Accuracy of Dose-Saving Artificial-Intelligence-Based 3D Angiography (3DA) for Grading of Intracranial Artery Stenoses: Preliminary Findings
Abstract
1. Introduction
2. Methods
2.1. Patient Selection
2.2. Three-Dimensional Angiography
2.3. Data Acquisition and Postprocessing
2.4. Image Evaluation
2.5. Assessment of 3DA and 3D-DSA Reconstructions
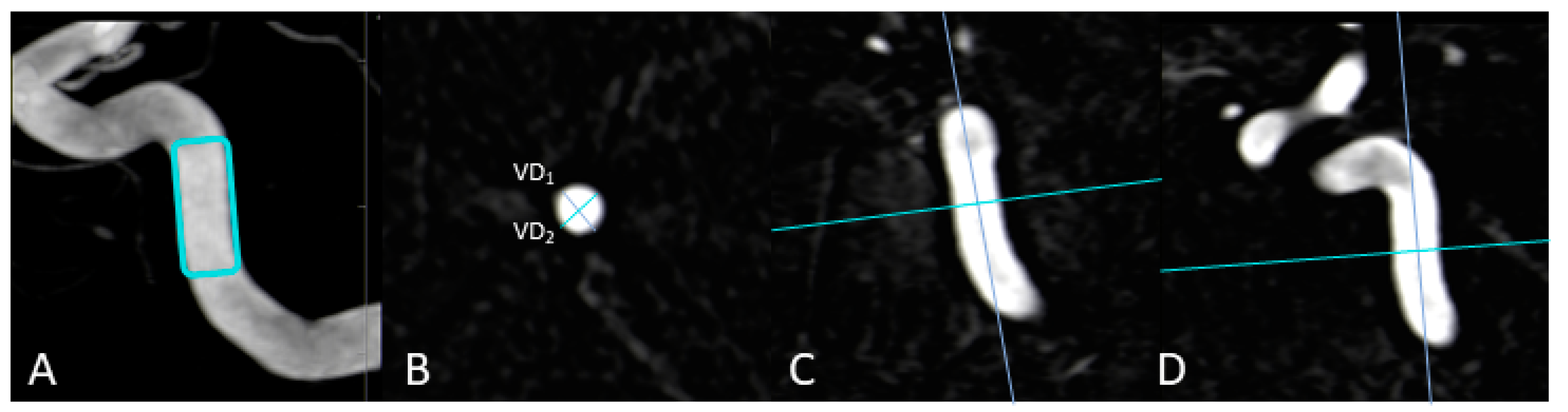
2.5.1. Vessel-Geometry Index (VGI)
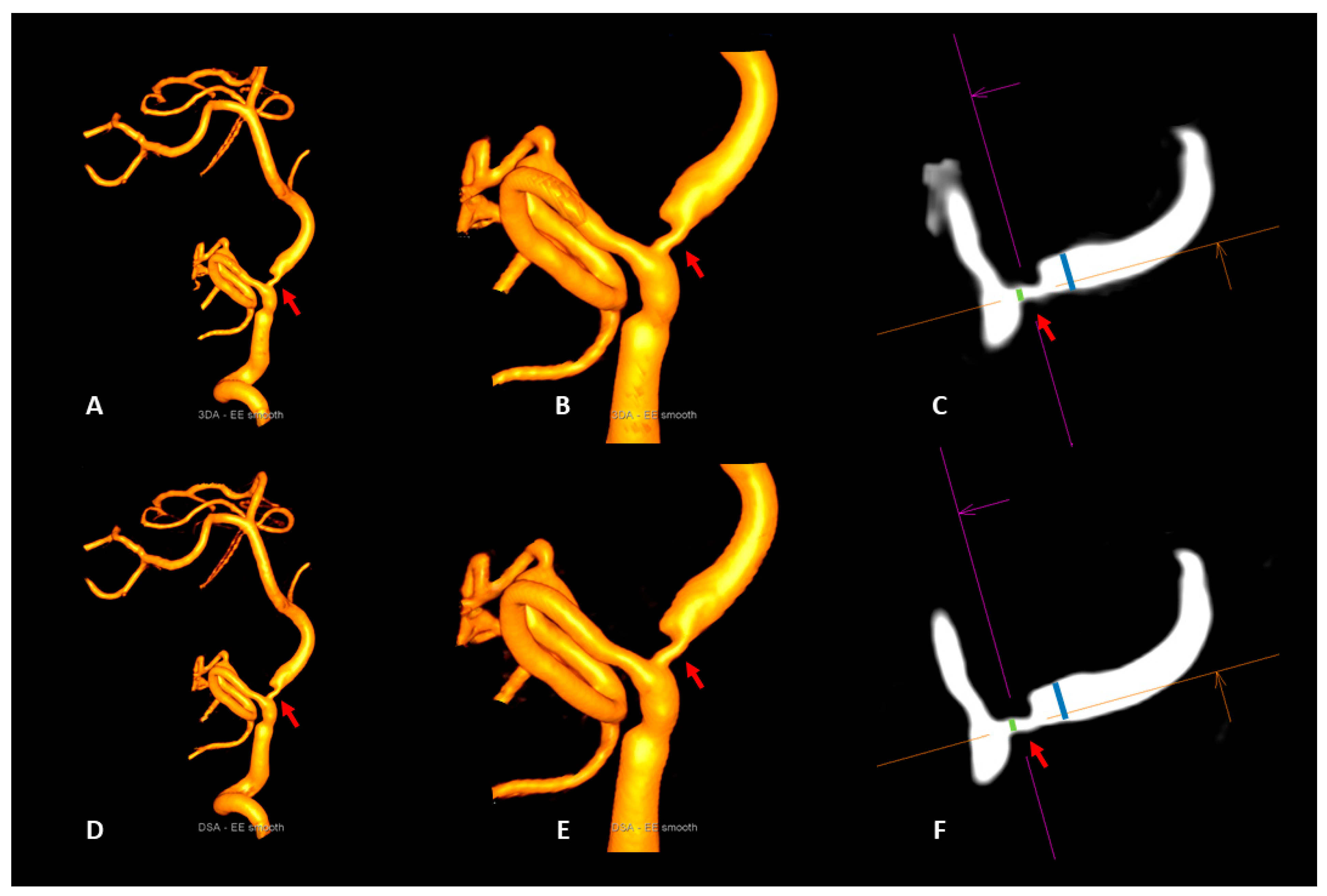
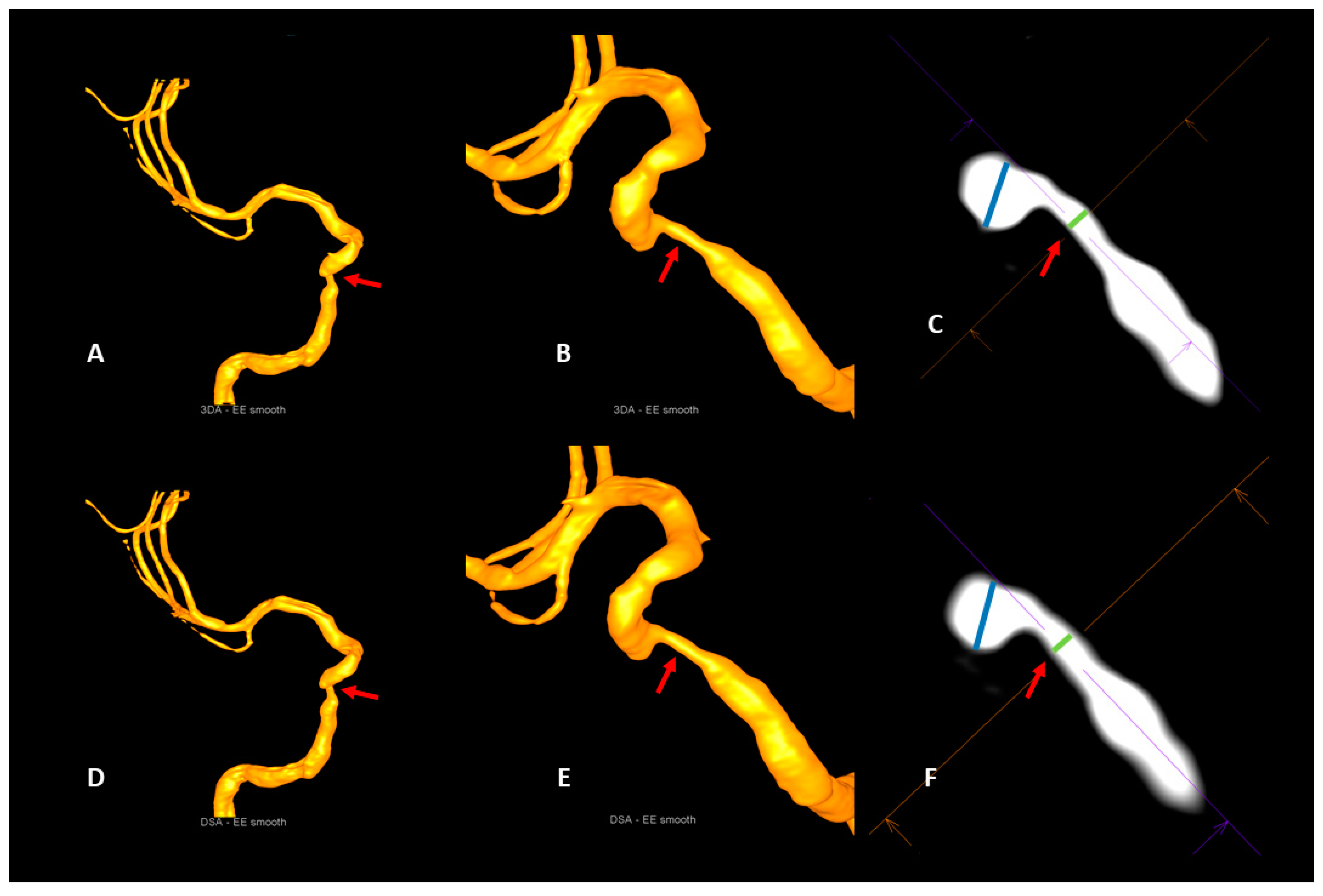
2.5.2. Intracranial Artery Stenosis
2.6. Statistical Analysis
3. Results
3.1. Image Quality
3.2. Qualitative and Quantitative Assessment of 3D-DSA and 3DA Reconstructions
3.3. Intracranial Artery Stenosis
4. Discussion
5. Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chen, X.Y.; Wong, K.S.; Lam, W.W.M.; Zhao, H.-L.; Ng, H.K. Middle cerebral artery atherosclerosis: Histological comparison between plaques associated with and not associated with infarct in a postmortem study. Cerebrovasc. Dis. 2007, 25, 74–80. [Google Scholar] [CrossRef]
- Mazighi, M.; Labreuche, J.; Gongora-Rivera, F.; Duyckaerts, C.; Hauw, J.-J.; Amarenco, P. Autopsy prevalence of intracranial atherosclerosis in patients with fatal stroke. Stroke 2008, 39, 1142–1147. [Google Scholar] [CrossRef] [PubMed]
- Sacco, R.; Kargman, D.; Gu, Q.; Zamaillo, M. Race-ethnicity and determinants of intracranial atherosclerotic cerebral infarction: The Northern Manhattan Stroke Study. Stroke 1995, 26, 14–20. [Google Scholar] [CrossRef] [PubMed]
- Suwanwela, N.C.; Chutinetr, A. Risk factors for atherosclerosis of cervicocerebral arteries: Intracranial versus extracranial. Neuroepidemiology 2003, 22, 37–40. [Google Scholar] [CrossRef]
- Wityk, R.J.; Lehman, D.; Klag, M.; Coresh, J.; Ahn, H.; Litt, B. Race and Sex Differences in the distribution of cerebral atherosclerosis. Stroke 1996, 27, 1974–1980. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.N.; Gao, S.; Li, S.W.; Li, J.F.; Wong, K.S.; Kay, R. Vascular lesions in Chinese patients with transient ischemic attacks. Neurology 1997, 48, 524–525. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.M.; Tu, Y.K.; Yip, P.K.; Su, C.T. Evaluation of intracranial and extracranial carotid steno-occlusive diseases in Taiwan Chinese patients with MR angiography: Preliminary experience. Stroke 1996, 27, 650–653. [Google Scholar] [CrossRef]
- Wong, K.S.; Huang, Y.N.; Gao, S.; Lam, W.; Chan, Y.L.; Kay, R. Intracranial stenosis in Chinese patients with acute stroke. Neurology 1998, 50, 812–813. [Google Scholar] [CrossRef] [PubMed]
- Wong, K.S.; Li, H.; Chan, Y.L.; Ahuja, A.; Lam, W.W.; Wong, A.; Kay, R. Use of transcranial Doppler ultrasound to predict outcome in patients with intracranial large-artery occlusive disease. Stroke 2000, 31, 2641–2647. [Google Scholar] [CrossRef]
- Hirai, T.; Korogi, Y.; Ono, K.; Nagano, M.; Maruoka, K.; Uemura, S.; Takahashi, M. Prospective evaluation of suspected stenoocclusive disease of the intracranial artery: Combined MR angiography and CT angiography compared with digital subtraction angiography. Am. J. Neuroradiol. 2002, 23, 93–101. [Google Scholar]
- Mturi, N.; Alcock, K.; Carter, J.A.; Newton, C.R.; Lange, J.H.; LaPorte, R.E.; Talbott, E.O.; Chang, Y.F.; Monsurro, M.R.; Aiello, I.E.; et al. Stroke outcome and neuroimaging of intracranial atherosclerosis (SONIA): Design of a prospective, multicenter trial of diagnostic tests. Neuroepidemiology 2004, 23, 23–32. [Google Scholar]
- Nguyen-Huynh, M.N.; Wintermark, M.; English, J.; Lam, J.; Vittinghoff, E.; Smith, W.S.; Johnston, S.C. How accurate is CT angiography in evaluating intracranial atherosclerotic disease? Stroke 2008, 39, 1184–1188. [Google Scholar] [CrossRef] [PubMed]
- Roubec, M.; Kuliha, M.; Jonszta, T.; Procházka, V.; Fadrná, T.; Filip, M.; Kaňovský, P.; Langová, K.; Herzig, R.; Školoudík, D. Detection of intracranial arterial stenosis using transcranial color-coded duplex sonography, computed tomographic angiography, and digital subtraction angiography. J. Ultrasound Med. 2011, 30, 1069–1075. [Google Scholar] [CrossRef]
- Duffis, E.J.; Jethwa, P.; Gupta, G.; Bonello, K.; Gandhi, C.D.; Prestigiacomo, C.J. Accuracy of computed tomographic angiography compared to digital subtraction angiography in the diagnosis of intracranial stenosis and its impact on clinical decision-making. J. Stroke Cerebrovasc. Dis. 2013, 22, 1013–1017. [Google Scholar] [CrossRef]
- Willinek, W.A.; von Falkenhausen, M.; Born, M.; Gieseke, J.; Höller, T.; Klockgether, T.; Textor, H.J.; Schild, H.H.; Urbach, H. Noninvasive detection of steno-occlusive disease of the supra-aortic arteries with three-dimensional contrast-enhanced magnetic resonance angiography: A prospective, intra-individual comparative analysis with digital subtraction angiography. Stroke 2005, 36, 38–43. [Google Scholar] [CrossRef] [PubMed]
- Baumgartner, R.W.; Mattle, H.P.; Schroth, G. Assessment of >/=50% and <50% intracranial stenoses by transcranial color-coded duplex sonography. Stroke 1999, 30, 87–92. [Google Scholar]
- Hurford, R.; Rothwell, P.M. Prevalence, prognosis, and treatment of atherosclerotic intracranial stenosis in Caucasians. Int. J. Stroke 2020, 16, 248–264. [Google Scholar] [CrossRef] [PubMed]
- Barr, J.D. cerebral angiography in the assessment of acute cerebral ischemia: Guidelines and recommendations. J. Vasc. Interv. Radiol. 2004, 15, S57–S66. [Google Scholar] [CrossRef]
- Rorick, M.B.; Nichols, F.T.; Adams, R.J. Transcranial Doppler correlation with angiography in detection of intracranial stenosis. Stroke 1994, 25, 1931–1934. [Google Scholar] [CrossRef]
- Lang, S.; Hoelter, P.; Schmidt, M.; Eisenhut, F.; Kaethner, C.; Kowarschik, M.; Lücking, H.; Doerfler, A. Evaluation of an artificial intelligence-based 3D-angiography for visualization of cerebral vasculature. Clin. Neuroradiol. 2019, 30, 705–712. [Google Scholar] [CrossRef]
- Struffert, T.; Hauer, M.; Banckwitz, R.; Köhler, C.; Royalty, K.; Doerfler, A. Effective dose to patient measurements in flat-detector and multislice computed tomography: A comparison of applications in neuroradiology. Eur. Radiol. 2014, 24, 1257–1265. [Google Scholar] [CrossRef]
- Montoya, J.; Li, Y.; Strother, C.; Chen, G.-H. 3D deep learning angiography (3D-DLA) from C-arm conebeam CT. Am. J. Neuroradiol. 2018, 39, 916–922. [Google Scholar] [CrossRef]
- Lang, S.; Hoelter, P.; Schmidt, M.; Strother, C.; Kaethner, C.; Kowarschik, M.; Doerfler, A. Artificial intelligence–based 3D angiography for visualization of complex cerebrovascular pathologies. Am. J. Neuroradiol. 2021, 42, 1762–1768. [Google Scholar] [CrossRef]
- Goodfellow, I.; Bengio, Y.; Courville, A. Deep Learning; MIT Press: Cambridge, MA, USA, 2016. [Google Scholar]
- Oord, A.V.D.; Dieleman, S.; Zen, H.; Simonyan, K.; Vinyals, O.; Graves, A.; Kalchbrenner, N.; Senior, A.; Kavukcuoglu, K. WaveNet: A generative model for raw audio. arXiv 2016, arXiv:1609.03499v2[cs.SD]. [Google Scholar]
- Ioffe, S.; Szegedy, C. Batch Normalization: Accelerating Deep Network Training by Reducing Internal Covariate Shift. In Proceedings of the 32nd International Conference on Machine Learning, Lille, France, 6–11 July 2015; PLMR; Volume 37, pp. 448–456. [Google Scholar]
- Kingma, D.; Ba, J. Adam: A Method for Stochastic Optimization. In Proceedings of the International Conference on Learning Representations (ICLR), San Diego, CA, USA, 7–9 May 2015. [Google Scholar]
- Bash, S.; Villablanca, J.P.; Jahan, R.; Duckwiler, G.; Tillis, M.; Kidwell, C.; Saver, J.; Sayre, J. Intracranial vascular stenosis and occlusive disease: Evaluation with CT angiography, MR angiography, and digital subtraction angiography. AJNR Am. J. Neuroradiol 2005, 26, 1012–1021. [Google Scholar]
- Grass, M.; Koppe, R.; Klotz, E.; Proksa, R.; Kuhn, M.; Aerts, H.; de Beek, J.O.; Kemkers, R. Three-dimensional reconstruction of high contrast objects using C-arm image intensifier projection data. Comput. Med. Imaging Graph. 1999, 23, 311–321. [Google Scholar] [CrossRef]
- Gosch, D.; Kurze, W.; Deckert, F.; Schulz, T.; Patz, A.; Kahn, T. Radiation exposure with 3D rotational angiography of the skull. RoFo: Fortschr. Auf Dem Geb. Der Rontgenstrahlen Und Der Nukl. 2006, 178, 880–885. [Google Scholar] [CrossRef]
- Raabe, A.; Beck, J.; Rohde, S.; Berkefeld, J.; Seifert, V. Three-dimensional rotational angiography guidance for aneurysm surgery. J. Neurosurg. 2006, 105, 406–411. [Google Scholar] [CrossRef]
- Hirai, T.; Korogi, Y.; Suginohara, K.; Ono, K.; Nishi, T.; Uemura, S.; Yamura, M.; Yamashita, Y. Clinical Usefulness of unsubtracted 3D digital angiography compared with rotational digital angiography in the pretreatment evaluation of intracranial aneurysms. Am. J. Neuroradiol. 2003, 24, 1067–1074. [Google Scholar]
- Pearl, M.S.; Torok, C.M.; Messina, A.S.; Radvany, M.; Rao, S.N.; Ehtiati, T.; Thompson, C.B.; Gailloud, P. Reducing radiation dose while maintaining diagnostic image quality of cerebral three-dimensional digital subtraction angiography: An in vivo study in swine. J. NeuroInterventional Surg. 2013, 6, 672–676. [Google Scholar] [CrossRef]
- Pearl, M.S.; Torok, C.; Katz, Z.; Messina, A.S.; Blasco, J.; Tamargo, R.J.; Huang, J.; Leigh, R.; Zeiler, S.; Radvany, M.; et al. Diagnostic quality and accuracy of low dose 3D-DSA protocols in the evaluation of intracranial aneurysms. J. NeuroInterventional Surg. 2014, 7, 386–390. [Google Scholar] [CrossRef]
- Rudilosso, S.; Rodríguez-Vázquez, A.; Urra, X.; Arboix, A. The potential impact of neuroimaging and translational research on the clinical management of lacunar stroke. Int. J. Mol. Sci. 2022, 23, 1497. [Google Scholar] [CrossRef]
| GradeIQ | Characteristics |
|---|---|
| 4 | excellent (high contrast, clear delineation of the stenosis, no artifacts) |
| 3 | good (high contrast; good delineation of the stenosis, minimal artifacts, e.g., due to movement) |
| 2 | compromised (e.g., noticeable movement artifacts and/or reduced homogeneity of the vessel contrast, delineation of the stenosis still acceptable) |
| 1 | heavily compromised (low contrast and/or strong movement artifacts, difficult delineation of the stenosis) |
| 0 | not diagnostic (no delineation of the stenosis) |
| Parameter | 3DA | 3D-DSA | r | p |
|---|---|---|---|---|
| VD1IAS | 4.41 ± 0.69 mm | 4.36 ± 0.64 mm | 0.994 | 0.0001 |
| VD2IAS | 4.65 ± 0.63 mm | 4.58 ± 0.61 mm | 0.994 | 0.0001 |
| VGIIAS | 0.95 ± 0.03 mm | 0.95 ± 0.03 mm | 0.899 | 0.0001 |
| dpoststenotic | 1.74 ± 0.58 mm | 1.68 ± 0.53 mm | 0.995 | 0.0001 |
| dintrastenotic | 3.12 ± 0.71 mm | 3.04 ± 0.67 mm | 0.993 | 0.0001 |
| NASCETIAS | 43 ± 0.16% | 44 ± 0.15% | 0.981 | 0.0001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lang, S.; Hoelter, P.; Schmidt, M.A.; Mrochen, A.; Kuramatsu, J.; Kaethner, C.; Roser, P.; Kowarschik, M.; Doerfler, A. Accuracy of Dose-Saving Artificial-Intelligence-Based 3D Angiography (3DA) for Grading of Intracranial Artery Stenoses: Preliminary Findings. Diagnostics 2023, 13, 712. https://doi.org/10.3390/diagnostics13040712
Lang S, Hoelter P, Schmidt MA, Mrochen A, Kuramatsu J, Kaethner C, Roser P, Kowarschik M, Doerfler A. Accuracy of Dose-Saving Artificial-Intelligence-Based 3D Angiography (3DA) for Grading of Intracranial Artery Stenoses: Preliminary Findings. Diagnostics. 2023; 13(4):712. https://doi.org/10.3390/diagnostics13040712
Chicago/Turabian StyleLang, Stefan, Philip Hoelter, Manuel Alexander Schmidt, Anne Mrochen, Joji Kuramatsu, Christian Kaethner, Philipp Roser, Markus Kowarschik, and Arnd Doerfler. 2023. "Accuracy of Dose-Saving Artificial-Intelligence-Based 3D Angiography (3DA) for Grading of Intracranial Artery Stenoses: Preliminary Findings" Diagnostics 13, no. 4: 712. https://doi.org/10.3390/diagnostics13040712
APA StyleLang, S., Hoelter, P., Schmidt, M. A., Mrochen, A., Kuramatsu, J., Kaethner, C., Roser, P., Kowarschik, M., & Doerfler, A. (2023). Accuracy of Dose-Saving Artificial-Intelligence-Based 3D Angiography (3DA) for Grading of Intracranial Artery Stenoses: Preliminary Findings. Diagnostics, 13(4), 712. https://doi.org/10.3390/diagnostics13040712






