Abstract
Urine sedimentation in the bladder can occur in various circumstances and can lead to urinary obstruction/stasis with associated pain. It is usually diagnosed with an ultrasound; however, CT is also used to assess the amount and to further check for urinary stones. Depending on the composition, urine sedimentation and stones can be treated medically by alkalinisation of the urine with potassium sodium hydrogen citrate in the case of uric acid-based sedimentation/stones. Due to technical developments and improved material differentiation and characterisation in CT imaging, dual-energy CT allows for differentiation of uric acid from calcium, which can be used for sedimentation/stone composition analysis. Subsequently, treatment decisions can be made based on the findings in dual-energy CT.
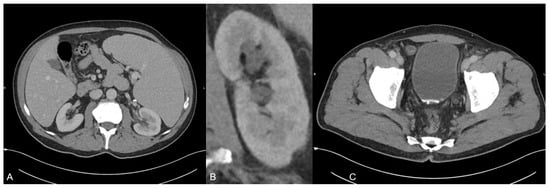
Figure 1.
A 70-year old man was presented to our emergeny department with acute left-sided strong flank pain and clearing of fragments since some weeks ago. The only known diseases included polycythaemia vera and actinic keratosis. The polycythaemia vera was treated with aspirin and ruxolitinib (janus kinase inhibitor). The initial body check showed no signs of hematoma or inflammation. The abdomen was soft during palpation, and the pain was not influencable during the check. Laboratory blood values showed no signs of infection or ischemia. The urinary pH was 5.1. Besides the urinary pH, all other urinary tests/values (no microhematuria and no elevated leucocyte levels) were inconspicuous. The initially performed ultrasound examination revealed no presence of any acute bowel pathology including appendicitis or diverticulitis or any presence of an urinary stone. Due to the persistent strong pain, a monophasic CT scan was performed in the portal venous phase on a third-generation dual-source dual-energy CT scanner (SOMATOM Force, Siemens Healthineers, Forchheim, Germany) to check for any missed pathologies. The CT scan protocol represents a standard protocol at our institution consisting of a scan 70 s after contrast material injection (Iomeron 400 mg, injected at a dose of 1.3 mL per kilogram body weight and at a flow rate of 2 mL/s through a superficial vein of the forearm) using a dual-energy mode with tube voltages of 90 and 150 kV due to the known several advantages of dual-energy CT in clinical routine [1,2,3,4,5,6,7,8,9]. The scan revealed a known splenomegaly but no signs of a splenic rupture or infarction (A). In addition, no free fluid was present. Instead, a second grade urinary stasis in the left kidney (B) and hyperdense material in the bladder adjacent to the posterior wall in terms of an urine sedimentation (C) were noted. Although there was no sign of an ureter obstruction by the sediment, the sediment was the only finding which may have caused decreased flow and corresponding stasis in the CT scan. No other acute pathologies were present in this scan.
Figure 1.
A 70-year old man was presented to our emergeny department with acute left-sided strong flank pain and clearing of fragments since some weeks ago. The only known diseases included polycythaemia vera and actinic keratosis. The polycythaemia vera was treated with aspirin and ruxolitinib (janus kinase inhibitor). The initial body check showed no signs of hematoma or inflammation. The abdomen was soft during palpation, and the pain was not influencable during the check. Laboratory blood values showed no signs of infection or ischemia. The urinary pH was 5.1. Besides the urinary pH, all other urinary tests/values (no microhematuria and no elevated leucocyte levels) were inconspicuous. The initially performed ultrasound examination revealed no presence of any acute bowel pathology including appendicitis or diverticulitis or any presence of an urinary stone. Due to the persistent strong pain, a monophasic CT scan was performed in the portal venous phase on a third-generation dual-source dual-energy CT scanner (SOMATOM Force, Siemens Healthineers, Forchheim, Germany) to check for any missed pathologies. The CT scan protocol represents a standard protocol at our institution consisting of a scan 70 s after contrast material injection (Iomeron 400 mg, injected at a dose of 1.3 mL per kilogram body weight and at a flow rate of 2 mL/s through a superficial vein of the forearm) using a dual-energy mode with tube voltages of 90 and 150 kV due to the known several advantages of dual-energy CT in clinical routine [1,2,3,4,5,6,7,8,9]. The scan revealed a known splenomegaly but no signs of a splenic rupture or infarction (A). In addition, no free fluid was present. Instead, a second grade urinary stasis in the left kidney (B) and hyperdense material in the bladder adjacent to the posterior wall in terms of an urine sedimentation (C) were noted. Although there was no sign of an ureter obstruction by the sediment, the sediment was the only finding which may have caused decreased flow and corresponding stasis in the CT scan. No other acute pathologies were present in this scan.

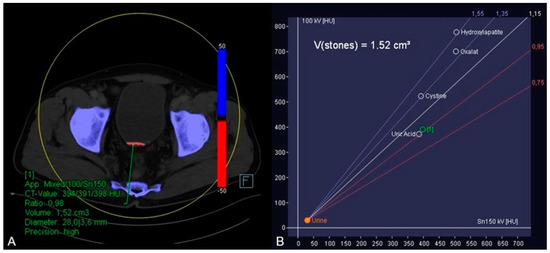
Figure 2.
In order to further analyze the urine sedimentation, dual-energy CT-based material decomposition originally developed for kidney stone analysis differentiating uric acid from calcium was applied [10,11]. In this context, soft tissue kernel reconstructions of the 90 and 150 kV scan were fused, and dedicated postprocessing software (syngo.via, version VB10B, Siemens Healthineers) was used to generate dedicated coloured reconstructions using default settings. By application of this algorithm, it was possible to identify the red coloured uric acid in the hyperdense material (blue colour shows calcium) (A) by placing a circular region of interest in the hyperdense material. Furthermore, the software displayed the dual-energy ratio of the region of interest in a diagram indicating its uric acid content (B). Subsequently, the urologists stared a therapy including alkalinisation of the urine with potassium sodium hydrogen citrate based on the results of this dual-energy CT-based material analysis.
Figure 2.
In order to further analyze the urine sedimentation, dual-energy CT-based material decomposition originally developed for kidney stone analysis differentiating uric acid from calcium was applied [10,11]. In this context, soft tissue kernel reconstructions of the 90 and 150 kV scan were fused, and dedicated postprocessing software (syngo.via, version VB10B, Siemens Healthineers) was used to generate dedicated coloured reconstructions using default settings. By application of this algorithm, it was possible to identify the red coloured uric acid in the hyperdense material (blue colour shows calcium) (A) by placing a circular region of interest in the hyperdense material. Furthermore, the software displayed the dual-energy ratio of the region of interest in a diagram indicating its uric acid content (B). Subsequently, the urologists stared a therapy including alkalinisation of the urine with potassium sodium hydrogen citrate based on the results of this dual-energy CT-based material analysis.

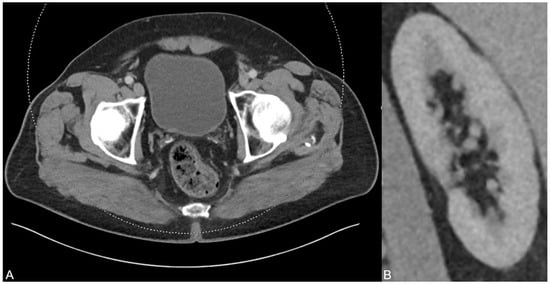
Figure 3.
After six months and permanent therapy with potassium sodium hydrogen citrate, a control CT scan was performed showing no residues of the uric acid-based urine sedimentation in the bladder (A). In addition, no urinary stasis was present anymore (B), indicating the treatment success based on the dual-energy CT findings and analysis results.
Figure 3.
After six months and permanent therapy with potassium sodium hydrogen citrate, a control CT scan was performed showing no residues of the uric acid-based urine sedimentation in the bladder (A). In addition, no urinary stasis was present anymore (B), indicating the treatment success based on the dual-energy CT findings and analysis results.
Author Contributions
Conceptualization, C.B.; methodology, I.Y.; software, C.B.; validation, J.L.W.; formal analysis, S.S.M.; investigation, C.B.; resources, C.B.; data curation, C.B., T.D.; writing—original draft preparation, C.B.; writing—review and editing, T.D.; visualization, C.B.; supervision, V.K., L.D.G., L.S.A., T.J.V.; project administration, C.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
This study was approved by the University Hospital Frankfurt. Protocol code is 20-549, date is 14 October 2020.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Data availability is possible upon request by C.B.
Acknowledgments
This work is dedicated to my father. Thank you for everything. I love and miss you.
Conflicts of Interest
C.B., I.Y. and J.L.W. received speaking fees from Siemens Healthineers. The rest of the authors has no conflict of interest.
References
- Cavallaro, M.; D’Angelo, T.; Albrecht, M.H.; Yel, I.; Martin, S.S.; Wichmann, J.L.; Lenga, L.; Mazziotti, S.; Blandino, A.; Ascenti, G.; et al. Comprehensive comparison of dual-energy computed tomography and magnetic resonance imaging for the assessment of bone marrow edema and fracture lines in acute vertebral fractures. Eur Radiol. 2022, 32, 561–571. [Google Scholar] [CrossRef] [PubMed]
- Booz, C.; Nöske, J.; Martin, S.S.; Albrecht, M.H.; Yel, I.; Lenga, L.; Gruber-Rouh, T.; Eichler, K.; D’Angelo, T.; Vogl, T.J.; et al. Virtual noncalcium dual-energy CT: Detection of lumbar disk herniation in comparison with standard gray-scale CT. Radiology 2019, 290, 446–455. [Google Scholar] [CrossRef] [PubMed]
- Booz, C.; Nöske, J.; Albrecht, M.H.; Lenga, L.; Martin, S.S.; Bucher, A.M.; Huizinga, N.A.; Wichmann, J.L.; Vogl, T.J.; Yel, I. Diagnostic accuracy of color-coded virtual noncalcium dual-energy CT for the assessment of bone marrow edema in sacral insufficiency fracture in comparison to MRI. Eur. J. Radiol. 2020, 129, 109046. [Google Scholar] [CrossRef] [PubMed]
- Koch, V.; Albrecht, M.H.; Gruenewald, L.D.; Yel, I.; Eichler, K.; Gruber-Rouh, T.; Hammerstingl, R.M.; Burck, I.; Wichmann, J.L.; Alizadeh, L.S.; et al. Diagnostic accuracy of color-coded virtual noncalcium reconstructions derived from portal venous phase dual-energy CT in the assessment of lumbar disk herniation. Eur. Radiol. 2022, 32, 2168–2177. [Google Scholar] [CrossRef] [PubMed]
- Martin, S.S.; Wichmann, J.L.; Scholtz, J.E.; Leithner, D.; D’Angelo, T.; Weyer, H.; Booz, C.; Lenga, L.; Vogl, T.J.; Albrecht, M.H. Noise-Optimized Virtual Monoenergetic Dual-Energy CT Improves Diagnostic Accuracy for the Detection of Active Arterial Bleeding of the Abdomen. J. Vasc. Interv. Radiol. 2017, 28, 1257–1266. [Google Scholar] [CrossRef] [PubMed]
- Mahmoudi, S.; Martin, S.; Koch, V.; Gruenewald, L.D.; Bernatz, S.; D’Angelo, T.; Vogl, T.J.; Booz, C.; Yel, I. Value of Dual-Energy CT Perfusion Analysis in Patients with Acute Pancreatitis: Correlation and Discriminative Diagnostic Accuracy with Varying Disease Severity. Diagnostics 2022, 12, 2601. [Google Scholar] [CrossRef] [PubMed]
- Gruenewald, L.D.; Koch, V.; Martin, S.S.; Yel, I.; Eichler, K.; Gruber-Rouh, T.; Lenga, L.; Wichmann, J.L.; Alizadeh, L.S.; Albrecht, M.H.; et al. Diagnostic accuracy of quantitative dual-energy CT-based volumetric bone mineral density assessment for the prediction of osteoporosis-associated fractures. Eur. Radiol. 2022, 32, 3076–3084. [Google Scholar] [CrossRef]
- D’Angelo, T.; Albrecht, M.H.; Caudo, D.; Mazziotti, S.; Vogl, T.J.; Wichmann, J.L.; Martin, S.; Yel, I.; Ascenti, G.; Koch, V.; et al. Virtual non-calcium dual-energy CT: Clinical applications. Eur. Radiol. Exp. 2021, 5, 38. [Google Scholar] [CrossRef] [PubMed]
- Martin, S.S.; Trapp, F.; Wichmann, J.L.; Albrecht, M.H.; Lenga, L.; Durden, J.; Booz, C.; Vogl, T.J. Dual-energy CT in early acute pancreatitis: Improved detection using iodine quantification. Eur. Radiol. 2019, 29, 2226–2232. [Google Scholar] [CrossRef] [PubMed]
- Primak, A.N.; Fletcher, J.G.; Vrtiska, T.J.; Dzyubak, O.P.; Lieske, J.C.; Jackson, M.E.; Williams, J.C., Jr.; McCollough, C.H. Noninvasive differentiation of uric acid versus non–uric acid kidney stones using dual-energy CT. Acad. Radiol. 2007, 14, 1441–1447. [Google Scholar] [CrossRef] [PubMed]
- Manglaviti, G.; Tresoldi, S.; Guerrer, C.S.; Di Leo, G.; Montanari, E.; Sardanelli, F.; Cornalba, G. In vivo evaluation of the chemical composition of urinary stones using dual-energy CT. Am. J. Roentgenol. 2011, 197, W76–W83. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
