Computed Tomography Indicators for Differentiating Stage 1 Borderline Ovarian Tumors from Stage I Malignant Epithelial Ovarian Tumors
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. CT Imaging
2.3. Image Analysis
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chiou, S.Y.; Lev-Toaff, A.S.; Masuda, E.; Feld, R.I.; Bergin, D. Adnexal torsion: New clinical and imaging observations by sonography, computed tomography, and magnetic resonance imaging. J. Ultrasound Med. 2007, 26, 1289–1301. [Google Scholar] [CrossRef] [PubMed]
- Hauptmann, S.; Friedrich, K.; Redline, R.; Avril, S. Ovarian borderline tumors in the 2014 WHO classification: Evolving concepts and diagnostic criteria. Virchows Arch. 2017, 470, 125–142. [Google Scholar] [PubMed]
- Morotti, M.; Menada, M.V.; Gillott, D.J.; Venturini, P.L.; Ferrero, S. The preoperative diagnosis of borderline ovarian tumors: A review of current literature. Arch. Gynecol. Obstet. 2012, 285, 1103–1112. [Google Scholar] [CrossRef] [PubMed]
- Silverberg, S.G.; Bell, D.A.; Kurman, R.J.; Seidman, J.D.; Prat, J.; Ronnett, B.M.; Copeland, L.; Silva, E.; Gorstein, F.; Young, R.H. Borderline ovarian tumors: Key points and workshop summary. Hum. Pathol. 2004, 35, 910–917. [Google Scholar] [CrossRef]
- Vasconcelos, I.; de Sousa Mendes, M. Conservative surgery in ovarian borderline tumours: A meta-analysis with emphasis on recurrence risk. Eur. J. Cancer 2015, 51, 620–631. [Google Scholar] [CrossRef]
- Bentivegna, E.; Gouy, S.; Maulard, A.; Pautier, P.; Leary, A.; Colombo, N.; Morice, P. Fertility-sparing surgery in epithelial ovarian cancer: A systematic review of oncological issues. Ann. Oncol. 2016, 27, 1994–2004. [Google Scholar] [CrossRef]
- Bent, C.L.; Sahdev, A.; Rockall, A.G.; Singh, N.; Sohaib, S.A.; Reznek, R.H. MRI appearances of borderline ovarian tumours. Clin. Radiol. 2009, 64, 430–438. [Google Scholar] [CrossRef]
- Denewar, F.A.; Takeuchi, M.; Urano, M.; Kamishima, Y.; Kawai, T.; Takahashi, N.; Takeuchi, M.; Kobayashi, S.; Honda, J.; Shibamoto, Y. Multiparametric MRI for differentiation of borderline ovarian tumors from stage I malignant epithelial ovarian tumors using multivariate logistic regression analysis. Eur. J. Radiol. 2017, 91, 116–123. [Google Scholar] [CrossRef]
- De Souza, N.M.; O’Neill, R.; McIndoe, G.A.; Dina, R.; Soutter, W.P. Borderline tumors of the ovary: CT and MRI features and tumor markers in differentiation from stage I disease. Am. J. Roentgenol. 2005, 184, 999–1003. [Google Scholar] [CrossRef]
- Sahin, H.; Akdogan, A.I.; Smith, J.; Zawaideh, J.P.; Addley, H. Serous borderline ovarian tumours: An extensive review on MR imaging features. Br. J. Radiol. 2021, 94, 20210116. [Google Scholar] [CrossRef]
- Timmerman, D.; Valentin, L.; Bourne, T.H.; Collins, W.P.; Verrelst, H.; Vergote, I. Terms, definitions and measurements to describe the sonographic features of adnexal tumors: A consensus opinion from the International Ovarian Tumor Analysis (IOTA) Group. Ultrasound Obstet. Gynecol. 2000, 16, 500–505. [Google Scholar] [CrossRef] [PubMed]
- Landis, J.R.; Koch, G.G. The measurement of observer agreement for categorical data. Biometrics 1977, 33, 159–174. [Google Scholar] [CrossRef] [PubMed]
- Cohen, J. Weighted kappa: Nominal scale agreement with provision for scaled disagreement or partial credit. Psychol. Bull. 1968, 70, 213–220. [Google Scholar] [CrossRef]
- Kaga, T.; Kato, H.; Hatano, Y.; Kawaguchi, M.; Furui, T.; Morishige, K.I.; Matsuo, M. Can MRI features differentiate ovarian mucinous carcinoma from mucinous borderline tumor? Eur. J. Radiol. 2020, 132, 109281. [Google Scholar] [CrossRef]
- Nougaret, S.; Lakhman, Y.; Molinari, N.; Feier, D.; Scelzo, C.; Vargas, H.A.; Sosa, R.E.; Hricak, H.; Soslow, R.A.; Grisham, R.N.; et al. CT Features of Ovarian Tumors: Defining Key Differences Between Serous Borderline Tumors and Low-Grade Serous Carcinomas. Am. J. Roentgenol. 2018, 210, 918–926. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Tang, H.; Xiao, F.; Zhu, J.; Hua, T.; Tang, G. Differentiation of borderline tumors from type I ovarian epithelial cancers on CT and MR imaging. Abdom. Radiol. 2020, 45, 3230–3238. [Google Scholar] [CrossRef]
- Yang, X.Y.; Li, X.; Ma, F.H.; Li, H.M.; Zhao, S.H.; Li, Y.A.; Qiang, J.W. MRI characteristics for differentiating mucinous borderline ovarian tumours from mucinous ovarian cancers. Clin. Radiol. 2022, 77, 142–147. [Google Scholar] [CrossRef]
- Borrelli, G.M.; de Mattos, L.A.; Andres, M.P.; Gonçalves, M.O.; Kho, R.M.; Abrão, M.S. Role of Imaging Tools for the Diagnosis of Borderline Ovarian Tumors: A Systematic Review and Meta-Analysis. J. Minim. Invasive Gynecol. 2017, 24, 353–363. [Google Scholar] [CrossRef] [PubMed]
- Grabowska-Derlatka, L.; Derlatka, P.; Palczewski, P.; Danska-Bidzinska, A.; Pacho, R. Differentiation of ovarian cancers from borderline ovarian tumors on the basis of evaluation of tumor vascularity in multi-row detector computed tomography—Comparison with histopathology. Int. J. Gynecol. Cancer 2013, 23, 1597–1602. [Google Scholar] [CrossRef]
- Zhang, Y.; Tan, J.; Wang, J.; Ai, C.; Jin, Y.; Wang, H.; Li, M.; Zhang, H.; Zhong, S. Are CT and MRI useful tools to distinguish between micropapillary type and typical type of ovarian serous borderline tumors? Abdom. Radiol. 2021, 46, 3354–3364. [Google Scholar] [CrossRef] [PubMed]
- Jung, S.E.; Lee, J.M.; Rha, S.E.; Byun, J.Y.; Jung, J.I.; Hahn, S.T. CT and MR imaging of ovarian tumors with emphasis on differential diagnosis. Radiographics 2002, 22, 1305–1325. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.E.; Choi, H.J.; Kim, M.H.; Cho, K.S. The serum CA-125 concentration data assists in evaluating CT imaging information when used to differentiate borderline ovarian tumor from malignant epithelial ovarian tumors. Korean J. Radiol. 2011, 12, 456–462. [Google Scholar] [CrossRef]
- Flicek, K.T.; VanBuren, W.; Dudiak, K.; Lahkman, Y.; Chen, L.W.; Butler, K.; Menias, C.O. Borderline epithelial ovarian tumors: What the radiologist should know. Abdom. Radiol. 2021, 46, 2350–2366. [Google Scholar] [CrossRef] [PubMed]
- Lalwani, N.; Shanbhogue, A.K.; Vikram, R.; Nagar, A.; Jagirdar, J.; Prasad, S.R. Current update on borderline ovarian neoplasms. Am. J. Roentgenol. 2010, 194, 330–336. [Google Scholar] [CrossRef] [PubMed]
- Shih Ie, M.; Kurman, R.J. Ovarian tumorigenesis: A proposed model based on morphological and molecular genetic analysis. Am. J. Pathol. 2004, 164, 1511–1518. [Google Scholar] [CrossRef]
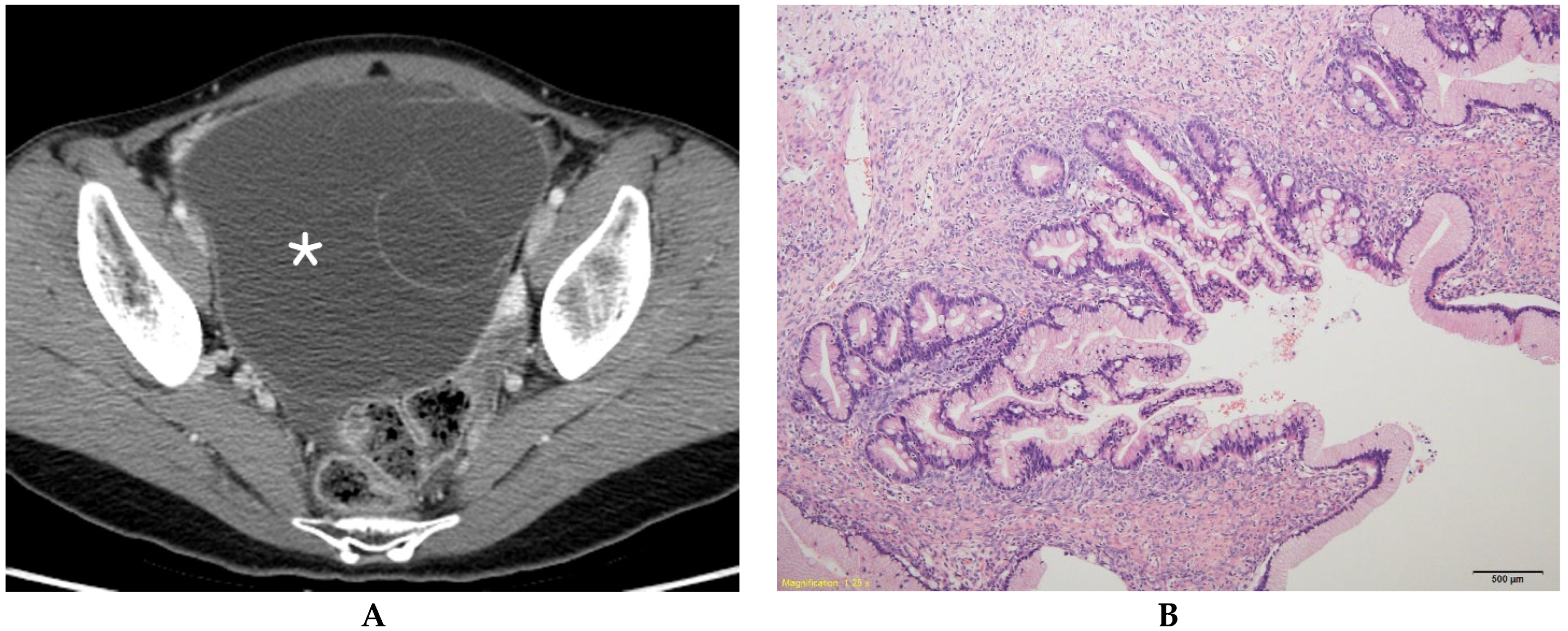
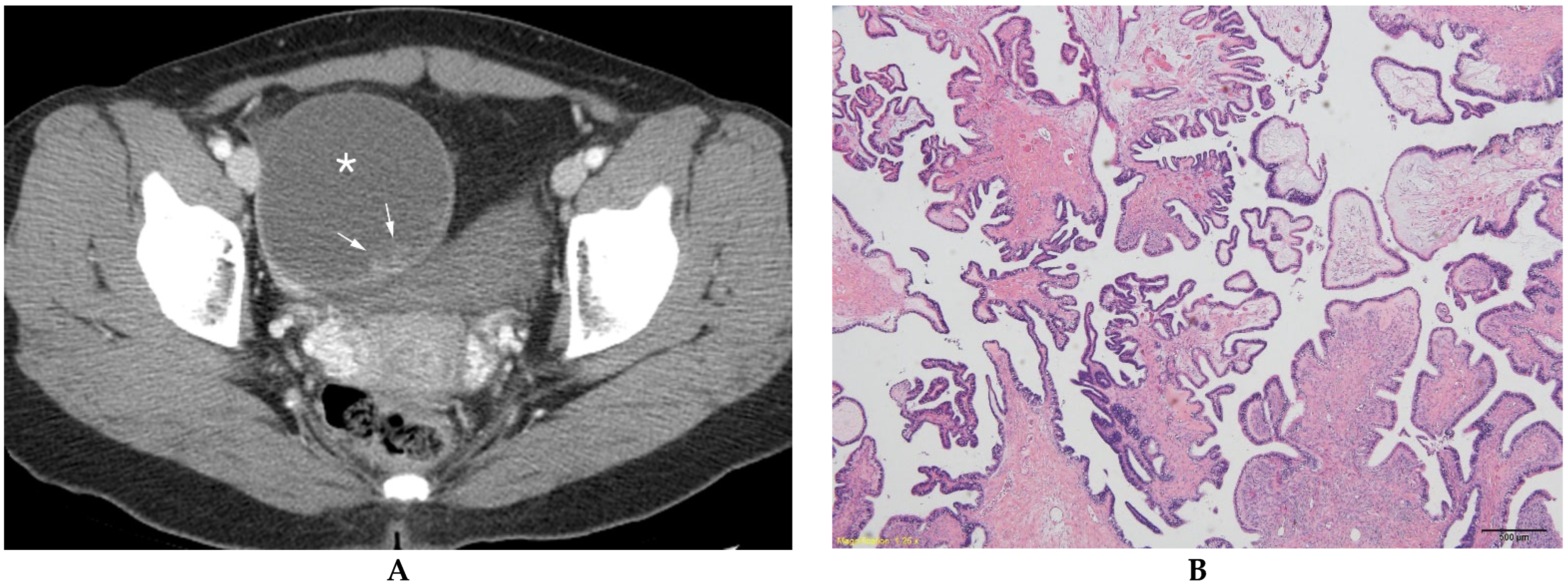
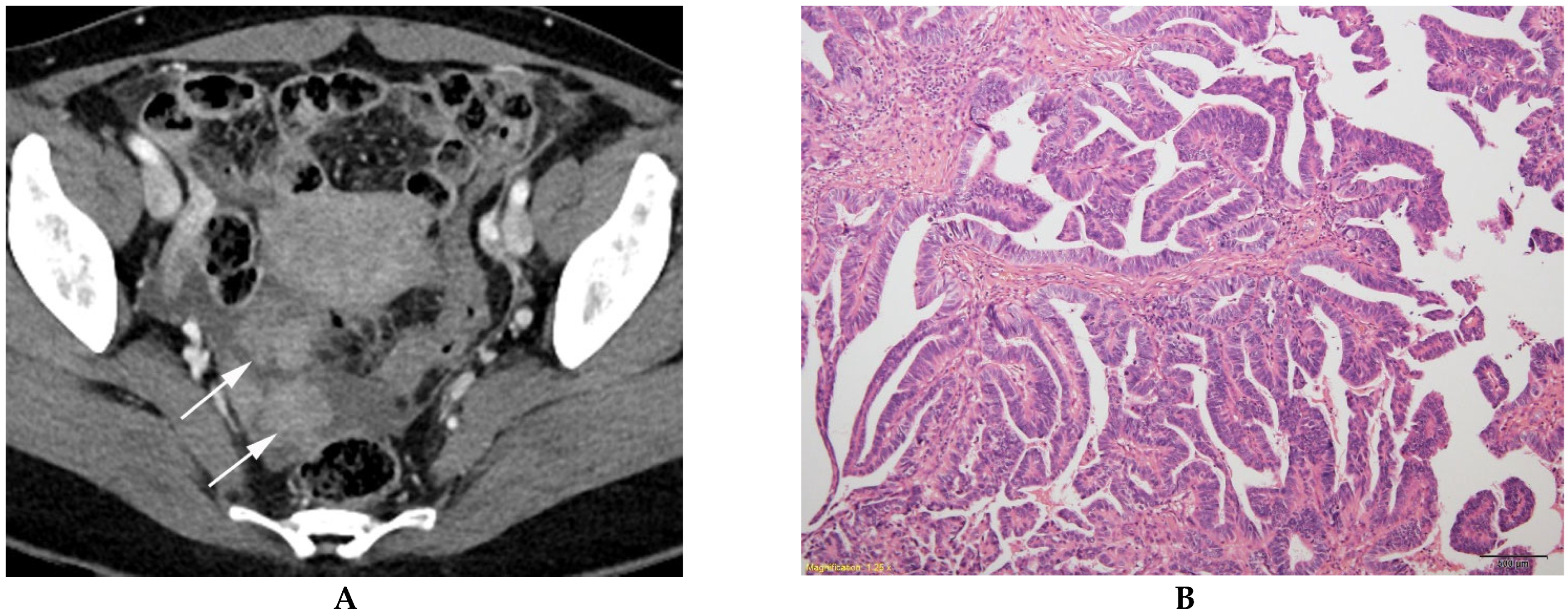
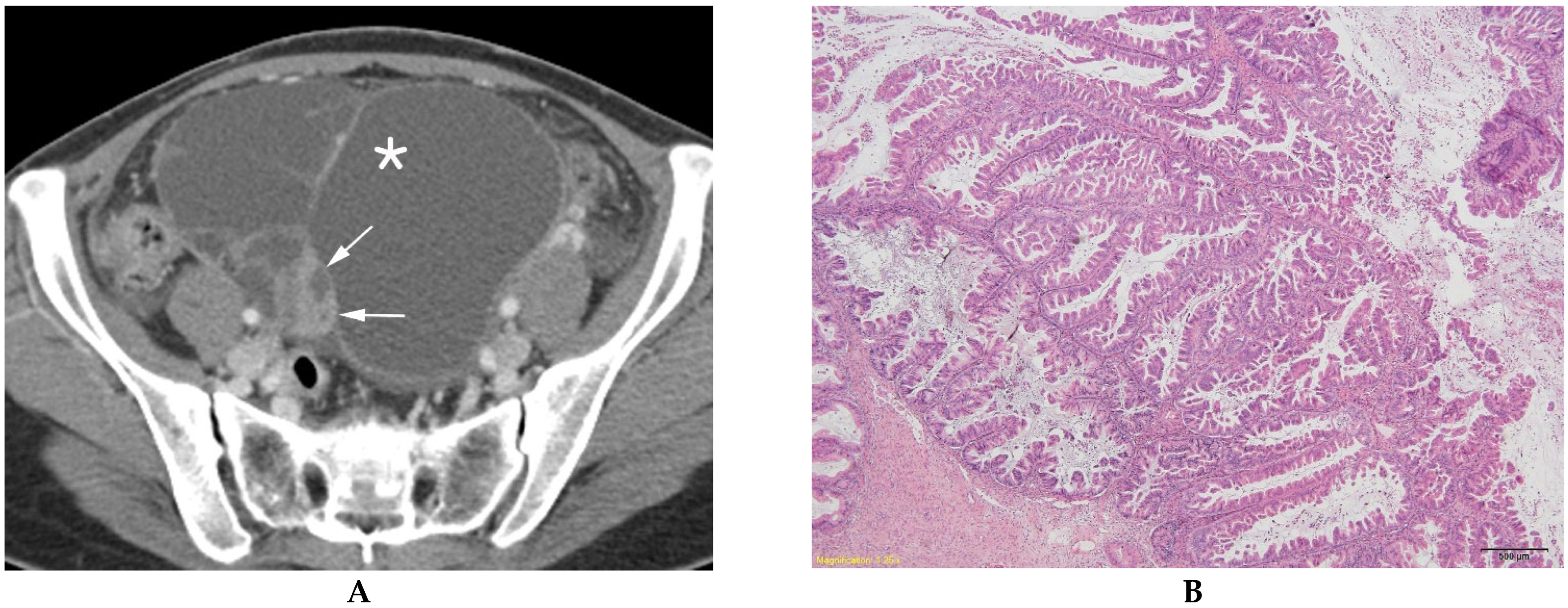
| BOT (n = 84) | MEOT (n = 57) | p Value | |
|---|---|---|---|
| Median age (years) | 45.5 (26.5–55.5) | 55.0 (49.0–61.5) | p = 0.0001 |
| Median CA-125 (U/mL) | 24.1 (15.2–56.0) | 75.2 (26.5–177.9) | p = 0.0001 |
| Laterality | p = 0.09 | ||
| Bilateral | 13 (15.5) | 16 (28.1) | |
| Unilateral | 71 (84.5) | 41 (71.9) | |
| Pathologic diagnosis | |||
| Serous borderline tumor | 27 (32.1) | NA | |
| Mucinous borderline tumor | 51 (60.7) | NA | |
| Seromucinous borderline tumor | 6 (7.1) | NA | |
| Serous carcinoma | NA | 43 (75.4) | |
| Mucinous carcinoma | NA | 8 (14.0) | |
| Clear cell carcinoma | NA | 2 (3.5) | |
| Endometrioid carcinoma | NA | 3 (5.3) | |
| Seromucinous carcinoma | NA | 2 (3.5) |
| Reader 1 | Reader 2 | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| BOT (n = 97) | MEOT (n = 73) | p Value | BOT (n = 97) | MEOT (n = 73) | p Value | ||||
| Univariate Analysis | Multivariate Analysis | Univariate Analysis | Multivariate Analysis | ||||||
| Tumor Morphology | <0.0001 | 0.5376 | Tumor Morphology | <0.0001 | 0.6780 | ||||
| Unilocular cyst | 8/97 (8.3) | 1/73 (1.4) | Unilocular cyst | 8/97 (8.2) | 1/73 (1.4) | ||||
| Unilocular-solid cyst | 6/97 (6.2) | 7/73 (9.6) | Unilocular-solid cyst | 6/97 (6.2) | 7/73 (9.6) | ||||
| Multilocular cyst | 50/97 (51.5) | 9/73 (12.3) | Multilocular cyst | 60/97 (61.9) | 9/73 (12.3) | ||||
| Multilocular-solid cyst | 32/97 (33.0) | 38/73 (52.1) | Multilocular-solid cyst | 22/97 (22.7) | 38/73 (52.1) | ||||
| Solid mass | 1/97 (1.0) | 18/73 (24.7) | Solid mass | 1/97 (1.0) | 18/73 (24.7) | ||||
| Calcification | 1.00 | Calcification | 1.00 | ||||||
| Present | 2/97 (2.1) | 2/73 (2.7) | Present | 2/97 (2.1) | 2/73 (2.7) | ||||
| Absent (reference) | 95/97 (97.9) | 71/73 (97.3) | Absent (reference) | 95/97 (97.9) | 71/73 (97.3) | ||||
| Solid component | <0.0001 | 1.00 | Solid component | <0.0001 | 1.00 | ||||
| Present | 39/97 (40.2) | 63/73 (86.3) | Present | 29/97 (29.9) | 63/73 (86.3) | ||||
| Absent (reference) | 58/97 (59.8) | 10/73 (13.7) | Absent (reference) | 68/97 (70.1) | 10/73 (13.7) | ||||
| Margin of tumor | 0.2333 | Margin of tumor | 0.2537 | ||||||
| Ill-defined | 2/97 (2.1) | 4/73 (5.5) | Ill-defined | 3/97 (3.1) | 5/73 (6.8) | ||||
| Well-defined (reference) | 95/97 (97.9) | 69/73 (94.5) | Well-defined (reference) | 94/97 (96.9) | 68/73 (93.2) | ||||
| Wall thickening | 0.5252 | Wall thickening | 0.3092 | ||||||
| Present | 15/97 (15.5) | 14/73 (19.2) | Present | 13/97 (13.4) | 14/73 (19.2) | ||||
| Absent (reference) | 82/97 (84.5) | 59/73 (80.8) | Absent (reference) | 84/97 (86.6) | 59/73 (80.8) | ||||
| Septal thickening | 0.2203 | Septal thickening | 0.3129 | ||||||
| Present | 20/97 (20.6) | 21/73 (28.8) | Present | 25/97 (25.8) | 24/73 (32.9) | ||||
| Absent (reference) | 77/97 (79.4) | 52/73 (71.2) | Absent (reference) | 72/97 (74.2) | 49/73 (67.1) | ||||
| Tumor size (cm) | 10.9 (6.6–14.9) | 7.1 (4.2–10.4) | <0.0001 | 0.0284 | Tumor size (cm) | 10.5 (6.4–14.1) | 7.3 (4.3–10.1) | <0.0001 | 0.0391 |
| Size of solid component (cm) | 2.1 (1.0–2.6) | 3.6 (2.3–5.2) | <0.0001 | 0.0007 | Size of solid component (cm) | 1.9 (1.1–3.4) | 4.0 (2.4–5.1) | <0.0001 | 0.0003 |
| Reader 1 | Reader 2 | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| BOT (n = 39) | MEOT (n = 63) | p Value | BOT (n = 29) | MEOT (n = 63) | p Value | ||||
| Univariate Analysis | Multivariate Analysis | Univariate Analysis | Multivariate Analysis | ||||||
| Tumor morphology | 0.0046 | 0.2542 | Tumor morphology | 0.0175 | 0.0979 | ||||
| Unilocular- solid cyst | 6/39 (15.4) | 7/63 (11.1) | Unilocular-solid cyst | 6/29 (15.4) | 7/63 (11.1) | ||||
| Multilocular-solid cyst | 32/39 (82.1) | 38/63 (60.3) | Multilocular-solid cyst | 22/29 (75.9) | 38/63 (60.3) | ||||
| Solid mass | 1/39 (2.6) | 18/63 (28.6) | Solid mass | 1/29 (3.4) | 18/63 (28.6) | ||||
| Enhancement of solid component | 0.153 | Enhancement of solid component | 0.187 | ||||||
| Mild | 31/39 (79.5) | 43/63 (68.3) | Mild | 24/29 (82.8) | 41/63 (65.1) | ||||
| Moderate | 6/39 (15.4) | 18/63 (28.6) | Moderate | 4/29 (13.8) | 20/63 (31.8) | ||||
| Avid | 2/39 (5.1) | 2/63 (3.2) | Avid | 1/29 (3.5) | 2/63 (3.2) | ||||
| Margin of solid component | <0.0001 | 0.0016 | Margin of solid component | 0.0234 | 0.0414 | ||||
| Ill-defined | 20/39 (51.3) | 6/63 (9.5) | Ill-defined | 16/29 (55.2) | 16/63 (25.4) | ||||
| Well-defined (reference) | 19/39 (48.7) | 57/63 (90.5) | Well-defined (reference) | 13/29 (44.8) | 47/63 (74.6) | ||||
| Wall thickening | 1.000 | Wall thickening | 0.370 | ||||||
| Present | 6/39 (15.4) | 11/63 (17.5) | Present | 3/29 (10.3) | 13/63 (20.6) | ||||
| Absent (reference) | 33/39 (84.6) | 52/63 (82.5) | Absent (reference) | 26/29 (89.7) | 50/63 (79.4) | ||||
| Septal thickening | 0.650 | Septal thickening | 0.238 | ||||||
| Present | 10/39 (25.6) | 19/63 (30.2) | Present | 7/29 (24.1) | 24/63 (38.1) | ||||
| Absent (reference) | 29/39 (74.4) | 44/63 (69.8) | Absent (reference) | 22/29 (75.9) | 39/63 (61.9) | ||||
| Tumor size (cm) | 8.5 (5.8–12.6) | 7.1 (4.3–10.5) | 0.1124 | Tumor size (cm) | 6.4 (5.0–10.4) | 7.4 (4.4–9.9) | 0.6559 | ||
| Size of solid component (cm) | 2.1 (1.0–2.6) | 3.7 (2.3–5.2) | <0.0001 | 0.0092 | Size of solid component (cm) | 1.9 (1.1–2.4) | 4.0 (2.4–5.1) | <0.0001 | 0.0014 |
| CT Finding | Reader 1 vs. Reader 2 |
|---|---|
| Tumor morphology | 0.78 (0.61–0.83) |
| Calcification of tumor | 0.93 (0.83–1.00) |
| Solid component or tumor | 0.85 (0.71–0.93) |
| Tumor margin | 0.95 (0.89–1.00) |
| Wall thickening of tumor | 0.71 (0.58–0.81) |
| Septal thickening of tumor | 0.73 (0.61–0.89) |
| Enhancement of solid component | 0.94 (0.88–0.99) |
| Margin of solid component | 0.79 (0.63–0.88) |
| Tumor size | 0.85 (0.81–0.92) |
| Size of solid component | 0.82 (0.73–0.88) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moon, M.H.; Park, H.S.; Kim, Y.J.; Yu, M.H.; Park, S.; Jung, S.I. Computed Tomography Indicators for Differentiating Stage 1 Borderline Ovarian Tumors from Stage I Malignant Epithelial Ovarian Tumors. Diagnostics 2023, 13, 480. https://doi.org/10.3390/diagnostics13030480
Moon MH, Park HS, Kim YJ, Yu MH, Park S, Jung SI. Computed Tomography Indicators for Differentiating Stage 1 Borderline Ovarian Tumors from Stage I Malignant Epithelial Ovarian Tumors. Diagnostics. 2023; 13(3):480. https://doi.org/10.3390/diagnostics13030480
Chicago/Turabian StyleMoon, Min Hoan, Hee Sun Park, Young Jun Kim, Mi Hye Yu, Sungeun Park, and Sung Il Jung. 2023. "Computed Tomography Indicators for Differentiating Stage 1 Borderline Ovarian Tumors from Stage I Malignant Epithelial Ovarian Tumors" Diagnostics 13, no. 3: 480. https://doi.org/10.3390/diagnostics13030480
APA StyleMoon, M. H., Park, H. S., Kim, Y. J., Yu, M. H., Park, S., & Jung, S. I. (2023). Computed Tomography Indicators for Differentiating Stage 1 Borderline Ovarian Tumors from Stage I Malignant Epithelial Ovarian Tumors. Diagnostics, 13(3), 480. https://doi.org/10.3390/diagnostics13030480






