Utilisation of 3D Printing in the Manufacturing of an Anthropomorphic Paediatric Head Phantom for the Optimisation of Scanning Parameters in CT
Abstract
1. Introduction
2. Materials and Methods
2.1. CT Scanner and Scanning Protocol
2.2. Anthropomorphic Phantom
2.3. Fabrication of the 3D Model
2.4. Assessment of Phantom Quality
2.5. Image Quality Model
2.6. Subjective Image Quality Assessment
3. Results
3.1. Accuracy of the Manufactured Phantom
3.2. Protocol Development
3.3. Patient Doses
3.4. Subjective Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Goo, H.W. CT radiation dose optimisation and estimation: An update for radiologists. Korean J. Radiol. 2012, 13, 1–11. [Google Scholar] [CrossRef]
- Mathews, J.D.; Forsythe, A.V.; Brady, Z.; Butler, M.W.; Goergen, S.K.; Byrnes, G.B.; Giles, G.G.; Wallace, A.B.; Anderson, P.R.; Guiver, T.A.; et al. Cancer risk in 680,000 people exposed to computed tomography scans in childhood or adolescence: Data linkage study of 11 million Australians. BMJ 2013, 346, f2360. [Google Scholar] [CrossRef]
- Čiva, L.M.; Beganović, A.; Busuladžić, M.; Jusufbegović, M.; Awad-Dedić, T.; Vegar-Zubović, S. Dose Descriptors and Assessment of Risk of Exposure-Induced Death in Patients Undergoing COVID-19 Related Chest Computed Tomography. Diagnostics 2022, 12, 2012. [Google Scholar] [CrossRef]
- ICRP. ICRP Publication 121: Radiological Protection in Paediatric Diagnostic and Interventional Radiology. Ann. ICRP 2013, 42, 1–63. [Google Scholar]
- Winslow, J.F.; Hyer, D.E.; Fisher, R.F.; Tien, C.J.; Hintenlang, D.E. Construction of anthropomorphic phantoms for use in dosimetry studies. J. Appl. Clin. Med. Phys. 2009, 10, 195–204. [Google Scholar] [CrossRef]
- Okkalidis, N. 3D printing methods for radiological anthropomorphic phantoms. Phys. Med. Biol. 2022, 67, 15TR04. [Google Scholar] [CrossRef]
- Ardila Pardo, G.L.; Conzelmann, J.; Genske, U.; Hamm, B.; Scheel, M.; Jahnke, P. 3D printing of anatomically realistic phantoms with detection tasks to assess the diagnostic performance of CT images. Eur. Radiol. 2020, 30, 4557–4563. [Google Scholar] [CrossRef]
- Bieniosek, M.F.; Lee, B.J.; Levin, C.S. Characterization of custom 3D printed multimodality imaging phantoms. Med. Phys. 2015, 42, 5913–5918. [Google Scholar] [CrossRef]
- Bücking, T.M.; Hill, E.R.; Robertson, J.L.; Maneas, E.; Plumb, A.A.; Nikitichev, D.I. From medical imaging data to 3D printed anatomical models. PLoS ONE 2017, 12, e0178540. [Google Scholar] [CrossRef] [PubMed]
- Leng, S.; McGee, K.; Morris, J.; Alexander, A.; Kuhlmann, J.; Vrieze, T.; McCollough, C.H.; Matsumoto, J. Anatomic modeling using 3D printing: Quality assurance and optimisation. 3D Print. Med. 2017, 3, 6. [Google Scholar] [CrossRef] [PubMed]
- Mahmood, U.; Apte, A.P.; Kanan, C.; Bates, D.D.; Corrias, G.; Manneli, L.; Oh, J.H.; Erdi, Y.E.; Nguyen, J.; Deasy, J.O.; et al. Quality control of radiomic features using 3D-printed CT phantoms. J. Med. Imaging 2021, 8, 033505. [Google Scholar] [CrossRef] [PubMed]
- Meyer-Szary, J.; Luis, M.S.; Mikulski, S.; Patel, A.; Schulz, F.; Tretiakow, D.; Fercho, J.; Jaguszewska, K.; Frankiewicz, M.; Pawłowska, E.; et al. The Role of 3D Printing in Planning Complex Medical Procedures and Training of Medical Professionals—Cross-Sectional Multispecialty Review. Int. J. Environ. Res. Public Health 2022, 19, 3331. [Google Scholar] [CrossRef] [PubMed]
- Yan, Q.; Dong, H.; Su, J.; Han, J.; Song, B.; Wei, Q.; Shi, Y. A review of 3D printing technology for medical applications. Engineering 2018, 4, 729–742. [Google Scholar] [CrossRef]
- Aimar, A.; Palermo, A.; Innocenti, B. The role of 3D printing in medical applications: A state of the art. J. Healthc. Eng. 2019, 2019, 5340616. [Google Scholar] [CrossRef] [PubMed]
- Dukov, N.; Bliznakova, K.; Teneva, T.; Marinov, S.; Bakic, P.; Bosmans, H.; Bliznakov, Z. Experimental evaluation of physical breast phantoms for 2D and 3D breast x-ray imaging techniques. In Proceedings of the European Medical and Biological Engineering Conference, Portoroz, Slovenia, 29 November–3 December 2020; Springer: Berlin/Heidelberg, Germany, 2020; pp. 544–552. [Google Scholar]
- Ikejimba, L.C.; Salad, J.; Graff, C.G.; Ghammraoui, B.; Cheng, W.C.; Lo, J.Y.; Glick, S.J. A four-alternative forced choice (4AFC) methodology for evaluating microcalcification detection in clinical full-field digital mammography (FFDM) and digital breast tomosynthesis (DBT) systems using an inkjet-printed anthropomorphic phantom. Med. Phys. 2019, 46, 3883–3892. [Google Scholar] [CrossRef]
- Aburjaile, W.; Mourao, A. Development of a chest phantom for testing in Computed Tomography scans. Radiat. Phys. Chem. 2017, 140, 275–277. [Google Scholar] [CrossRef]
- Irnstorfer, N.; Unger, E.; Hojreh, A.; Homolka, P. An anthropomorphic phantom representing a prematurely born neonate for digital x-ray imaging using 3D printing: Proof of concept and comparison of image quality from different systems. Sci. Rep. 2019, 9, 14357. [Google Scholar] [CrossRef]
- Leary, M.; Tino, R.; Keller, C.; Franich, R.; Yeo, A.; Lonski, P.; Kyriakou, E.; Kron, T.; Brandt, M. Additive manufacture of lung equivalent anthropomorphic phantoms: A method to control hounsfield number utilising partial volume effect. ASME J. Med. Diagn. 2020, 3, 011001. [Google Scholar] [CrossRef]
- Jusufbegović, M.; Pandžić, A.; Šehić, A.; Jašić, R.; Julardžija, F.; Vegar-Zubović, S.; Beganović, A. Computed tomography tissue equivalence of 3D printing materials. Radiography (Lond.) 2022, 28, 788–792. [Google Scholar] [CrossRef]
- McGarry, C.K.; Grattan, L.J.; Ivory, A.M.; Leek, F.; Liney, G.P.; Liu, Y.; Miloro, P.; Rai, R.; Robinson, A.P.; Shih, A.J.; et al. Tissue mimicking materials for imaging and therapy phantoms: A review. Phys. Med. Biol. 2020, 65, 23TR01. [Google Scholar] [CrossRef]
- Niebuhr, N.; Johnen, W.; Echner, G.; Runz, A.; Bach, M.; Stoll, M.; Giske, K.; Greilich, S.; Pfaffenberger, A. The ADAM-pelvis phantom—An anthropomorphic, deformable and multimodal phantom for MRgRT. Phys. Med. Biol. 2019, 64, 04NT05. [Google Scholar] [CrossRef]
- Ali, A.M.; Hogg, P.; Johansen, S.; England, A. Construction and validation of a low cost paediatric pelvis phantom. Eur. J. Radiol. 2018, 108, 84–91. [Google Scholar]
- AAPM. Pediatric Routine Head CT Protocols, Version 1.1; American Association of Physicists in Medicine: Alexandria, VA, USA, 2015. [Google Scholar]
- European Commission. Radiation Protection N° 180: Diagnostic Reference Levels in Thirty-Six European Countries; European Union: Luxembourg, 2014. [Google Scholar]
- Awad-Dedić, T.; Čiva M., L.; Beganović, A.; Busuladžić, M.; Đedović, E.; Vegar-Zubović, S. Local Diagnostic Reference Levels in Emergency Computed Tomography of the Head. IFMBE 2021, 84, 768–776. [Google Scholar]
- Genisa, M.; Shuib, S.; Rajion, Z.A.; Arief, E.M.; Hermana, M. Density estimation based on the Hounsfield unit value of cone beam computed tomography imaging of the jawbone system. Proc. Inst. Mech. Eng. H 2018, 232, 1168–1175. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.Y.; Choi, I.; Lee, S.J.; Jeong, I.S.; Kim, Y.O.; Woo, Y.J.; Cho, H.J. Clinical Significance of Gray to White Matter Ratio after Cardiopulmonary Resuscitation in Children. Children 2022, 9, 36. [Google Scholar] [CrossRef] [PubMed]
- CIRS. ATOM Max Dental and Diagnostic Head Phantom Model 711-HN. 2013. Available online: https://bit.ly/3CFXdpf (accessed on 15 October 2022).
- Saeed, M.; Almalki, Y. Assessment of computed tomography radiation doses for paediatric head and chest examinations using paediatric phantoms of three different ages. Radiography 2021, 27, 332–339. [Google Scholar] [CrossRef]
- Filippou, V.; Tsoumpas, C. Recent advances on the development of phantoms using 3D printing for imaging with CT, MRI, PET, SPECT, and ultrasound. Med. Phys. 2018, 45, e740–e760. [Google Scholar] [CrossRef]
- Hatamikia, S.; Kronreif, G.; Unger, A.; Oberoi, G.; Jaksa, L.; Unger, E.; Koschitz, S.; Gulyas, I.; Irnstorfer, N.; Buschmann, M.; et al. 3D printed patient-specific thorax phantom with realistic heterogenous bone radiopacity using filament printer technology. Z. Med. Phys. 2022, 32, 438–452. [Google Scholar] [CrossRef]
- Ehler, E.; Craft, D.; Rong, Y. 3D printing technology will eventually eliminate the need of purchasing commercial phantoms for clinical medical physics QA procedures. J. Appl. Clin. Med. Phys. 2018, 19, 8–12. [Google Scholar] [CrossRef]
- Holmes, R.B.; Negus, I.S.; Wiltshire, S.J.; Thorne, G.C.; Young, P.; Initiative, A.D.N. Creation of an anthropomorphic CT head phantom for verification of image segmentation. Med. Phys. 2020, 47, 2380–2391. [Google Scholar] [CrossRef]
- Kikinis, R.; Pieper, S.D.; Vosburgh, K.G. 3D Slicer: A platform for subject-specific image analysis, visualization, and clinical support. In Intraoperative Imaging and Image-Guided Therapy; Springer: Berlin/Heidelberg, Germany, 2014; pp. 277–289. [Google Scholar]
- Pandzic, A. Influence of layer height, build orientation and post curing on tensile mechanical properties of SLA 3D printed material. In Proceedings of the 32nd DAAAM International Symposium on Intelligent Manufacturing and Automation, Vienna, Austria, 28–29 October 2021; DAAAM International: Vienna, Austria, 2021; pp. 0200–0208. [Google Scholar]
- Chandramohan, D.; Cao, P.; Han, M.; An, H.; Sunderland, J.; Kinahan, P.; Laforest, R.; Hope, T.; Larson, P. Anthropomorphic skull phantom using quantitatively accurate bone mimic material. J. Nucl. Med. 2019, 60, 1356. [Google Scholar]
- Bryant, J.; Drage, N.A.; Richmond, S. CT number definition. Radiat. Phys. Chem. 2012, 81, 358–361. [Google Scholar] [CrossRef]
- Verdun, F.; Racine, D.; Ott, J.; Tapiovaara, M.; Toroi, P.; Bochud, F.; Veldkamp, W.; Schegerer, A.; Bouwman, R.; Giron, I.H.; et al. Image quality in CT: From physical measurements to model observers. Phys. Med. 2015, 31, 823–843. [Google Scholar] [CrossRef]
- Diwakar, M.; Kumar, M. A review on CT image noise and its denoising. Biomed. Signal Proces. Control 2018, 42, 73–88. [Google Scholar] [CrossRef]
- Brooks, R.A.; Di Chiro, G. Statistical limitations in x-ray reconstructive tomography. Med. Phys. 1976, 3, 237–240. [Google Scholar] [CrossRef]
- Rodríguez-Sánchez, Á.; Thompson, A.; Körner, L.; Brierley, N.; Leach, R. Review of the influence of noise in X-ray computed tomography measurement uncertainty. Precis. Eng. 2020, 66, 382–391. [Google Scholar] [CrossRef]
- Duan, X.; Wang, J.; Leng, S.; Schmidt, B.; Allmendinger, T.; Grant, K.; Flohr, T.; McCollough, C.H. Electronic noise in CT detectors: Impact on image noise and artifacts. AJR Am. J. Roentgenol. 2013, 201, W626–W632. [Google Scholar] [CrossRef]
- Ma, J.; Liang, Z.; Fan, Y.; Liu, Y.; Huang, J.; Chen, W.; Lu, H. Variance analysis of x-ray CT sinograms in the presence of electronic noise background. Med. Phys. 2012, 39, 4051–4065. [Google Scholar] [CrossRef]
- Karappara, J.; Koteshwar, P.; Panakkal, N.C.; Sukumar, S. Optimization of paediatric CT brain protocol to achieve reduced patient dose. Biomed. Pharmacol. J. 2020, 13, 391–397. [Google Scholar] [CrossRef]
- Jaramillo-Garzón, W.; Caballero, M.; Alvarez-Aldana, D. Size-specific dose estimates for paediatric non-contrast head CT scans: A retrospective patient study in Tunja, Colombia. Radiat. Prot. Dosim. 2021, 193, 221–227. [Google Scholar] [CrossRef]
- Carmichael, J.; Moores, B.; Maccia, C. European Guidelines on Quality Criteria for Diagnostic Radiographic Images; European Commission and Directorate-General for Research and Innovation, Publications Office: Brussels, Belgium, 2000. [Google Scholar]
- Vasung, L.; Turk, E.A.; Ferradal, S.L.; Sutin, J.; Stout, J.N.; Ahtam, B.; Lin, P.Y.; Grant, P.E. Exploring early human brain development with structural and physiological neuroimaging. Neuroimage 2019, 187, 226–254. [Google Scholar] [CrossRef] [PubMed]
- Brooks, T.; Choi, J.E.; Garnich, M.; Hammer, N.; Waddell, J.N.; Duncan, W.; Jermy, M. Finite element models and material data for analysis of infant head impacts. Heliyon 2018, 4, e01010. [Google Scholar] [CrossRef] [PubMed]
- Bartholomeusz, H.; Courchesne, E.; Karns, C. Relationship between head circumference and brain volume in healthy normal toddlers, children, and adults. Neuropaediatrics 2002, 33, 239–241. [Google Scholar] [CrossRef] [PubMed]
- Pearce, M.S.; Salotti, J.A.; Little, M.P.; McHugh, K.; Lee, C.; Kim, K.P.; Howe, N.L.; Ronckers, C.M.; Rajaraman, P.; Craft, A.W.S.; et al. Radiation exposure from CT scans in childhood and subsequent risk of leukaemia and brain tumours: A retrospective cohort study. Lancet 2012, 380, 499–505. [Google Scholar] [CrossRef]
- Gao, Y.; Quinn, B.; Pandit-Taskar, N.; Behr, G.; Mahmood, U.; Long, D.; Xu, G.X.; Germain, J.S.; Dauer, L.T. Patient-specific organ and effective dose estimates in paediatric oncology computed tomography. Phys. Med. 2018, 45, 146–155. [Google Scholar] [CrossRef]
- Spampinato, M.V.; Stalcup, S.; Matheus, M.G.; Byington, K.; Tyler, M.; Bickley, S.; Tipnis, S. Radiation dose and image quality in paediatric head CT. Radiat. Prot. Dosim. 2018, 182, 310–316. [Google Scholar] [CrossRef]
- Mohammadbeigi, A.; Khoshgard, K.; Haghparast, A.; Eivazi, M.T. Local DRLs for paediatric CT examinations based on size-specific dose estimates in Kermanshah, Iran. Radiat. Prot. Dosim. 2019, 186, 496–506. [Google Scholar] [CrossRef]
- Abdulkadir, M.K.; Shuaib, I.L.; Achuthan, A.; Nasirudin, R.A.; Samsudin, A.H.Z.; Osman, N.D. Estimation of paediatric dose descriptors adapted to individual specific size from CT examinations. Radiat. Prot. Dosim. 2022, 198, 1292–1302. [Google Scholar] [CrossRef]
- Granata, C.; Origgi, D.; Palorini, F.; Matranga, D.; Salerno, S. Radiation dose from multidetector CT studies in children: Results from the first Italian nationwide survey. Pediatr. Radiol. 2015, 45, 695–705. [Google Scholar] [CrossRef]
- Célier, D.; Roch, P.; Etard, C.; Le Pointe, H.D.; Brisse, H.J. Multicentre survey on patient dose in paediatric imaging and proposal for updated diagnostic reference levels for France. Part 1: Computed tomography. Eur. Radiol. 2020, 30, 1156–1165. [Google Scholar] [CrossRef]
- EuropeanCommission. European Guidelines on Diagnostic Reference Levels for Paediatric Imaging; European Union: Luxembourg, 2018. [Google Scholar]
- Burton, C.S.; Szczykutowicz, T.P. Evaluation of AAPM Reports 204 and 220: Estimation of effective diameter, water-equivalent diameter, and ellipticity ratios for chest, abdomen, pelvis, and head CT scans. J. Appl. Clin. Med. Phys. 2018, 19, 228–238. [Google Scholar] [CrossRef] [PubMed]
- Keat, N. CT scanner automatic exposure control systems. MHRA Rep. 05016 2005. [Google Scholar] [CrossRef]
- Söderberg, M.; Gunnarsson, M. Automatic exposure control in computed tomography–an evaluation of systems from different manufacturers. Acta Radiol. 2010, 51, 625–634. [Google Scholar] [CrossRef] [PubMed]
- Tack, D.; Kalra, M.K.; Gevenois, P.A. Radiation Dose from Multidetector CT; Springer Science & Business Media: New York, NY, USA, 2012. [Google Scholar]
- Inoue, Y.; Itoh, H.; Shiibashi, N.; Sasa, R.; Mitsui, K. Sample Size and Estimation of Standard Radiation Doses for Pediatric Brain CT. Tomography 2022, 8, 2486–2497. [Google Scholar] [CrossRef]
- McNitt-Gray, M.F. AAPM/RSNA physics tutorial for residents: Topics in CT: Radiation dose in CT. Radiographics 2002, 22, 1541–1553. [Google Scholar] [CrossRef]
- Abdulkadir, M.K.; Rahim, N.A.Y.M.; Mazlan, N.S.; Daud, N.M.; Shuaib, I.L.; Osman, N.D. Dose optimisation in paediatric CT examination: Assessment on current scanning protocols associated with radiation dose. Radiat. Phys. Chem. 2020, 171, 108740. [Google Scholar] [CrossRef]
- Kim, S.Y.; Park, J.W.; Park, J.; Yea, J.W.; Oh, S.A. Fabrication of 3D printed head phantom using plaster mixed with polylactic acid powder for patient-specific QA in intensity-modulated radiotherapy. Sci. Rep. 2022, 12, 17500. [Google Scholar] [CrossRef]
- Wang, Y.; Dankelman, J.; Ruijters, D. Cost-efficient anthropomorphic head phantom for quantitative image quality assessment in cone beam CT. Biomed. Phys. Eng. Express 2022, 8, 065038. [Google Scholar] [CrossRef]

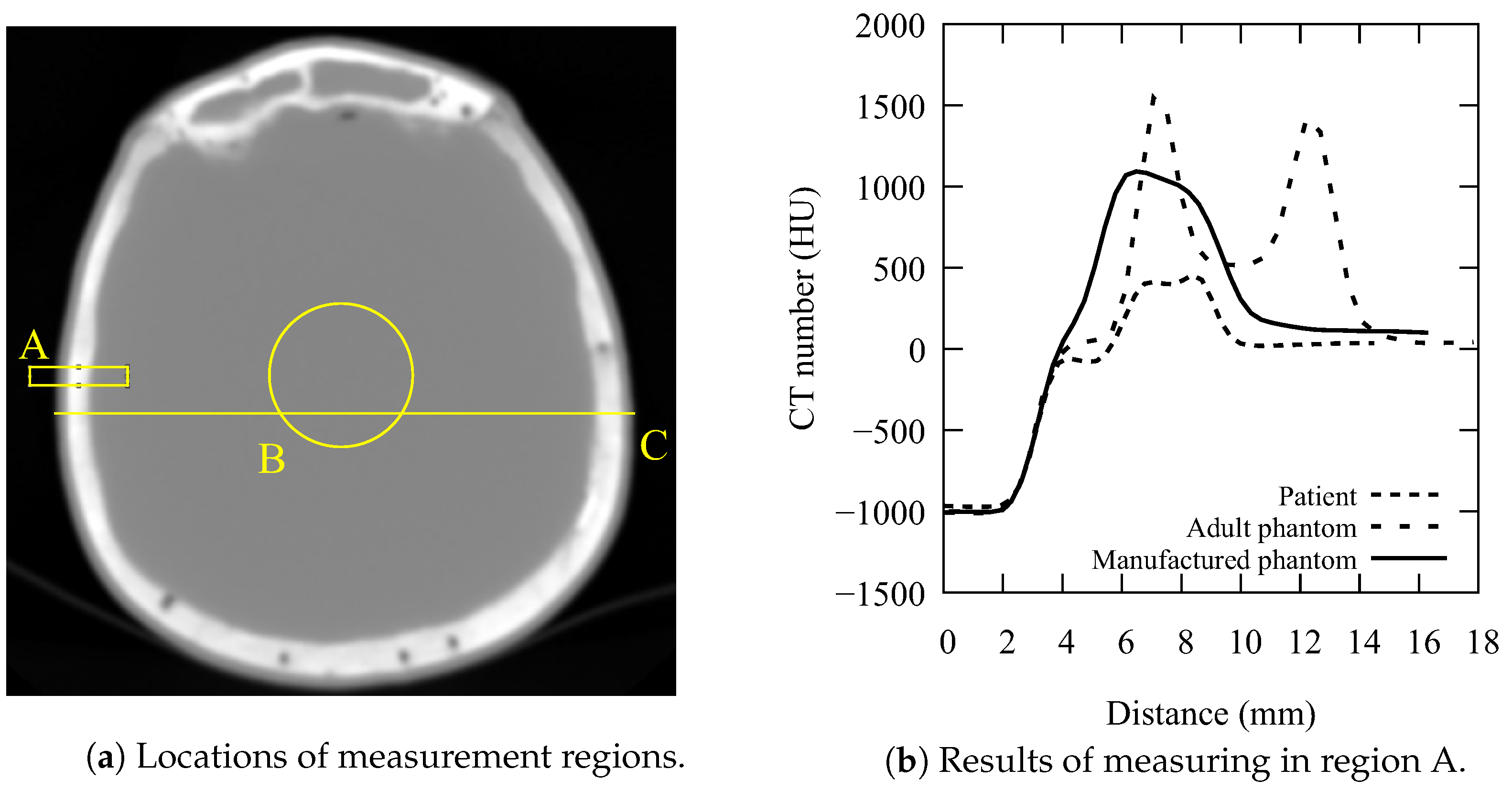


| Mode | U | I | t | w | p | T | Conv. Kernel |
|---|---|---|---|---|---|---|---|
| Helical | 120 kV | 200 mA | 750 ms | 16 mm | 0.69 | 3 mm/1 mm | FC26/FC30 |
| U | Q | 1 | Adult Phantom | Paediatric Phantom | |||||
|---|---|---|---|---|---|---|---|---|---|
| (kV) | (mAs) | (mGy) | |||||||
| 80 | 37.0 | 3.60 | 34.7 | 20.3 | 411 | 48.3 | 10.8 | 118 | 1.31% |
| 75.0 | 7.20 | 33.8 | 14.1 | 199 | 46.2 | 7.74 | 59.9 | 1.20% | |
| 112 | 10.8 | 33.6 | 11.5 | 132 | 46.5 | 6.93 | 48.0 | 1.25% | |
| 150 | 14.4 | 33.6 | 9.71 | 94.3 | 46.7 | 5.74 | 32.9 | 1.26% | |
| 187 | 18.0 | 33.6 | 8.68 | 75.3 | 48.2 | 5.49 | 30.1 | 1.41% | |
| 100 | 37.0 | 6.20 | 45.8 | 12.4 | 154 | 72.2 | 7.29 | 53.1 | 2.52% |
| 75.0 | 12.4 | 44.1 | 9.58 | 91.8 | 71.8 | 4.96 | 24.6 | 2.65% | |
| 112 | 18.6 | 44.6 | 7.62 | 58.1 | 72.7 | 4.22 | 17.8 | 2.69% | |
| 150 | 24.8 | 44.7 | 6.40 | 41.0 | 72.9 | 3.83 | 14.7 | 2.69% | |
| 187 | 34.0 | 44.4 | 5.62 | 31.6 | 71.9 | 3.50 | 12.3 | 2.64% | |
| 120 | 37.0 | 9.10 | 45.7 | 9.97 | 99.4 | 82.0 | 5.68 | 32.3 | 3.47% |
| 75.0 | 18.3 | 46.4 | 7.31 | 53.4 | 81.8 | 3.98 | 15.8 | 3.38% | |
| 112 | 27.4 | 47.3 | 6.08 | 37.0 | 81.8 | 3.35 | 11.2 | 3.29% | |
| 150 | 36.5 | 45.9 | 4.89 | 23.9 | 82.6 | 2.99 | 8.94 | 3.51% | |
| 187 | 50.2 | 45.3 | 4.39 | 19.3 | 81.6 | 2.63 | 6.92 | 3.47% | |
| 135 | 37.0 | 11.6 | 43.4 | 9.12 | 83.2 | 84.1 | 5.19 | 26.9 | 3.90% |
| 75.0 | 23.2 | 42.7 | 6.37 | 40.6 | 84.8 | 3.67 | 13.5 | 4.04% | |
| 112 | 34.8 | 45.5 | 5.17 | 26.7 | 84.6 | 3.25 | 10.6 | 3.74% | |
| 150 | 51.0 | 43.5 | 4.18 | 17.5 | 83.7 | 2.89 | 8.35 | 3.85% | |
| 187 | 63.8 | 43.0 | 3.75 | 14.1 | 84.3 | 2.65 | 7.02 | 3.96% | |
| Q (mAs) | |||||||
|---|---|---|---|---|---|---|---|
| (mGy) | (HU2) | (HU) | (mGy) | 80 kV | 100 kV | 120 kV | 135 kV |
| 20 | 52.86 | 7.27 | 7.2 | 74.2 | 46.3 | 33.9 | 28.2 |
| 30 | 35.50 | 5.96 | 10.4 | 107.9 | 64.0 | 45.8 | 37.3 |
| 40 | 27.24 | 5.22 | 13.4 | 139.6 | 80.7 | 57.1 | 46.0 |
| 50 | 22.42 | 4.74 | 16.3 | 169.7 | 96.4 | 67.8 | 54.1 |
| 60 | 19.27 | 4.39 | 19.0 | 198.1 | 111.4 | 77.9 | 61.8 |
| 70 | 17.04 | 4.13 | 21.6 | 225.1 | 125.5 | 87.5 | 69.1 |
| 80 | 15.39 | 3.92 | 24.1 | 250.7 | 139.0 | 96.6 | 76.1 |
| 90 | 14.11 | 3.76 | 26.4 | 275.1 | 151.8 | 105.3 | 82.7 |
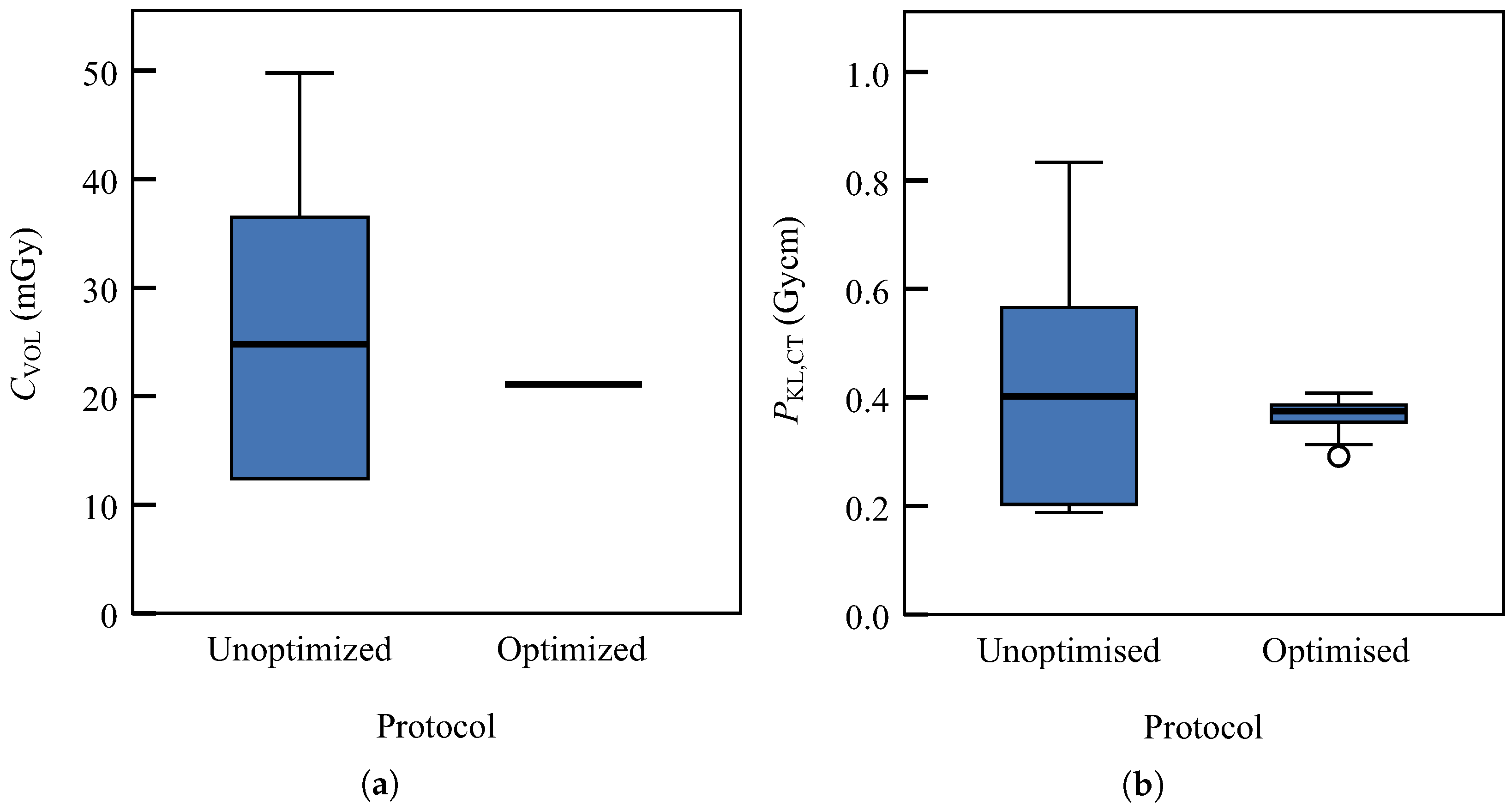
| Protocol | Patient | Age (y) | L (cm) | U (kV) | I (mA) | (mA) | b (mGy) | (mGycm) | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Unoptimised | 12 | 0.3 | 159 | 107 a | 167 | 167 | 27 | 14 | 432 | 243 |
| Optimised | 30 | 0.5 | 171 | 100 | 170 | 170 | 21 | 0 | 365 | 33 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jusufbegović, M.; Pandžić, A.; Busuladžić, M.; Čiva, L.M.; Gazibegović-Busuladžić, A.; Šehić, A.; Vegar-Zubović, S.; Jašić, R.; Beganović, A. Utilisation of 3D Printing in the Manufacturing of an Anthropomorphic Paediatric Head Phantom for the Optimisation of Scanning Parameters in CT. Diagnostics 2023, 13, 328. https://doi.org/10.3390/diagnostics13020328
Jusufbegović M, Pandžić A, Busuladžić M, Čiva LM, Gazibegović-Busuladžić A, Šehić A, Vegar-Zubović S, Jašić R, Beganović A. Utilisation of 3D Printing in the Manufacturing of an Anthropomorphic Paediatric Head Phantom for the Optimisation of Scanning Parameters in CT. Diagnostics. 2023; 13(2):328. https://doi.org/10.3390/diagnostics13020328
Chicago/Turabian StyleJusufbegović, Merim, Adi Pandžić, Mustafa Busuladžić, Lejla M. Čiva, Azra Gazibegović-Busuladžić, Adnan Šehić, Sandra Vegar-Zubović, Rahima Jašić, and Adnan Beganović. 2023. "Utilisation of 3D Printing in the Manufacturing of an Anthropomorphic Paediatric Head Phantom for the Optimisation of Scanning Parameters in CT" Diagnostics 13, no. 2: 328. https://doi.org/10.3390/diagnostics13020328
APA StyleJusufbegović, M., Pandžić, A., Busuladžić, M., Čiva, L. M., Gazibegović-Busuladžić, A., Šehić, A., Vegar-Zubović, S., Jašić, R., & Beganović, A. (2023). Utilisation of 3D Printing in the Manufacturing of an Anthropomorphic Paediatric Head Phantom for the Optimisation of Scanning Parameters in CT. Diagnostics, 13(2), 328. https://doi.org/10.3390/diagnostics13020328






