. MRI-based brain tumour image detection using CNN based deep learning method. Neurosci. Inform. 2022, 2, 100060. [Google Scholar] [CrossRef]
- Vankdothu, R.; Hameed, M.A.; Fatima, H. A Brain Tumor Identification and Classification Using Deep Learning based on CNN-LSTM Method. Comput. Electr. Eng. 2022, 101, 107960. [Google Scholar] [CrossRef]
- Deepak, S.; Ameer, P.M. Brain tumor classification using deep CNN features via transfer learning. Comput. Biol. Med. 2019, 111, 103345. [Google Scholar] [CrossRef]
- Konwar, P.; Bhadra, J.; Dutta, M.; Dowari, J. Detecting Brain Tumour in Early Stage Using Deep Learning. J. Inform. Tech. Softw. Eng. 2022, 12, 297. [Google Scholar]
- Haq, A.; Li, J.P.; Khan, S.; Alshara, M.A.; Alotaibi, R.M.; Mawuli, C.B. DACBT: Deep learning approach for classification of brain tumors using MRI data in IoT healthcare environment. Sci. Rep. 2022, 12, 15331. [Google Scholar] [CrossRef]
- Rao, P.K.; Rao, A.B.; Patro, K.K.; Prakash, A.J.; Jamjoom, M.M.; Abdel Samee, N. A novel approach for brain tumour detection using deep learning based technique. Biomed. Signal Process. Control 2023, 82, 104549. [Google Scholar]
- ZainEldin, H.; Gamel, S.A.; El-Kenawy, E.M.; Alharbi, A.H.; Khafaga, D.S.; Ibrahim, A.; Talaat, F.M. Brain Tumor Detection and Classification Using Deep Learning and Sine-Cosine Fitness GreyWolf Optimization. Bioengineering 2023, 10, 18. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J. Brain Tumor Dataset; Figshare: London, UK, 2018. [Google Scholar] [CrossRef]
- Szegedy, C.; Liu, W.; Jia, Y.; Sermanet, P.; Reed, S.; Anguelov, D.; Erhan, D.; Rabinovich, A. Going deeper with convolutions. In Proceedings of the 2015 IEEE Conference on Computer Vision and Pattern Recognition (CVPR), Boston, MA, USA, 7–12 June 2015. [Google Scholar]
- Krizhevsky, A.; Sutskever, I.; Hinton, G.E. ImageNet Classification with Deep Convolutional Neural Networks. In Proceedings of the 25th International Conference on Neural Information Processing Systems, Lake Tahoe, NV, USA, 3–6 December 2012; Volume 1, pp. 1097–1105. [Google Scholar]
- Omar, E.Z. A refined denoising method for noisy phaseshifting interference fringe patterns. Opt. Quantum Electron. 2021, 53, 464. [Google Scholar] [CrossRef]
- Kotsiantis, S.B. Supervised machine learning: A review of classification techniques. Informatica 2007, 31, 249–268. [Google Scholar]
- Ali, R.; Li, H.; Dillman, J.R.; Altaye, M.; Wang, H.; Parikh, N.A.; He, L. A self-training deep neural network for early prediction of cognitive deficits in very preterm infants using brain functional connectome data. Pediatr. Radiol. 2022, 52, 2227–2240. [Google Scholar] [CrossRef] [PubMed]
- Aamir, M.; Rahman, Z.; Ahmed Dayo, Z.; Ahmed Abro, W.; Irfan Uddin, M.; Khan, I.; Shariq Imran, A.; Ali, Z.; Ishfaq, M.; Guan, Y.; et al. A deep learning approach for brain tumor classification using MRI images. Comput. Electr. Eng. 2022, 101, 108105. [Google Scholar] [CrossRef]
- Ali, R.; Hardie, R.C.; Narayanan, B.N.; Kebede, T.M. IMNets: Deep Learning Using an Incremental Modular Network Synthesis Approach for Medical Imaging Applications. Appl. Sci. 2022, 12, 5500. [Google Scholar] [CrossRef]
- Ait Amou, M.; Xia, K.; Kamhi, S.; Mouhafid, M. A Novel MRI Diagnosis Method for Brain Tumor Classification Based on CNN and Bayesian Optimization. Healthcare 2022, 10, 494. [Google Scholar] [CrossRef]
- Bousquet, O.; Boucheron, S.; Lugosi, G. Theory of classification: A survey of recent advances. ESAIM Probab. Stat. 2005, 9, 249–268. [Google Scholar]
- Srinivas, B.; Rao, G.S. A Hybrid CNN-KNN model for MRI brain tumor Classification. Int. J. Recent Technol. Eng. 2019, 8, 2277–3878. [Google Scholar] [CrossRef]
- Swati, Z.N.K.; Zhao, Q.; Ali, F.; Ali, Z.; Ahmed, S.; Lu, J. Brain tumor classification for MR images using transfer learning and fine-tuning. Comput. Med. Imaging Graph. 2019, 75, 34–46. [Google Scholar] [CrossRef] [PubMed]
- Gurbina, M.; Lascu, M.; Lascu, D. Tumor detection and classification of MRI brain image using different wavelet transforms and support vector machines. In Proceedings of the 2019 42nd International Conference on Telecommunications and Signal Processing (TSP), Budapest, Hungary, 1–3 July 2019; IEEE: Piscataway, NJ, USA, 2019; pp. 505–508. [Google Scholar]
- Özyurt, F.; Sert, E.; Avci, E.; Dogantekin, E. Brain tumor detection based on Convolutional Neural Network with neutrosophic expert maximum fuzzy sure entropy. Measurement 2020, 147, 106830. [Google Scholar] [CrossRef]
- Sajjad, M.; Khan, S.; Muhammad, K.; Wu, W.; Ullah, A.; Baik, S.W. Multi-grade brain tumor classification using deep CNN with extensive data augmentation. J. Comput. Sci. 2020, 30, 174–182. [Google Scholar] [CrossRef]
- Salçin, K. Detection and classification of brain tumours from MRI images using faster R-CNN. Teh. Glas. 2019, 13, 337–342. [Google Scholar]
- Amin, J.; Sharif, M.; Haldorai, A.; Yasmin, A.; Nayak, R.S. Brain tumour detection and classification using machine learning: A comprehensive survey. Complex Intell. Syst. 2021, 8, 3161–3183. [Google Scholar] [CrossRef]



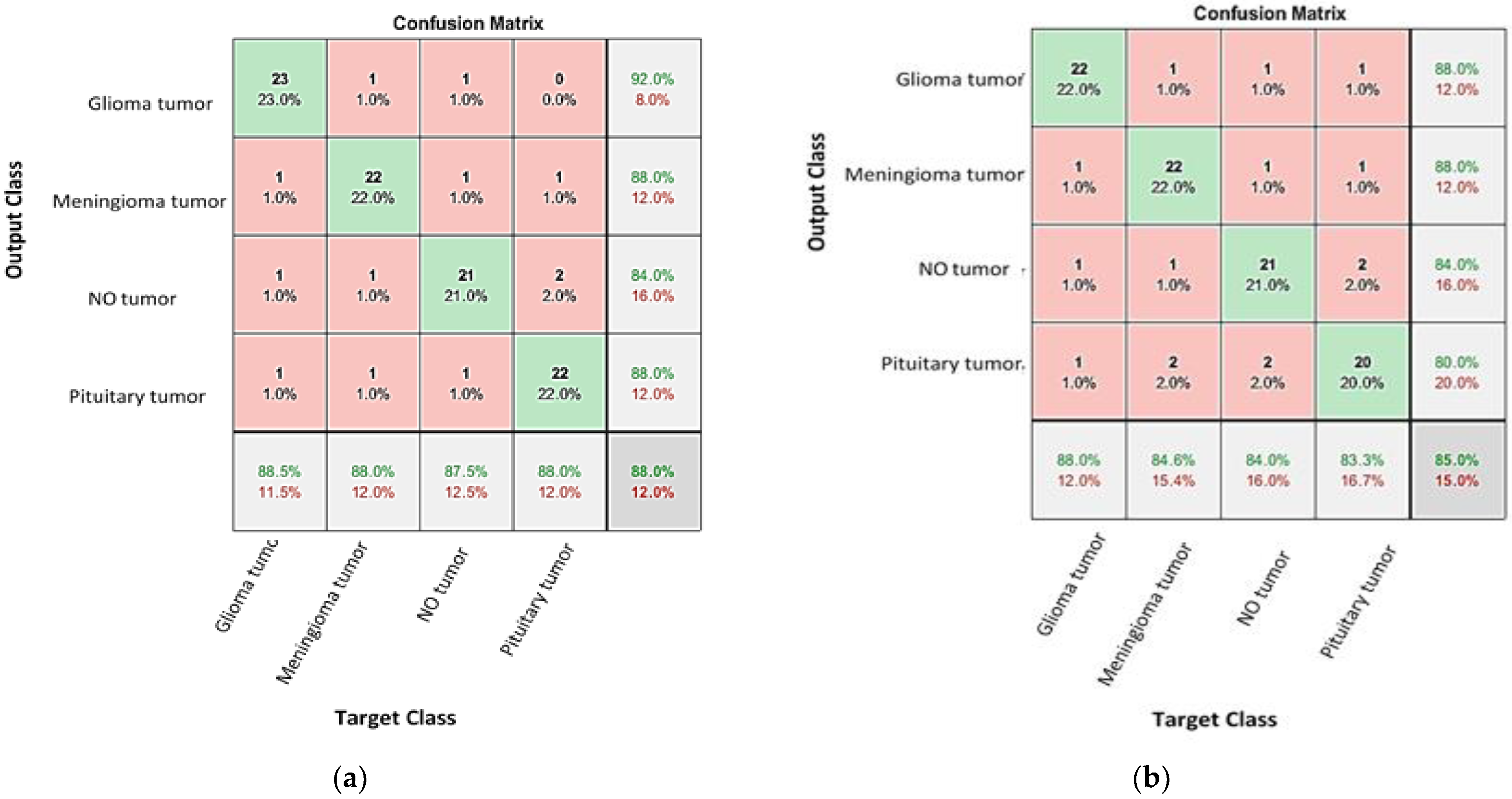
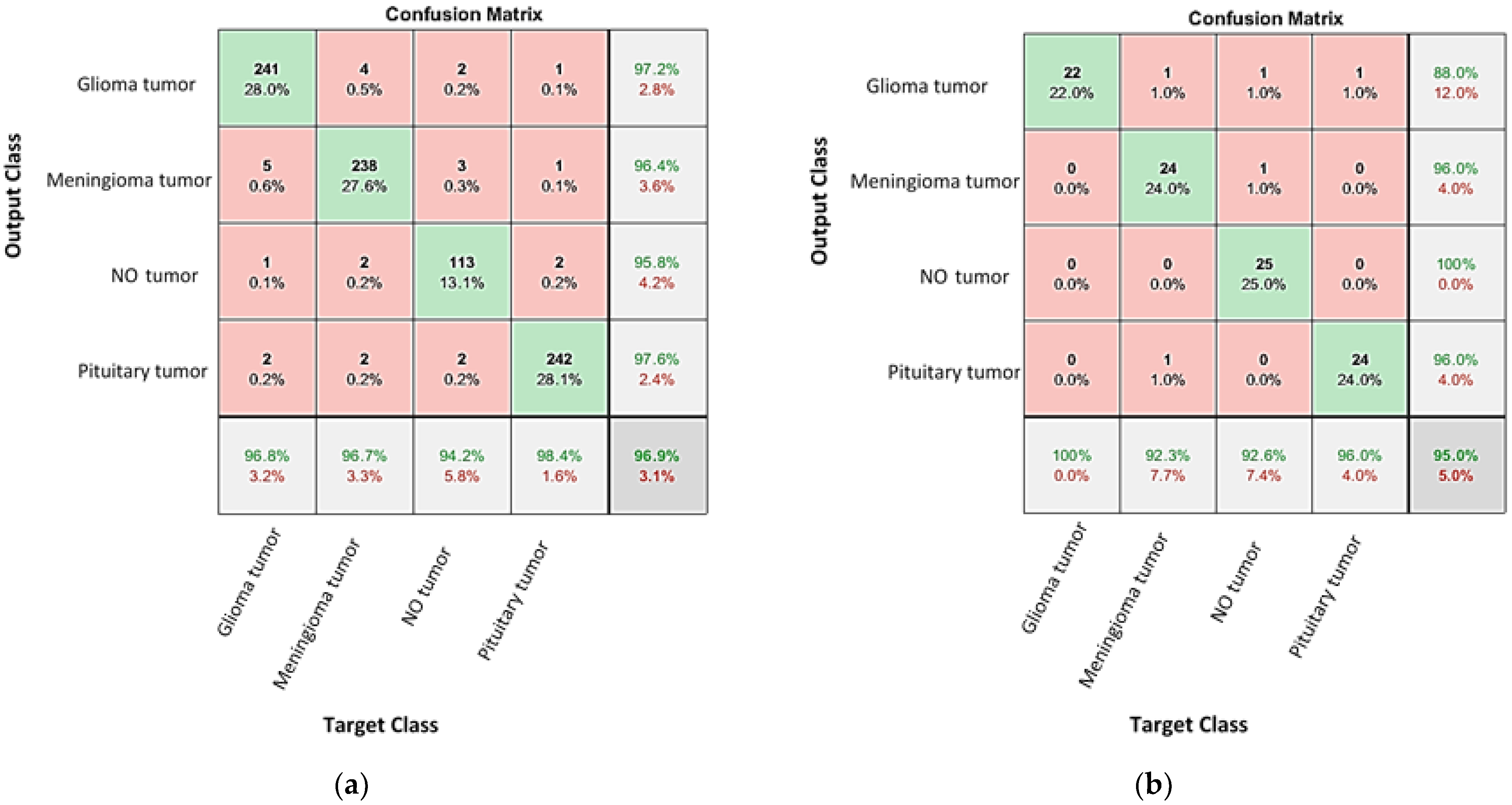
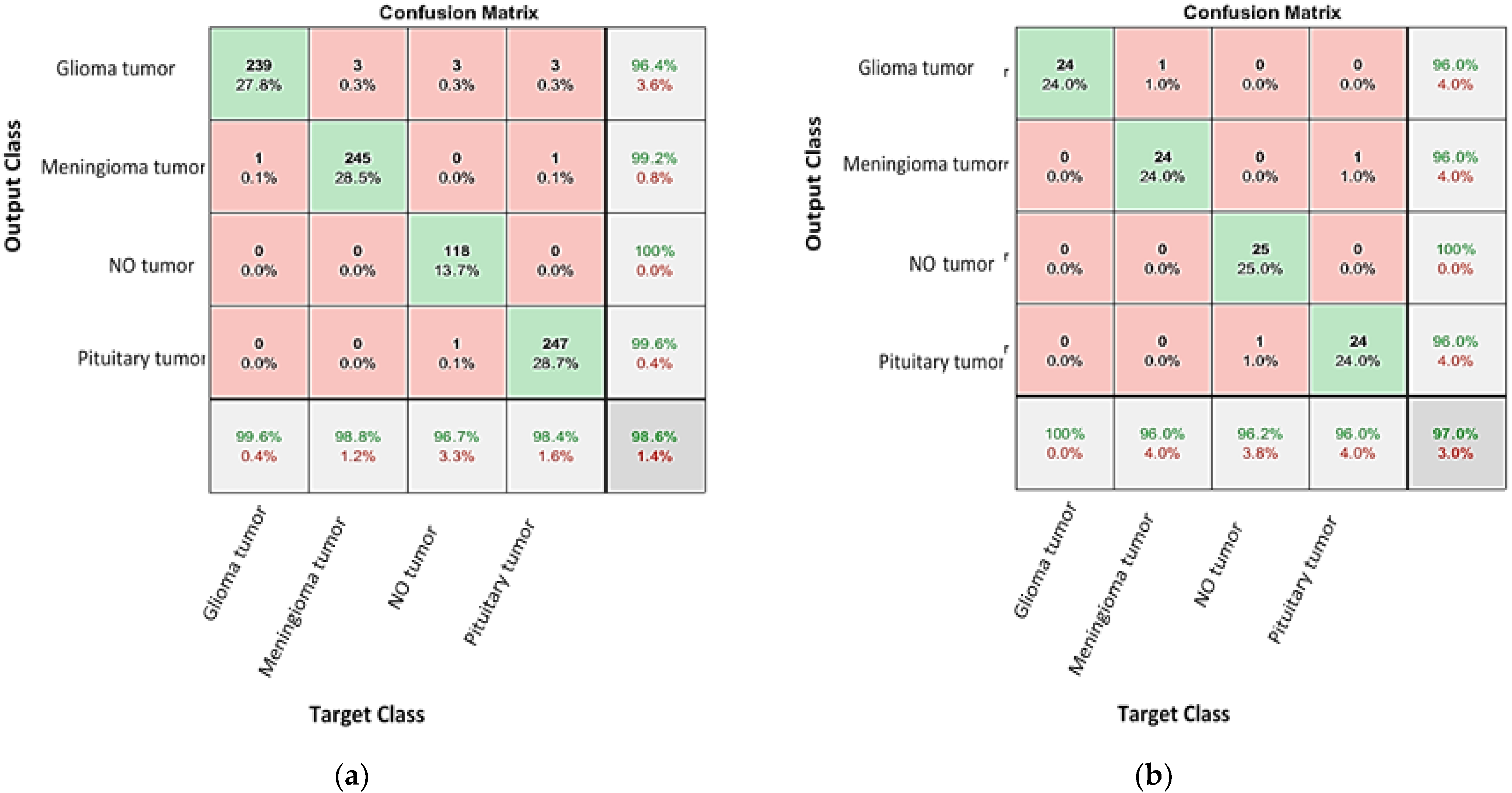
| Network | Validation (%) 1 | Testing Accuracy (%) | Precession (%) | Recall (%) | Specificity (%) | F1-Score (%) |
|---|---|---|---|---|---|---|
| GoogleNet | 91.5 | 84–92 | 88.46 | 88 | 96.05 | 88.46 |
| AlexNet | 90.2 | 80–88 | 88 | 84.62 | 95.95 | 86.27 |
| AlexNet-SVM | 96.9 | 88–100 | 88 | 100 | 96.15 | 93.62 |
| AlexNet-KNN | 98.6 | 96–100 | 96 | 100 | 98.68 | 97.96 |
| Reference | Tumor Classes | The Used Classifier Model | Accuracy |
|---|---|---|---|
| B. Srinivas et al. [36] | Malign and Benign | CNN-KNN | 96.25% |
| Nawab et al. [37] | Glioma, meningioma, and pituitary | Block-wise transfer learning | 94.82%. |
| Mircea et al. [38] | Benign or low-grade (1, 2) and malignant or high-grade (3, 4) | Wavelet transforms and support vector machines | 91% |
| F. Özyurt et al. [39] | Malign and Benign | NS-EMFSE–CNN–(KNN & SVM) | 90.62% 95.62% |
| M. Sajjad et al. [40] | Benign or low-grade (1, 2) and malignant or high-grade (3, 4) | Fine-tune- VGG-19/Softmax classifer | 90.67%. |
| K. Salçin [41] | Glioma, meningioma, and pituitary | Faster R-CNN | 91.66% |
| J. Amine et al. [42] | Benign or low-grade (1, 2) and malignant or high-grade (3, 4) | Inception-V3 and DensNet201 | 89% |
| Our work | Glioma, meningioma, pituitary, and no tumor cases | AlexNet-KNN | 98.6% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
AlTahhan, F.E.; Khouqeer, G.A.; Saadi, S.; Elgarayhi, A.; Sallah, M. Refined Automatic Brain Tumor Classification Using Hybrid Convolutional Neural Networks for MRI Scans. Diagnostics 2023, 13, 864. https://doi.org/10.3390/diagnostics13050864
AlTahhan FE, Khouqeer GA, Saadi S, Elgarayhi A, Sallah M. Refined Automatic Brain Tumor Classification Using Hybrid Convolutional Neural Networks for MRI Scans. Diagnostics. 2023; 13(5):864. https://doi.org/10.3390/diagnostics13050864
Chicago/Turabian StyleAlTahhan, Fatma E., Ghada A. Khouqeer, Sarmad Saadi, Ahmed Elgarayhi, and Mohammed Sallah. 2023. "Refined Automatic Brain Tumor Classification Using Hybrid Convolutional Neural Networks for MRI Scans" Diagnostics 13, no. 5: 864. https://doi.org/10.3390/diagnostics13050864
APA StyleAlTahhan, F. E., Khouqeer, G. A., Saadi, S., Elgarayhi, A., & Sallah, M. (2023). Refined Automatic Brain Tumor Classification Using Hybrid Convolutional Neural Networks for MRI Scans. Diagnostics, 13(5), 864. https://doi.org/10.3390/diagnostics13050864







