Review on Pediatric Malignant Focal Liver Lesions with Imaging Evaluation: Part I
Abstract
1. Introduction
2. Imaging Evaluation on Pediatric Malignant Focal Liver Lesions
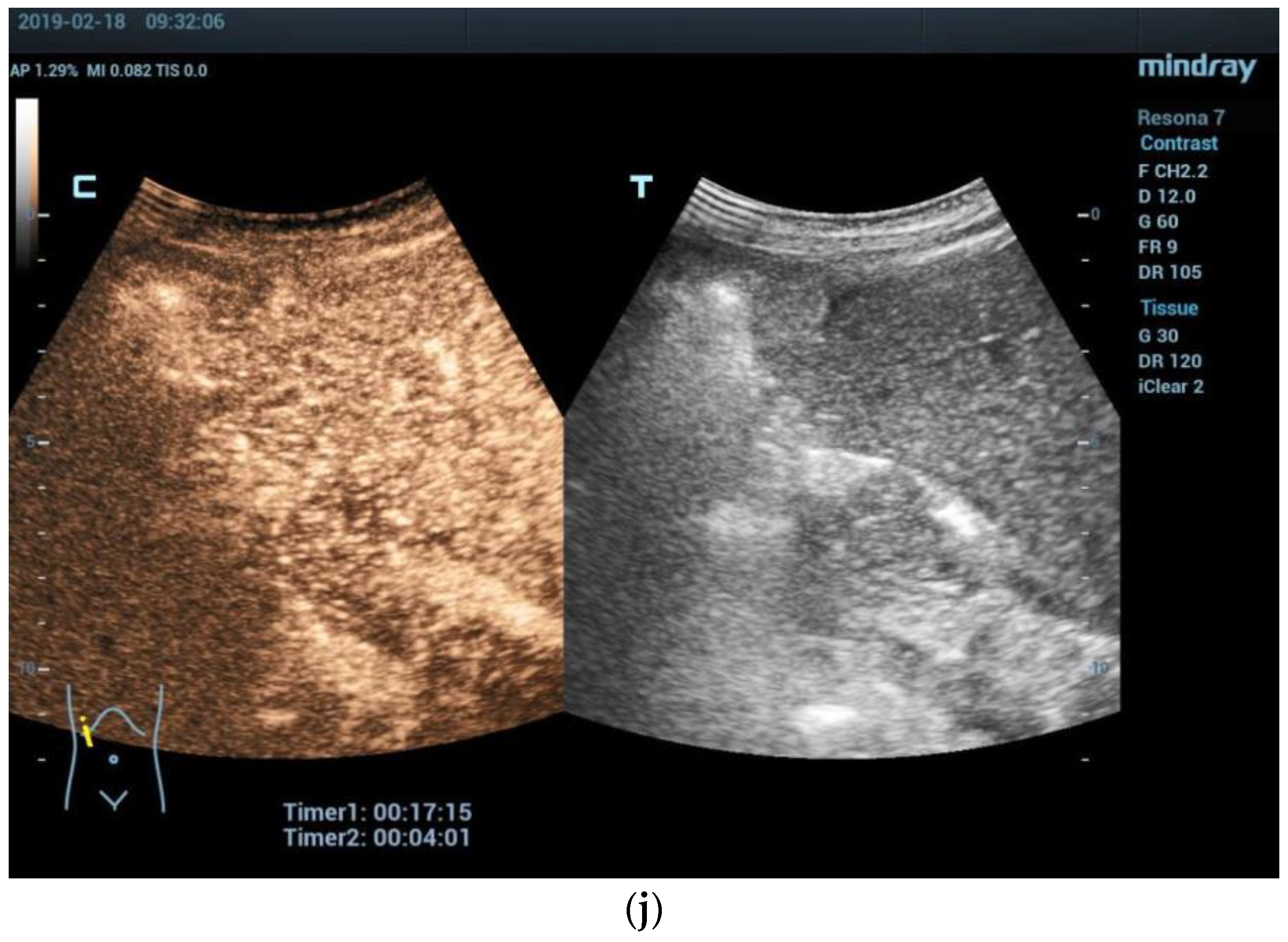
2.1. Ultrasound
2.2. Computed Tomography (CT)
2.3. Magnetic Resonance Imaging (MRI)
2.4. PRE-Treatment EXTent of Tumor (PRETEXT) System
3. Hepatoblastoma
3.1. Epidemiology, Clinical Features, and Pathological Features
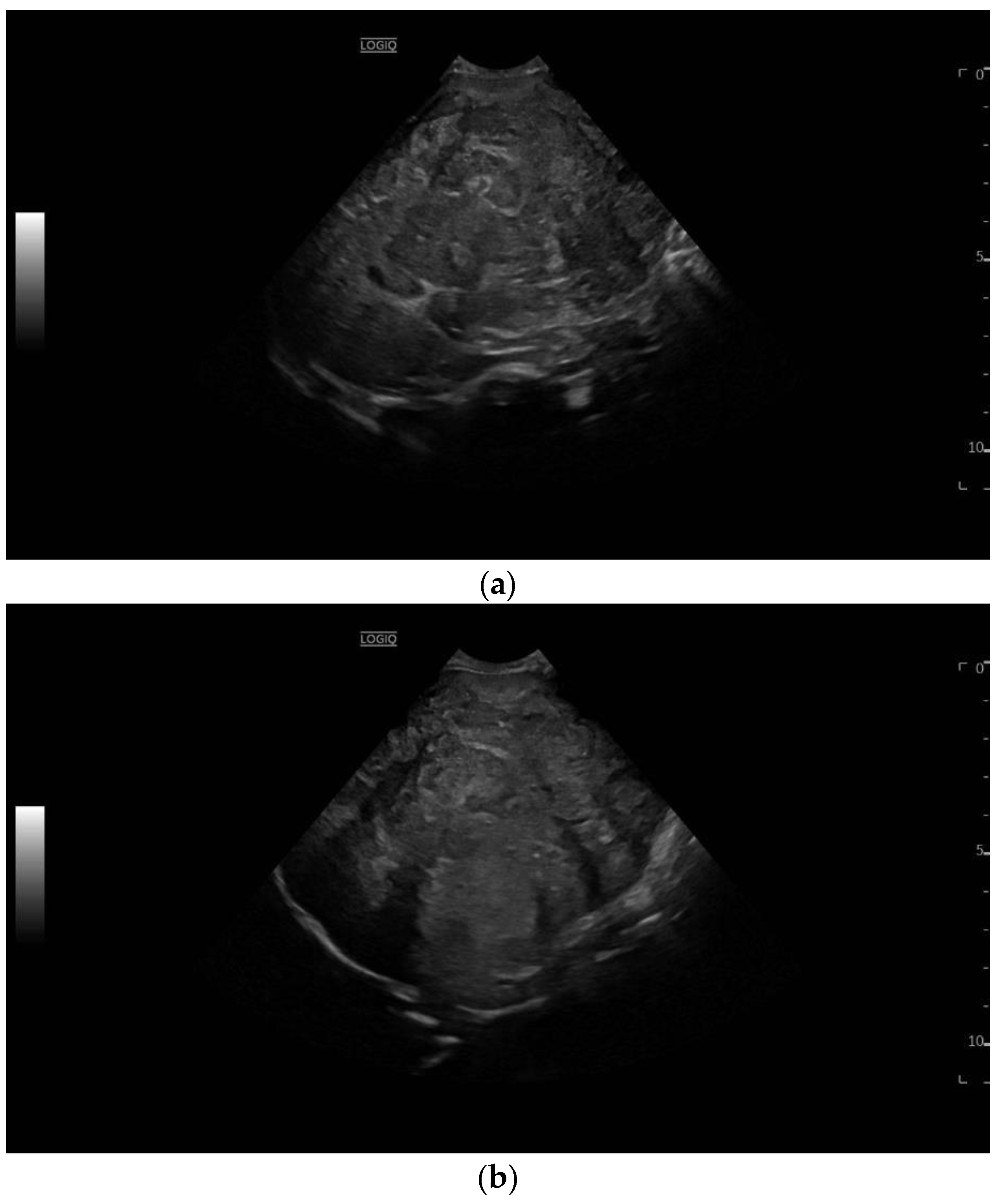
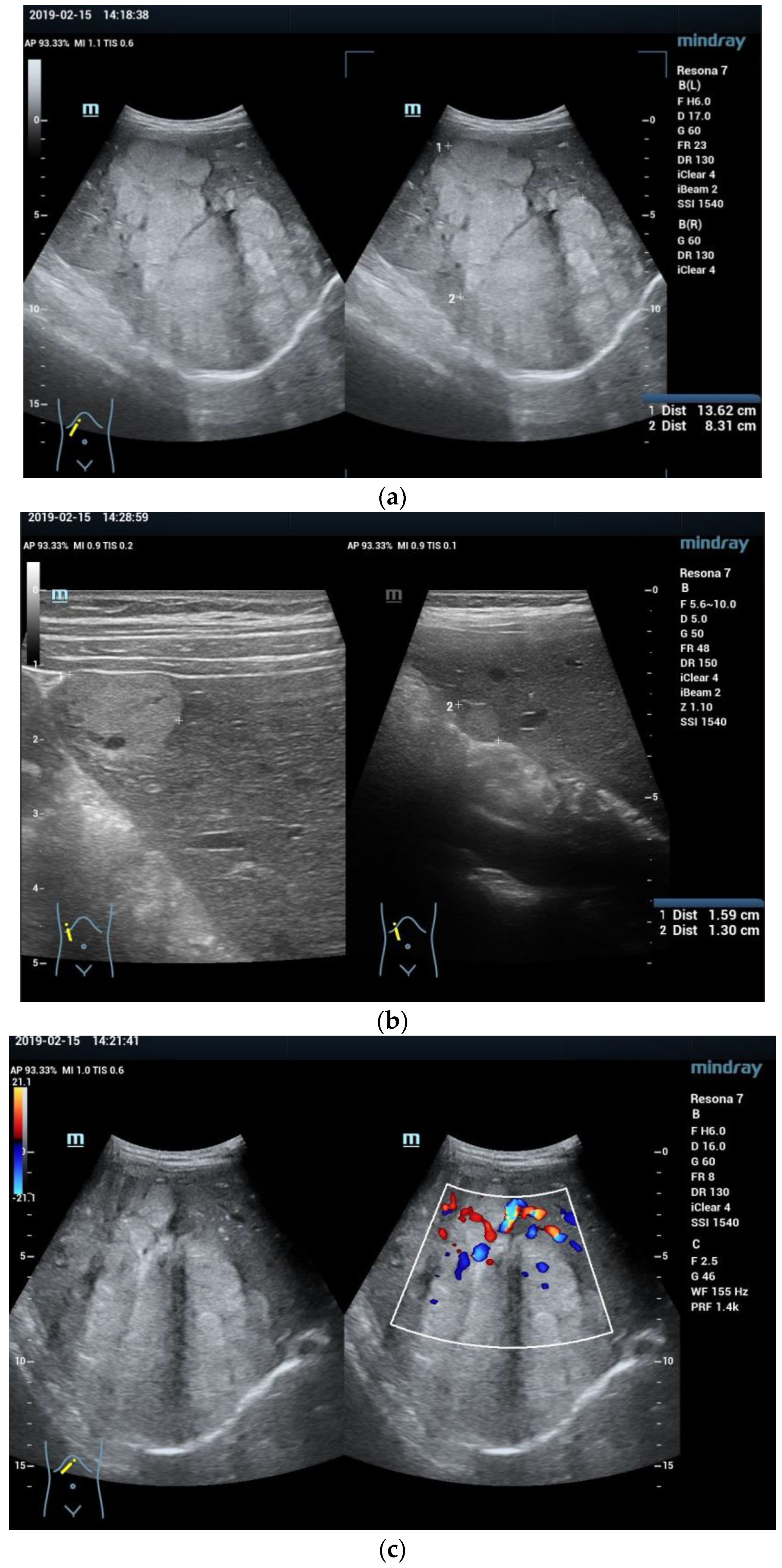
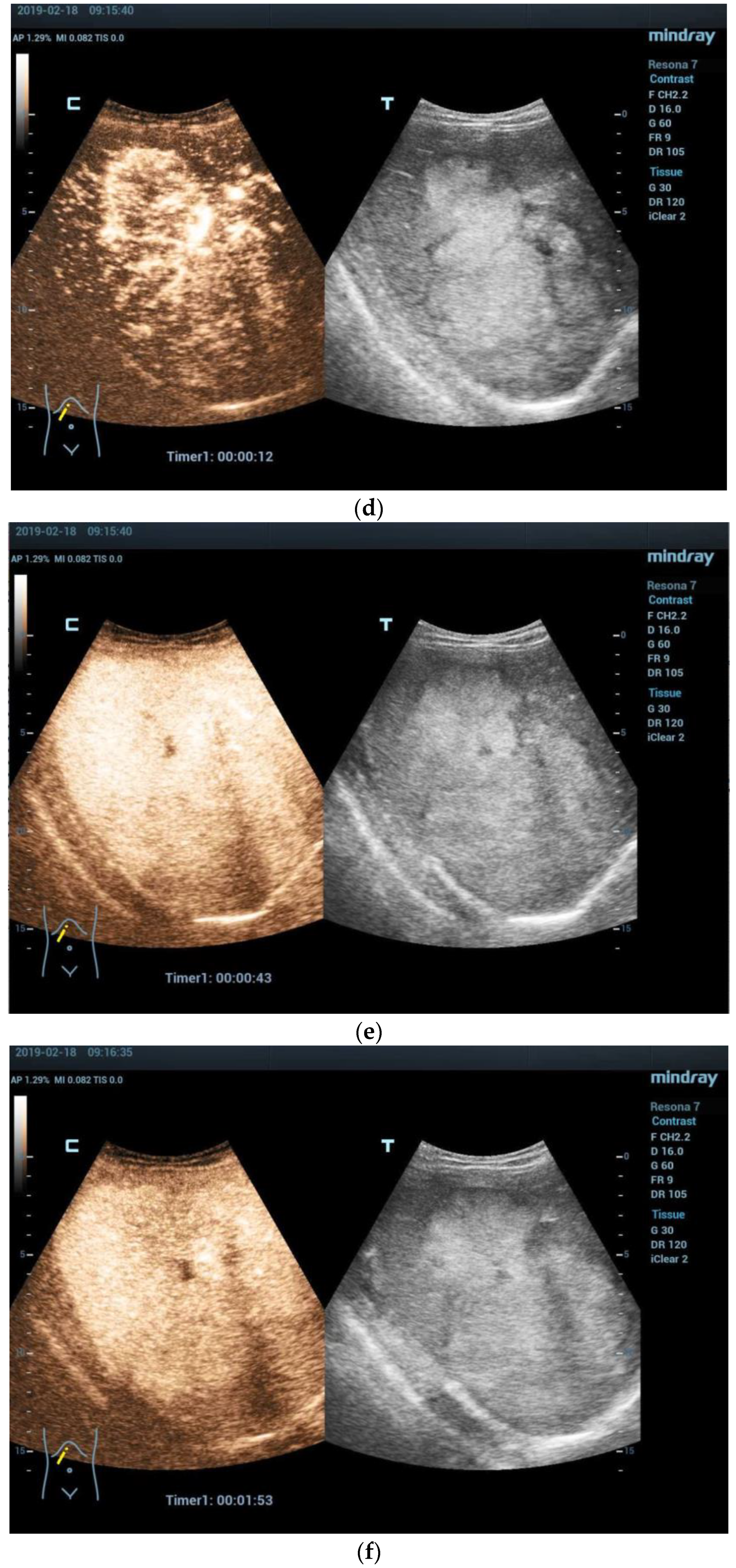
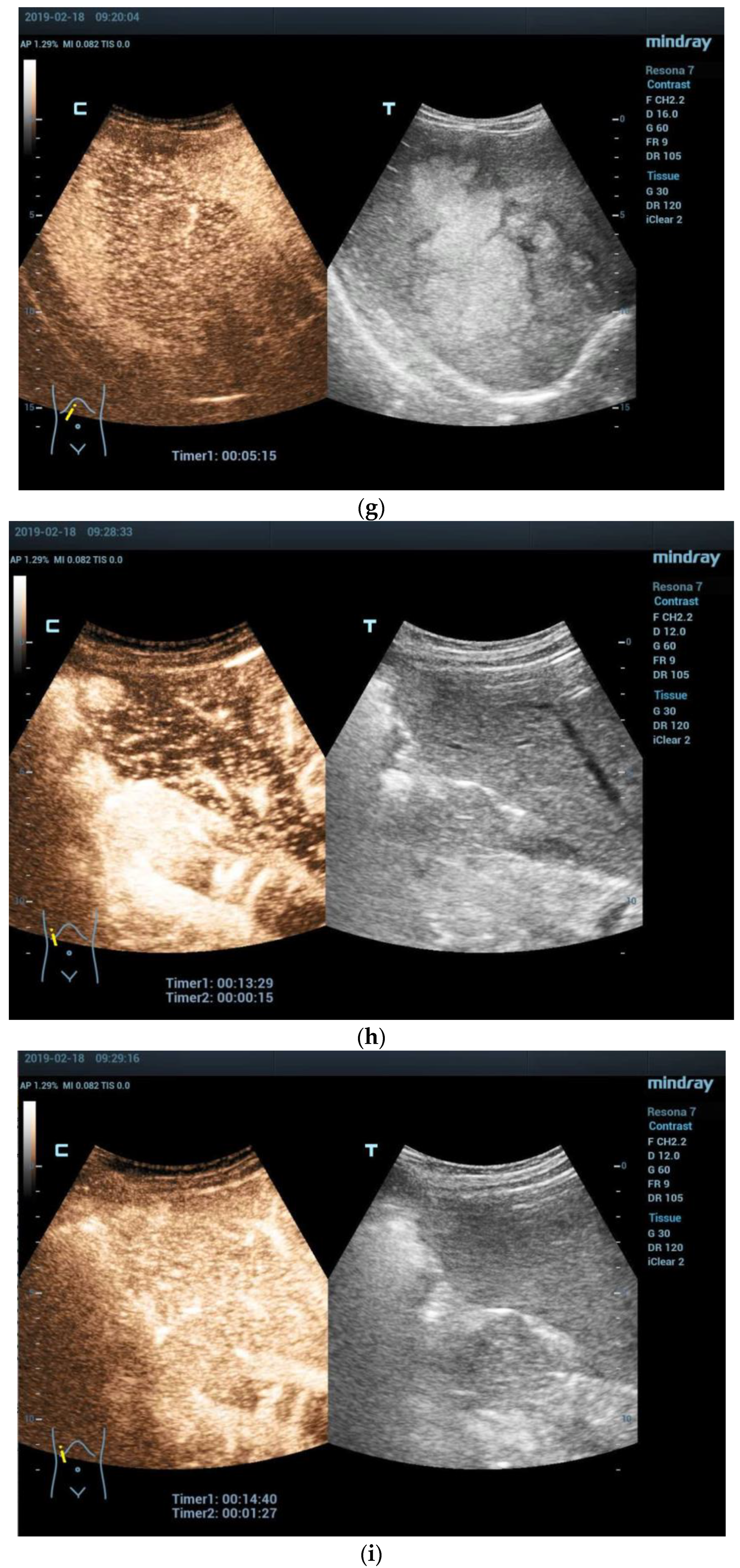
3.2. Imaging Features
3.3. Management and Prognosis
4. Hepatocellular Carcinoma (HCC)
4.1. Epidemiology, Clinical Features, and Pathological Features
4.2. Imaging Features
4.3. Management and Prognosis
5. Cholangiocarcinoma (CCA)
5.1. Epidemiology, Clinical Features, and Pathological Features
5.2. Imaging Features
5.3. Management and Prognosis
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Meyers, R.L. Tumors of the liver in children. Surg. Oncol. 2007, 16, 195–203. [Google Scholar] [CrossRef] [PubMed]
- Ng, K.; Mogul, D.B. Pediatric Liver Tumors. Clin. Liver Dis. 2018, 22, 753–772. [Google Scholar] [CrossRef] [PubMed]
- Jha, P.; Chawla, S.C.; Tavri, S.; Patel, C.; Gooding, C.; Daldrup-Link, H. Pediatric liver tumors—A pictorial review. Eur. Radiol. 2009, 19, 209–219. [Google Scholar] [CrossRef]
- López-Terrada, D.; Alaggio, R.; de Dávila, M.T.; Czauderna, P.; Hiyama, E.; Katzenstein, H.; Leuschner, I.; Malogolowkin, M.; Meyers, R.; Ranganathan, S.; et al. Towards an international pediatric liver tumor consensus classification: Proceedings of the Los Angeles COG liver tumors symposium. Mod. Pathol. 2014, 27, 472–491. [Google Scholar] [CrossRef] [PubMed]
- Khanna, R.; Verma, S.K. Pediatric hepatocellular carcinoma. World J. Gastroenterol. 2018, 24, 3980–3999. [Google Scholar] [CrossRef]
- Chiorean, L.; Cui, X.W.; Tannapfel, A.; Franke, D.; Stenzel, M.; Kosiak, W.; Schreiber-Dietrich, D.; Jüngert, J.; Chang, J.M.; Dietrich, C.F. Benign liver tumors in pediatric patients–Review with emphasis on imaging features. World J. Gastroenterol. 2015, 21, 8541–8561. [Google Scholar] [CrossRef] [PubMed]
- Towbin, A.J.; Meyers, R.L.; Woodley, H.; Miyazaki, O.; Weldon, C.B.; Morland, B.; Hiyama, E.; Czauderna, P.; Roebuck, D.J.; Tiao, G.M. 2017 PRETEXT: Radiologic staging system for primary hepatic malignancies of childhood revised for the Paediatric Hepatic International Tumour Trial (PHITT). Pediatr. Radiol. 2018, 48, 536–554. [Google Scholar] [CrossRef]
- Chung, E.M.; Lattin, G.E., Jr.; Cube, R.; Lewis, R.B.; Marichal-Hernandez, C.; Shawhan, R.; Conran, R.M. From the archives of the AFIP: Pediatric liver masses: Radiologic-pathologic correlation. Part 2. Malignant tumors. Radiographics 2011, 31, 483–507. [Google Scholar] [CrossRef]
- Pugmire, B.S.; Towbin, A.J. Magnetic resonance imaging of primary pediatric liver tumors. Pediatr. Radiol. 2016, 46, 764–777. [Google Scholar] [CrossRef]
- Courtier, J.L.; Perito, E.R.; Rhee, S.; Tsai, P.; Heyman, M.B.; MacKenzie, J.D. Targeted MRI contrast agents for pediatric hepatobiliary disease. J. Pediatr. Gastroenterol. Nutr. 2012, 54, 454–462. [Google Scholar] [CrossRef]
- El-Ali, A.M.; Davis, J.C.; Cickelli, J.M.; Squires, J.H. Contrast-enhanced ultrasound of liver lesions in children. Pediatr. Radiol. 2019, 49, 1422–1432. [Google Scholar] [CrossRef]
- Dietrich, C.F.; Augustiniene, R.; Batko, T.; Cantisani, V.; Cekuolis, A.; Deganello, A.; Dong, Y.; Franke, D.; Harkanyi, Z.; Humphries, P.D.; et al. European Federation of Societies for Ultrasound in Medicine and Biology (EFSUMB): An Update on the Pediatric CEUS Registry on Behalf of the “EFSUMB Pediatric CEUS Registry Working Group”. Ultraschall Der Med. 2021, 42, 270–277. [Google Scholar] [CrossRef] [PubMed]
- Hwang, M.; Piskunowicz, M.; Darge, K. Advanced Ultrasound Techniques for Pediatric Imaging. Pediatrics 2019, 143, e20182609. [Google Scholar] [CrossRef] [PubMed]
- Dietrich, C.F.; Ferraioli, G.; Sirli, R.; Popescu, A.; Sporea, I.; Pienar, C.; Kunze, C.; Taut, H.; Schrading, S.; Bota, S.; et al. General advice in ultrasound based elastography of pediatric patients. Med. Ultrason. 2019, 21, 315–326. [Google Scholar] [CrossRef] [PubMed]
- Westheim, B.H.; Østensen, A.B.; Aagenæs, I.; Sanengen, T.; Almaas, R. Evaluation of risk factors for bleeding after liver biopsy in children. J. Pediatr. Gastroenterol. Nutr. 2012, 55, 82–87. [Google Scholar] [CrossRef]
- Sidhu, P.S.; Cantisani, V.; Deganello, A.; Dietrich, C.F.; Duran, C.; Franke, D.; Harkanyi, Z.; Kosiak, W.; Miele, V.; Ntoulia, A.; et al. Role of Contrast-Enhanced Ultrasound (CEUS) in Paediatric Practice: An EFSUMB Position Statement. Ultraschall Der Med. 2017, 38, 33–43. [Google Scholar] [CrossRef] [PubMed]
- Fang, C.; Anupindi, S.A.; Back, S.J.; Franke, D.; Green, T.G.; Harkanyi, Z.; Jungert, J.; Kwon, J.K.; Paltiel, H.J.; Squires, J.H.; et al. Contrast-enhanced ultrasound of benign and malignant liver lesions in children. Pediatr. Radiol. 2021, 51, 2181–2197. [Google Scholar] [CrossRef]
- Darge, K.; Papadopoulou, F.; Ntoulia, A.; Bulas, D.I.; Coley, B.D.; Fordham, L.A.; Paltiel, H.J.; McCarville, B.; Volberg, F.M.; Cosgrove, D.O.; et al. Safety of contrast-enhanced ultrasound in children for non-cardiac applications: A review by the Society for Pediatric Radiology (SPR) and the International Contrast Ultrasound Society (ICUS). Pediatr. Radiol. 2013, 43, 1063–1073. [Google Scholar] [CrossRef]
- Rosado, E.; Riccabona, M. Off-Label Use of Ultrasound Contrast Agents for Intravenous Applications in Children: Analysis of the Existing Literature. J. Ultrasound Med. 2016, 35, 487–496. [Google Scholar] [CrossRef]
- Coleman, J.L.; Navid, F.; Furman, W.L.; McCarville, M.B. Safety of ultrasound contrast agents in the pediatric oncologic population: A single-institution experience. AJR Am. J. Roentgenol. 2014, 202, 966–970. [Google Scholar] [CrossRef]
- Menichini, G.; Sessa, B.; Trinci, M.; Galluzzo, M.; Miele, V. Accuracy of contrast-enhanced ultrasound (CEUS) in the identification and characterization of traumatic solid organ lesions in children: A retrospective comparison with baseline US and CE-MDCT. La Radiol. Medica 2015, 120, 989–1001. [Google Scholar] [CrossRef] [PubMed]
- McCarville, M.B.; Kaste, S.C.; Hoffer, F.A.; Khan, R.B.; Walton, R.C.; Alpert, B.S.; Furman, W.L.; Li, C.; Xiong, X. Contrast-enhanced sonography of malignant pediatric abdominal and pelvic solid tumors: Preliminary safety and feasibility data. Pediatr. Radiol. 2012, 42, 824–833. [Google Scholar] [CrossRef] [PubMed]
- Piskunowicz, M.; Kosiak, W.; Batko, T.; Piankowski, A.; Połczyńska, K.; Adamkiewicz-Drożyńska, E. Safety of intravenous application of second-generation ultrasound contrast agent in children: Prospective analysis. Ultrasound Med. Biol. 2015, 41, 1095–1099. [Google Scholar] [CrossRef] [PubMed]
- Dietrich, C.F.; Nolsøe, C.P.; Barr, R.G.; Berzigotti, A.; Burns, P.N.; Cantisani, V.; Chammas, M.C.; Chaubal, N.; Choi, B.I.; Clevert, D.A.; et al. Guidelines and Good Clinical Practice Recommendations for Contrast Enhanced Ultrasound (CEUS) in the Liver–Update 2020–WFUMB in Cooperation with EFSUMB, AFSUMB, AIUM, and FLAUS. Ultraschall Der Med. 2020, 41, 562–585. [Google Scholar] [CrossRef] [PubMed]
- Ntoulia, A.; Anupindi, S.A.; Back, S.J.; Didier, R.A.; Hwang, M.; Johnson, A.M.; McCarville, M.B.; Papadopoulou, F.; Piskunowicz, M.; Sellars, M.E.; et al. Contrast-enhanced ultrasound: A comprehensive review of safety in children. Pediatr. Radiol. 2021, 51, 2161–2180. [Google Scholar] [CrossRef] [PubMed]
- Stenzel, M.; Mentzel, H.J. Ultrasound elastography and contrast-enhanced ultrasound in infants, children and adolescents. Eur. J. Radiol. 2014, 83, 1560–1569. [Google Scholar] [CrossRef] [PubMed]
- Fang, C.; Bernardo, S.; Sellars, M.E.; Deganello, A.; Sidhu, P.S. Contrast-enhanced ultrasound in the diagnosis of pediatric focal nodular hyperplasia and hepatic adenoma: Interobserver reliability. Pediatr. Radiol. 2019, 49, 82–90. [Google Scholar] [CrossRef] [PubMed]
- Jacob, J.; Deganello, A.; Sellars, M.E.; Hadzic, N.; Sidhu, P.S. Contrast enhanced ultrasound (CEUS) characterization of grey-scale sonographic indeterminate focal liver lesions in pediatric practice. Ultraschall Der Med. 2013, 34, 529–540. [Google Scholar] [CrossRef]
- Young, C.; Owens, C.M. Pediatric computed tomography imaging guideline. Acta Radiol. 2013, 54, 998–1006. [Google Scholar] [CrossRef]
- Yekeler, E. Pediatric abdominal applications of multidetector-row CT. Eur. J. Radiol. 2004, 52, 31–43. [Google Scholar] [CrossRef]
- Pappas, J.N.; Donnelly, L.F.; Frush, D.P. Reduced frequency of sedation of young children with multisection helical CT. Radiology 2000, 215, 897–899. [Google Scholar] [CrossRef]
- Chavhan, G.B.; Siddiqui, I.; Ingley, K.M.; Gupta, A.A. Rare malignant liver tumors in children. Pediatr. Radiol. 2019, 49, 1404–1421. [Google Scholar] [CrossRef] [PubMed]
- Shelmerdine, S.C.; Roebuck, D.J.; Towbin, A.J.; McHugh, K. MRI of paediatric liver tumours: How we review and report. Cancer Imaging 2016, 16, 21. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, C.L.; Vasanawala, S.S. An approach to pediatric liver MRI. AJR Am. J. Roentgenol. 2011, 196, W519–W526. [Google Scholar] [CrossRef] [PubMed]
- Meyers, R.L.; Maibach, R.; Hiyama, E.; Häberle, B.; Krailo, M.; Rangaswami, A.; Aronson, D.C.; Malogolowkin, M.H.; Perilongo, G.; von Schweinitz, D.; et al. Risk-stratified staging in paediatric hepatoblastoma: A unified analysis from the Children’s Hepatic tumors International Collaboration. Lancet Oncol. 2017, 18, 122–131. [Google Scholar] [CrossRef] [PubMed]
- Darcy, D.G.; Malek, M.M.; Kobos, R.; Klimstra, D.S.; DeMatteo, R.; La Quaglia, M.P. Prognostic factors in fibrolamellar hepatocellular carcinoma in young people. J. Pediatr. Surg. 2015, 50, 153–156. [Google Scholar] [CrossRef] [PubMed]
- Qiao, G.; Cucchetti, A.; Li, J.; Cescon, M.; Ercolani, G.; Liu, G.; Pinna, A.D.; Li, L.; Shen, F.; Ren, J. Applying of pretreatment extent of disease system in patients with hepatocellular carcinoma after curative partial hepatectomy. Oncotarget 2016, 7, 30408–30419. [Google Scholar] [CrossRef][Green Version]
- Weeda, V.B.; Murawski, M.; McCabe, A.J.; Maibach, R.; Brugières, L.; Roebuck, D.; Fabre, M.; Zimmermann, A.; Otte, J.B.; Sullivan, M.; et al. Fibrolamellar variant of hepatocellular carcinoma does not have a better survival than conventional hepatocellular carcinoma—Results and treatment recommendations from the Childhood Liver Tumour Strategy Group (SIOPEL) experience. Eur. J. Cancer 2013, 49, 2698–2704. [Google Scholar] [CrossRef]
- Darbari, A.; Sabin, K.M.; Shapiro, C.N.; Schwarz, K.B. Epidemiology of primary hepatic malignancies in U.S. children. Hepatology 2003, 38, 560–566. [Google Scholar] [CrossRef]
- Spector, L.G.; Birch, J. The epidemiology of hepatoblastoma. Pediatr. Blood Cancer 2012, 59, 776–779. [Google Scholar] [CrossRef]
- Tulla, M.; Berthold, F.; Graf, N.; Rutkowski, S.; von Schweinitz, D.; Spix, C.; Kaatsch, P. Incidence, Trends, and Survival of Children With Embryonal Tumors. Pediatrics 2015, 136, e623–e632. [Google Scholar] [CrossRef] [PubMed]
- Dasgupta, P.; Henshaw, C.; Youlden, D.R.; Aitken, J.F.; Sullivan, A.; Irving, H.; Baade, P.D. Global trends in incidence rates of childhood liver cancers: A systematic review and meta-analysis. Paediatr. Perinat. Epidemiol. 2020, 34, 609–617. [Google Scholar] [CrossRef] [PubMed]
- Cohen, M.M., Jr. Beckwith-Wiedemann syndrome: Historical, clinicopathological, and etiopathogenetic perspectives. Pediatr. Dev. Pathol. 2005, 8, 287–304. [Google Scholar] [CrossRef] [PubMed]
- Cereda, A.; Carey, J.C. The trisomy 18 syndrome. Orphanet J. Rare Dis. 2012, 7, 81. [Google Scholar] [CrossRef] [PubMed]
- Half, E.; Bercovich, D.; Rozen, P. Familial adenomatous polyposis. Orphanet J. Rare Dis. 2009, 4, 22. [Google Scholar] [CrossRef] [PubMed]
- Herzog, C.E.; Andrassy, R.J.; Eftekhari, F. Childhood cancers: Hepatoblastoma. Oncologist 2000, 5, 445–453. [Google Scholar] [CrossRef] [PubMed]
- Wu, P.V.; Rangaswami, A. Current Approaches in Hepatoblastoma-New Biological Insights to Inform Therapy. Curr. Oncol. Rep. 2022, 24, 1209–1218. [Google Scholar] [CrossRef] [PubMed]
- Towbin, A.J.; Braojos Braga, F.D.C.; Zhang, B.; Geller, J.I.; Tiao, G.M.; Podberesky, D.J. Fractures in children with newly diagnosed hepatoblastoma. Pediatr. Radiol. 2018, 48, 581–585. [Google Scholar] [CrossRef]
- Stocker, J.T. Hepatic tumors in children. Clin. Liver Dis. 2001, 5, 259–281, viii–ix. [Google Scholar] [CrossRef]
- Lai, M.; Burjonrappa, S. Perinatal hemorrhage complicating neonatal hepatoblastoma: Case report. J. Pediatr. Surg. 2012, 47, e29–e32. [Google Scholar] [CrossRef]
- von Schweinitz, D. Management of liver tumors in childhood. Semin. Pediatr. Surg. 2006, 15, 17–24. [Google Scholar] [CrossRef]
- Yhoshu, E.; Lone, Y.A.; Mahajan, J.K.; Singh, U.B. Hepatoblastoma with Precocious Puberty. J. Indian Assoc. Pediatr. Surg. 2019, 24, 68–71. [Google Scholar] [PubMed]
- De Ioris, M.; Brugieres, L.; Zimmermann, A.; Keeling, J.; Brock, P.; Maibach, R.; Pritchard, J.; Shafford, L.; Zsiros, J.; Czaudzerna, P.; et al. Hepatoblastoma with a low serum alpha-fetoprotein level at diagnosis: The SIOPEL group experience. Eur. J. Cancer 2008, 44, 545–550. [Google Scholar] [CrossRef] [PubMed]
- Woodward, P.J.; Sohaey, R.; Kennedy, A.; Koeller, K.K. From the archives of the AFIP: A comprehensive review of fetal tumors with pathologic correlation. Radiographics 2005, 25, 215–242. [Google Scholar] [CrossRef] [PubMed]
- Haas, J.E.; Feusner, J.H.; Finegold, M.J. Small cell undifferentiated histology in hepatoblastoma may be unfavorable. Cancer 2001, 92, 3130–3134. [Google Scholar] [CrossRef] [PubMed]
- McCarville, M.B.; Roebuck, D.J. Diagnosis and staging of hepatoblastoma: Imaging aspects. Pediatr. Blood Cancer 2012, 59, 793–799. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, H.; Peng, Y.L.; Chen, T.W.; Jiang, Y.; Luo, Y.; Zhang, Q.; Yang, Z.G. The comparison of grey-scale ultrasonic and clinical features of hepatoblastoma and hepatocellular carcinoma in children: A retrospective study for ten years. BMC Gastroenterol. 2011, 11, 78. [Google Scholar] [CrossRef] [PubMed]
- Faingold, R.; Albuquerque, P.A.; Carpineta, L. Hepatobiliary tumors. Radiol. Clin. N. Am. 2011, 49, 679–687. [Google Scholar] [CrossRef]
- Pan, F.S.; Xu, M.; Wang, W.; Zhou, L.Y.; Xie, X.Y. Infantile hepatic hemangioendothelioma in comparison with hepatoblastoma in children: Clinical and ultrasound features. Hepat. Mon. 2013, 13, e11103. [Google Scholar] [CrossRef]
- Anupindi, S.A.; Biko, D.M.; Ntoulia, A.; Poznick, L.; Morgan, T.A.; Darge, K.; Back, S.J. Contrast-enhanced US Assessment of Focal Liver Lesions in Children. Radiographics 2017, 37, 1632–1647. [Google Scholar] [CrossRef]
- Wang, G.; Xie, X.; Chen, H.; Zhong, Z.; Zhou, W.; Jiang, H.; Xie, X.; Zhou, L. Development of a pediatric liver CEUS criterion to classify benign and malignant liver lesions in pediatric patients: A pilot study. Eur. Radiol. 2021, 31, 6747–6757. [Google Scholar] [CrossRef] [PubMed]
- McCarville, M.B. Contrast-enhanced sonography in pediatrics. Pediatr. Radiol. 2011, 41 (Suppl. S1), S238–S242. [Google Scholar] [CrossRef] [PubMed]
- Baheti, A.D.; Luana Stanescu, A.; Li, N.; Chapman, T. Contrast-enhanced CT features of hepatoblastoma: Can we predict histopathology? Clin. Imaging 2017, 44, 33–37. [Google Scholar] [CrossRef] [PubMed]
- Kim, P.H.; Hwang, J.; Yoon, H.M.; Shin, H.J.; Yoon, H.; Lee, M.J.; Jung, A.Y.; Cho, Y.A.; Lee, J.S.; Koh, K.N.; et al. Outcome of staging chest CT and identification of factors associated with lung metastasis in children with hepatoblastoma. Eur. Radiol. 2021, 31, 8850–8857. [Google Scholar] [CrossRef]
- Sharma, K.; Agarwala, S.; Kandasamy, D.; Jana, M.; Sharma, R.; Dhua, A.; Jain, V.; Bhatnagar, V. Role of Diffusion Weighted MRI (DW-MR) in Detection of Satellite Lesions Not Detected with Multiphase CT Scans in Hepatoblastoma and Its Implications for Management. Indian J. Pediatr. 2022, 89, 968–974. [Google Scholar] [CrossRef] [PubMed]
- Meyers, A.B.; Towbin, A.J.; Serai, S.; Geller, J.I.; Podberesky, D.J. Characterization of pediatric liver lesions with gadoxetate disodium. Pediatr. Radiol. 2011, 41, 1183–1197. [Google Scholar] [CrossRef]
- Zhang, B.; He, Q.; Shi, X.; Wang, X.; Zhang, X. Recurrent Scapular Metastasis From Hepatoblastoma Shown on FDG PET/CT and F-DOPA PET/CT. Clin. Nucl. Med. 2017, 42, e449–e451. [Google Scholar] [CrossRef]
- Yang, T.; Whitlock, R.S.; Vasudevan, S.A. Surgical Management of Hepatoblastoma and Recent Advances. Cancers 2019, 11, 1944. [Google Scholar] [CrossRef]
- Sharma, D.; Subbarao, G.; Saxena, R. Hepatoblastoma. Semin. Diagn. Pathol. 2017, 34, 192–200. [Google Scholar] [CrossRef]
- Lim, I.I.P.; Bondoc, A.J.; Geller, J.I.; Tiao, G.M. Hepatoblastoma-The Evolution of Biology, Surgery, and Transplantation. Children 2018, 6, 1. [Google Scholar] [CrossRef]
- Meyers, R.L.; Tiao, G.M.; Dunn, S.P.; Langham, M.R., Jr. Liver transplantation in the management of unresectable hepatoblastoma in children. Front. Biosci. 2012, 4, 1293–1302. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.; Polychronidis, G.; Heger, U.; Frongia, G.; Mehrabi, A.; Hoffmann, K. Incidence trends and survival prediction of hepatoblastoma in children: A population-based study. Cancer Commun. 2019, 39, 62. [Google Scholar] [CrossRef] [PubMed]
- Ke, M.; Zhou, Y.; Chang-Zhen, Y.; Li, L.; Diao, M. A nomogram model to predict prognosis of patients with hepatoblastoma. Pediatr. Blood Cancer 2022, 69, e29932. [Google Scholar] [CrossRef] [PubMed]
- Semeraro, M.; Branchereau, S.; Maibach, R.; Zsiros, J.; Casanova, M.; Brock, P.; Domerg, C.; Aronson, D.C.; Zimmermann, A.; Laithier, V.; et al. Relapses in hepatoblastoma patients: Clinical characteristics and outcome--experience of the International Childhood Liver Tumour Strategy Group (SIOPEL). Eur. J. Cancer 2013, 49, 915–922. [Google Scholar] [CrossRef] [PubMed]
- Kelly, D.; Sharif, K.; Brown, R.M.; Morland, B. Hepatocellular carcinoma in children. Clin. Liver Dis. 2015, 19, 433–447. [Google Scholar] [CrossRef] [PubMed]
- Hadzic, N.; Finegold, M.J. Liver neoplasia in children. Clin. Liver Dis. 2011, 15, 443–462. [Google Scholar] [CrossRef]
- Czauderna, P.; Mackinlay, G.; Perilongo, G.; Brown, J.; Shafford, E.; Aronson, D.; Pritchard, J.; Chapchap, P.; Keeling, J.; Plaschkes, J.; et al. Hepatocellular carcinoma in children: Results of the first prospective study of the International Society of Pediatric Oncology group. J. Clin. Oncol. 2002, 20, 2798–2804. [Google Scholar] [CrossRef]
- Liu, Y.; Luo, Y.; Xia, L.; Qiu, B.J.; Zhou, T.; Feng, M.X.; Xue, F.; Chen, X.S.; Han, L.S.; Zhang, J.J.; et al. Living-donor liver transplantation for children with tyrosinemia type I. J. Dig. Dis. 2020, 21, 189–194. [Google Scholar] [CrossRef]
- Arikan, C.; Kilic, M.; Nart, D.; Ozgenc, F.; Ozkan, T.; Tokat, Y.; Yagci, R.V.; Aydogdu, S. Hepatocellular carcinoma in children and effect of living-donor liver transplantation on outcome. Pediatr. Transplant. 2006, 10, 42–47. [Google Scholar] [CrossRef]
- Hadžić, N.; Quaglia, A.; Portmann, B.; Paramalingam, S.; Heaton, N.D.; Rela, M.; Mieli-Vergani, G.; Davenport, M. Hepatocellular carcinoma in biliary atresia: King’s College Hospital experience. J. Pediatr. 2011, 159, 617–622.e1. [Google Scholar] [CrossRef]
- Katzenstein, H.M.; Krailo, M.D.; Malogolowkin, M.H.; Ortega, J.A.; Liu-Mares, W.; Douglass, E.C.; Feusner, J.H.; Reynolds, M.; Quinn, J.J.; Newman, K.; et al. Hepatocellular carcinoma in children and adolescents: Results from the Pediatric Oncology Group and the Children’s Cancer Group intergroup study. J. Clin. Oncol. 2002, 20, 2789–2797. [Google Scholar] [CrossRef] [PubMed]
- Aronson, D.C.; Meyers, R.L. Malignant tumors of the liver in children. Semin. Pediatr. Surg. 2016, 25, 265–275. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.J. Pediatric Liver Tumors: Updates in Classification. Surg. Pathol. Clin. 2020, 13, 601–623. [Google Scholar] [CrossRef]
- Seda Neto, J.; Leite, K.M.; Porta, A.; Fonseca, E.A.; Feier, F.H.; Pugliese, R.; Miura, I.K.; Chapchap, P.; Porta, G. HCC prevalence and histopathological findings in liver explants of patients with hereditary tyrosinemia type 1. Pediatr. Blood Cancer 2014, 61, 1584–1589. [Google Scholar] [CrossRef] [PubMed]
- Zen, Y.; Vara, R.; Portmann, B.; Hadzic, N. Childhood hepatocellular carcinoma: A clinicopathological study of 12 cases with special reference to EpCAM. Histopathology 2014, 64, 671–682. [Google Scholar] [CrossRef] [PubMed]
- Ismail, H.; Broniszczak, D.; Kaliciński, P.; Markiewicz-Kijewska, M.; Teisseyre, J.; Stefanowicz, M.; Szymczak, M.; Dembowska-Bagińska, B.; Kluge, P.; Perek, D.; et al. Liver transplantation in children with hepatocellular carcinoma. Do Milan criteria apply to pediatric patients? Pediatr. Transplant. 2009, 13, 682–692. [Google Scholar] [CrossRef] [PubMed]
- Torbenson, M.S.; Ng, I.O.L.; Park, Y.N.; Roncalli, M.; Sakamato, M. Hepatocellular Carcinoma. In Tumours of the Liver and Intrahepatic Bile Ducts, 5th ed.; Paradis, V., Fukayama, M., Park, Y.N., Schirmacher, P., Eds.; WHO Classification of Tumours; IARC Publications: Lyon, France, 2019. [Google Scholar]
- Yu, S.C.; Yeung, D.T.; So, N.M. Imaging features of hepatocellular carcinoma. Clin. Radiol. 2004, 59, 145–156. [Google Scholar] [CrossRef]
- Weeda, V.B.; Aronson, D.C.; Verheij, J.; Lamers, W.H. Is hepatocellular carcinoma the same disease in children and adults? Comparison of histology, molecular background, and treatment in pediatric and adult patients. Pediatr. Blood Cancer 2019, 66, e27475. [Google Scholar] [CrossRef]
- Rees, M.A.; Schooler, G.R.; Chavhan, G.B.; Towbin, A.J.; Alazraki, A.L.; Squires, J.H.; Fraum, T.J.; Zhang, C.; Khanna, G. Imaging Features of Hepatocellular Carcinoma in Children With and Without Underlying Risk Factors. AJR Am. J. Roentgenol. 2022, 219, 647–654. [Google Scholar] [CrossRef]
- Dietrich, C.F.; Nolsøe, C.P.; Barr, R.G.; Berzigotti, A.; Burns, P.N.; Cantisani, V.; Chammas, M.C.; Chaubal, N.; Choi, B.I.; Clevert, D.A.; et al. Guidelines and Good Clinical Practice Recommendations for Contrast-Enhanced Ultrasound (CEUS) in the Liver-Update 2020 WFUMB in Cooperation with EFSUMB, AFSUMB, AIUM, and FLAUS. Ultrasound Med. Biol. 2020, 46, 2579–2604. [Google Scholar] [CrossRef]
- Squires, J.H.; McCarville, M.B. Contrast-Enhanced Ultrasound in Children: Implementation and Key Diagnostic Applications. AJR Am. J. Roentgenol. 2021, 217, 1217–1231. [Google Scholar] [CrossRef] [PubMed]
- Arias, G.A.; Siddiqui, I.; Navarro, O.M.; Shaikh, F.; Sayed, B.A.; Chavhan, G.B. Imaging and clinical features of pediatric hepatocellular carcinoma. Pediatr. Radiol. 2021, 51, 1339–1347. [Google Scholar] [CrossRef]
- Alemayehu, T.; Hailu, D. Hepatocellular carcinoma with vertebral metastasis in a child with hepatitis B virus infection: A case report. J. Med. Case Rep. 2019, 13, 158. [Google Scholar] [CrossRef]
- Chavhan, G.B.; Shelmerdine, S.; Jhaveri, K.; Babyn, P.S. Liver MR Imaging in Children: Current Concepts and Technique. Radiographics 2016, 36, 1517–1532. [Google Scholar] [CrossRef] [PubMed]
- Levy, A.D. Malignant liver tumors. Clin. Liver Dis. 2002, 6, 147–164. [Google Scholar] [CrossRef] [PubMed]
- Hussain, S.M.; Zondervan, P.E.; JN, I.J.; Schalm, S.W.; de Man, R.A.; Krestin, G.P. Benign versus malignant hepatic nodules: MR imaging findings with pathologic correlation. Radiographics 2002, 22, 1023–1036, discussion 1037–1039. [Google Scholar] [CrossRef]
- Omata, M.; Cheng, A.L.; Kokudo, N.; Kudo, M.; Lee, J.M.; Jia, J.; Tateishi, R.; Han, K.H.; Chawla, Y.K.; Shiina, S.; et al. Asia-Pacific clinical practice guidelines on the management of hepatocellular carcinoma: A 2017 update. Hepatol. Int. 2017, 11, 317–370. [Google Scholar] [CrossRef]
- Chernyak, V.; Fowler, K.J.; Kamaya, A.; Kielar, A.Z.; Elsayes, K.M.; Bashir, M.R.; Kono, Y.; Do, R.K.; Mitchell, D.G.; Singal, A.G.; et al. Liver Imaging Reporting and Data System (LI-RADS) Version 2018: Imaging of Hepatocellular Carcinoma in At-Risk Patients. Radiology 2018, 289, 816–830. [Google Scholar] [CrossRef]
- Khanna, G.; Chavhan, G.B.; Schooler, G.R.; Fraum, T.J.; Alazraki, A.L.; Squires, J.H.; Salter, A.; Podberesky, D.J.; Towbin, A.J. Diagnostic Performance of LI-RADS Version 2018 for Evaluation of Pediatric Hepatocellular Carcinoma. Radiology 2021, 299, 190–199. [Google Scholar] [CrossRef]
- Pessanha, I.; Heitor, F.; Furtado, E.; Campos, A.P.; Gonçalves, I. Long-term survival after choriocarcinoma transmitted by liver graft: A successful report in pediatric transplantation. Pediatr. Transplant. 2022, 26, e14135. [Google Scholar] [CrossRef]
- McLarney, J.K.; Rucker, P.T.; Bender, G.N.; Goodman, Z.D.; Kashitani, N.; Ros, P.R. Fibrolamellar carcinoma of the liver: Radiologic-pathologic correlation. Radiographics 1999, 19, 453–471. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Wang, W.P.; Mao, F.; Zhang, Q.; Yang, D.; Tannapfel, A.; Meloni, M.F.; Neye, H.; Clevert, D.A.; Dietrich, C.F. Imaging Features of Fibrolamellar Hepatocellular Carcinoma with Contrast-Enhanced Ultrasound. Ultraschall Der Med. 2021, 42, 306–313. [Google Scholar] [CrossRef] [PubMed]
- Ganeshan, D.; Szklaruk, J.; Kaseb, A.; Kattan, A.; Elsayes, K.M. Fibrolamellar hepatocellular carcinoma: Multiphasic CT features of the primary tumor on pre-therapy CT and pattern of distant metastases. Abdom. Radiol. 2018, 43, 3340–3348. [Google Scholar] [CrossRef]
- Zhou, S.; Venkatramani, R.; Gupta, S.; Wang, K.; Stein, J.E.; Wang, L.; Mascarenhas, L. Hepatocellular malignant neoplasm, NOS: A clinicopathological study of 11 cases from a single institution. Histopathology 2017, 71, 813–822. [Google Scholar] [CrossRef] [PubMed]
- Angelico, R.; Grimaldi, C.; Saffioti, M.C.; Castellano, A.; Spada, M. Hepatocellular carcinoma in children: Hepatic resection and liver transplantation. Transl. Gastroenterol. Hepatol. 2018, 3, 59. [Google Scholar] [CrossRef]
- Schmid, I.; von Schweinitz, D. Pediatric hepatocellular carcinoma: Challenges and solutions. J. Hepatocell. Carcinoma 2017, 4, 15–21. [Google Scholar] [CrossRef]
- Lau, C.S.; Mahendraraj, K.; Chamberlain, R.S. Hepatocellular Carcinoma in the Pediatric Population: A Population Based Clinical Outcomes Study Involving 257 Patients from the Surveillance, Epidemiology, and End Result (SEER) Database (1973–2011). HPB Surg. 2015, 2015, 670728. [Google Scholar] [CrossRef]
- Gupta, A.A.; Gerstle, J.T.; Ng, V.; Wong, A.; Fecteau, A.; Malogolowkin, M.H.; Meyers, R.L.; Grant, D.; Grant, R.M. Critical review of controversial issues in the management of advanced pediatric liver tumors. Pediatr. Blood Cancer 2011, 56, 1013–1018. [Google Scholar] [CrossRef]
- Schmid, I.; Häberle, B.; Albert, M.H.; Corbacioglu, S.; Fröhlich, B.; Graf, N.; Kammer, B.; Kontny, U.; Leuschner, I.; Scheel-Walter, H.G.; et al. Sorafenib and cisplatin/doxorubicin (PLADO) in pediatric hepatocellular carcinoma. Pediatr. Blood Cancer 2012, 58, 539–544. [Google Scholar] [CrossRef]
- Kohorst, M.A.; Warad, D.M.; Matsumoto, J.M.; Heimbach, J.K.; El-Youssef, M.; Arndt, C.A.S.; Rodriguez, V.; Nageswara Rao, A.A. Management of pediatric hepatocellular carcinoma: A multimodal approach. Pediatr. Transplant. 2017, 21, e13007. [Google Scholar] [CrossRef]
- D’Souza, A.M.; Shah, R.; Gupta, A.; Towbin, A.J.; Alonso, M.; Nathan, J.D.; Bondoc, A.; Tiao, G.; Geller, J.I. Surgical management of children and adolescents with upfront completely resected hepatocellular carcinoma. Pediatr. Blood Cancer 2018, 65, e27293. [Google Scholar] [CrossRef]
- Wang, J.; Mao, Y.; Liu, Y.; Chen, Z.; Chen, M.; Lao, X.; Li, S. Hepatocellular Carcinoma in Children and Adolescents: Clinical Characteristics and Treatment. J. Gastrointest. Surg. 2017, 21, 1128–1135. [Google Scholar] [CrossRef] [PubMed]
- McAteer, J.P.; Goldin, A.B.; Healey, P.J.; Gow, K.W. Surgical treatment of primary liver tumors in children: Outcomes analysis of resection and transplantation in the SEER database. Pediatr. Transplant. 2013, 17, 744–750. [Google Scholar] [CrossRef] [PubMed]
- Baumann, U.; Adam, R.; Duvoux, C.; Mikolajczyk, R.; Karam, V.; D’Antiga, L.; Chardot, C.; Coker, A.; Colledan, M.; Ericzon, B.G.; et al. Survival of children after liver transplantation for hepatocellular carcinoma. Liver Transplant. 2018, 24, 246–255. [Google Scholar] [CrossRef] [PubMed]
- Monsereenusorn, C.; Satayasoontorn, K.; Rujkijyanont, P.; Traivaree, C. Cholangiocarcinoma in a Child with Progressive Abdominal Distension and Secondary Hypercalcemia. Case Rep. Pediatr. 2018, 2018, 6828037. [Google Scholar] [CrossRef] [PubMed]
- Newsome, J.R.; Venkatramani, R.; Heczey, A.; Danysh, H.E.; Fishman, D.S.; Miloh, T. Cholangiocarcinoma Among Children and Adolescents: A Review of the Literature and Surveillance, Epidemiology, and End Results Program Database Analysis. J. Pediatr. Gastroenterol. Nutr. 2018, 66, e12–e18. [Google Scholar] [CrossRef] [PubMed]
- Bismuth, H.; Krissat, J. Choledochal cystic malignancies. Ann. Oncol. 1999, 10 (Suppl. S4), 94–98. [Google Scholar] [CrossRef] [PubMed]
- Yoon, H.J.; Jeon, T.Y.; Yoo, S.Y.; Kim, J.H.; Eo, H.; Lee, S.K.; Kim, J.S. Hepatic tumours in children with biliary atresia: Single-centre experience in 13 cases and review of the literature. Clin. Radiol. 2014, 69, e113–e119. [Google Scholar] [CrossRef]
- Liu, R.; Cox, K.; Guthery, S.L.; Book, L.; Witt, B.; Chadwick, B.; Adler, D.G. Cholangiocarcinoma and high-grade dysplasia in young patients with primary sclerosing cholangitis. Dig. Dis. Sci. 2014, 59, 2320–2324. [Google Scholar] [CrossRef]
- Björnsson, E.; Angulo, P. Cholangiocarcinoma in young individuals with and without primary sclerosing cholangitis. Am. J. Gastroenterol. 2007, 102, 1677–1682. [Google Scholar] [CrossRef]
- Deneau, M.; Adler, D.G.; Schwartz, J.J.; Hutson, W.; Sorensen, J.; Book, L.; Lowichik, A.; Guthery, S. Cholangiocarcinoma in a 17-year-old boy with primary sclerosing cholangitis and inflammatory bowel disease. J. Pediatr. Gastroenterol. Nutr. 2011, 52, 617–620. [Google Scholar] [CrossRef] [PubMed]
- Hayward, A.R.; Levy, J.; Facchetti, F.; Notarangelo, L.; Ochs, H.D.; Etzioni, A.; Bonnefoy, J.Y.; Cosyns, M.; Weinberg, A. Cholangiopathy and tumors of the pancreas, liver, and biliary tree in boys with X-linked immunodeficiency with hyper-IgM. J. Immunol. 1997, 158, 977–983. [Google Scholar] [CrossRef] [PubMed]
- Mangeya, N.; Mafukidze, A.T.; Pascoe, M.; Mbuwayesango, B.; Madziva, D.; Ndlovu, N.; Corbett, E.L.; Miller, R.F.; Ferrand, R.A. Cholangiocarcinoma presenting in an adolescent with vertically acquired HIV infection. Int. J. STD AIDS 2008, 19, 717–718. [Google Scholar] [CrossRef] [PubMed]
- Cunningham, J.J. Cholangiocarcinoma occurring after childhood radiotherapy for Wilm’s tumor. Hepato-Gastroenterology 1990, 37, 395–397. [Google Scholar] [PubMed]
- Nakanuma, Y.; Kakuda, Y. Pathologic classification of cholangiocarcinoma: New concepts. Best Pract. Res. Clin. Gastroenterol. 2015, 29, 277–293. [Google Scholar] [CrossRef] [PubMed]
- González, I.A.; Linn, R.L.; Wilkins, B.J. Solid-Tubulocystic Variant of Intrahepatic Cholangiocarcinoma: Report of a Pediatric Case With Molecular Characterization. Pediatr. Dev. Pathol. 2022, 25, 661–667. [Google Scholar] [CrossRef] [PubMed]
- Beaufrère, A.; Calderaro, J.; Paradis, V. Combined hepatocellular-cholangiocarcinoma: An update. J. Hepatol. 2021, 74, 1212–1224. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, S.; Kubota, M.; Yagi, M.; Okuyama, N.; Ohtaki, M.; Yamazaki, S.; Hirayama, Y.; Kurosaki, I.; Hatakeyama, K. An 11-year-old male patient demonstrating cholangiocarcinoma associated with congenital biliary dilatation. J. Pediatr. Surg. 2006, 41, e15–e19. [Google Scholar] [CrossRef]
- Yuan, M.; Li, R.; Zhang, Y.; Yang, L.; Zhang, X.; Tang, C.; Guo, D. Enhancement Patterns of Intrahepatic Cholangiocarcinoma on Contrast-Enhanced Ultrasound: Correlation with Clinicopathologic Findings and Prognosis. Ultrasound Med. Biol. 2019, 45, 26–34. [Google Scholar] [CrossRef]
- Hall, C.; Mamlok, V.; Al-Khalil, I. A Sporadic Case of Advanced Metastatic Cholangiocarcinoma in a Child: A Case Report and Review of Literature. J. Pediatr. Hematol./Oncol. 2015, 37, e333–e335. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dong, Y.; Cekuolis, A.; Schreiber-Dietrich, D.; Augustiniene, R.; Schwarz, S.; Möller, K.; Nourkami-Tutdibi, N.; Chen, S.; Cao, J.-Y.; Huang, Y.-L.; et al. Review on Pediatric Malignant Focal Liver Lesions with Imaging Evaluation: Part I. Diagnostics 2023, 13, 3568. https://doi.org/10.3390/diagnostics13233568
Dong Y, Cekuolis A, Schreiber-Dietrich D, Augustiniene R, Schwarz S, Möller K, Nourkami-Tutdibi N, Chen S, Cao J-Y, Huang Y-L, et al. Review on Pediatric Malignant Focal Liver Lesions with Imaging Evaluation: Part I. Diagnostics. 2023; 13(23):3568. https://doi.org/10.3390/diagnostics13233568
Chicago/Turabian StyleDong, Yi, Andrius Cekuolis, Dagmar Schreiber-Dietrich, Rasa Augustiniene, Simone Schwarz, Kathleen Möller, Nasenien Nourkami-Tutdibi, Sheng Chen, Jia-Ying Cao, Yun-Lin Huang, and et al. 2023. "Review on Pediatric Malignant Focal Liver Lesions with Imaging Evaluation: Part I" Diagnostics 13, no. 23: 3568. https://doi.org/10.3390/diagnostics13233568
APA StyleDong, Y., Cekuolis, A., Schreiber-Dietrich, D., Augustiniene, R., Schwarz, S., Möller, K., Nourkami-Tutdibi, N., Chen, S., Cao, J.-Y., Huang, Y.-L., Wang, Y., Taut, H., Grevelding, L., & Dietrich, C. F. (2023). Review on Pediatric Malignant Focal Liver Lesions with Imaging Evaluation: Part I. Diagnostics, 13(23), 3568. https://doi.org/10.3390/diagnostics13233568






