Is Marfan Syndrome Associated with Primary Structural Changes in the Left Atrium?
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Patient Selection
2.2. Echocardiography—Technique and Measurements
2.3. Blood Sampling and Analysis
2.4. Genetic Examination
2.5. Statistical Analysis
3. Results
3.1. Study Cohort
3.2. Left and Right Ventricular Function
3.3. Left and Right Atrial Size
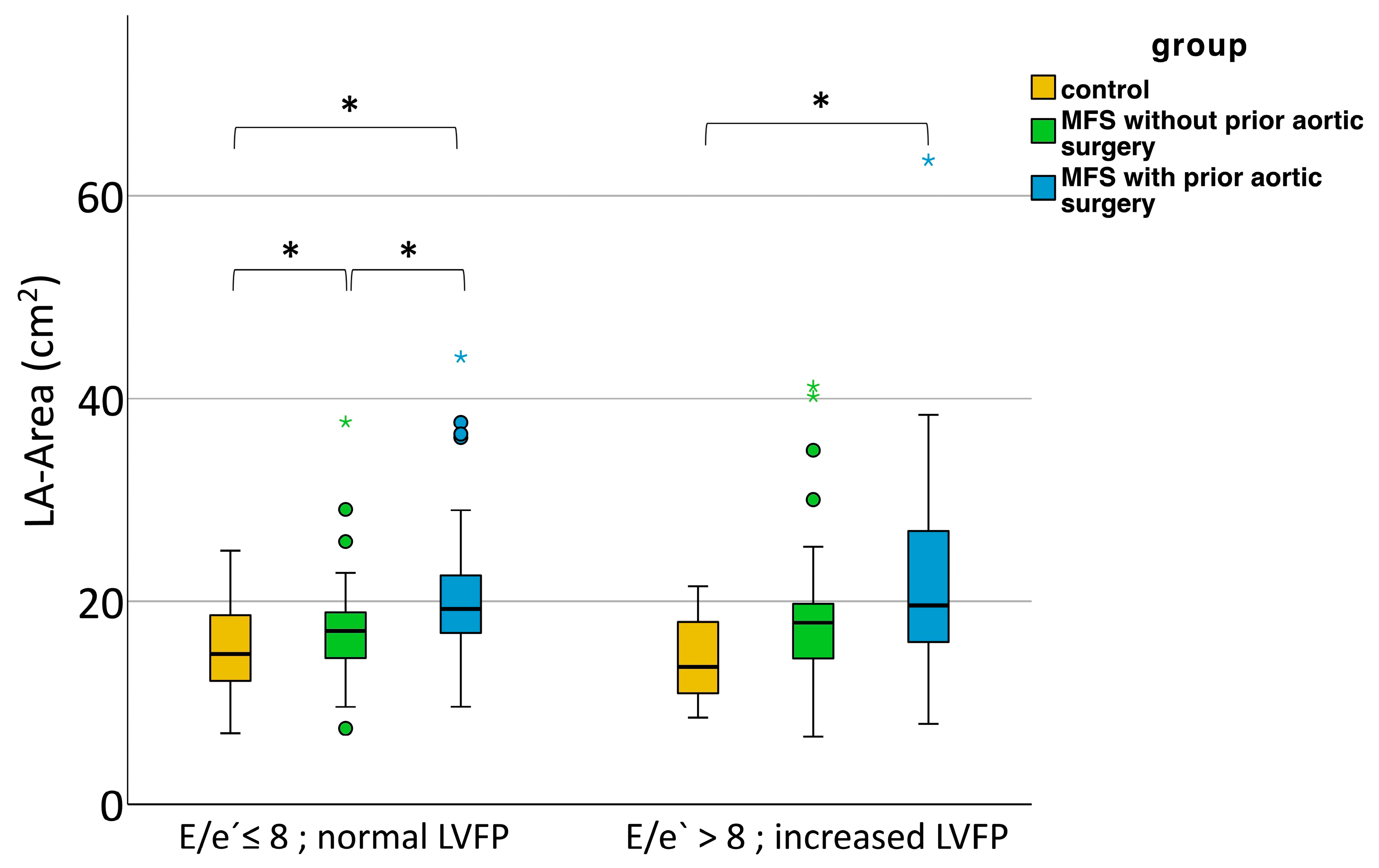
3.4. Increase in LA Size at Normal Filling Pressures
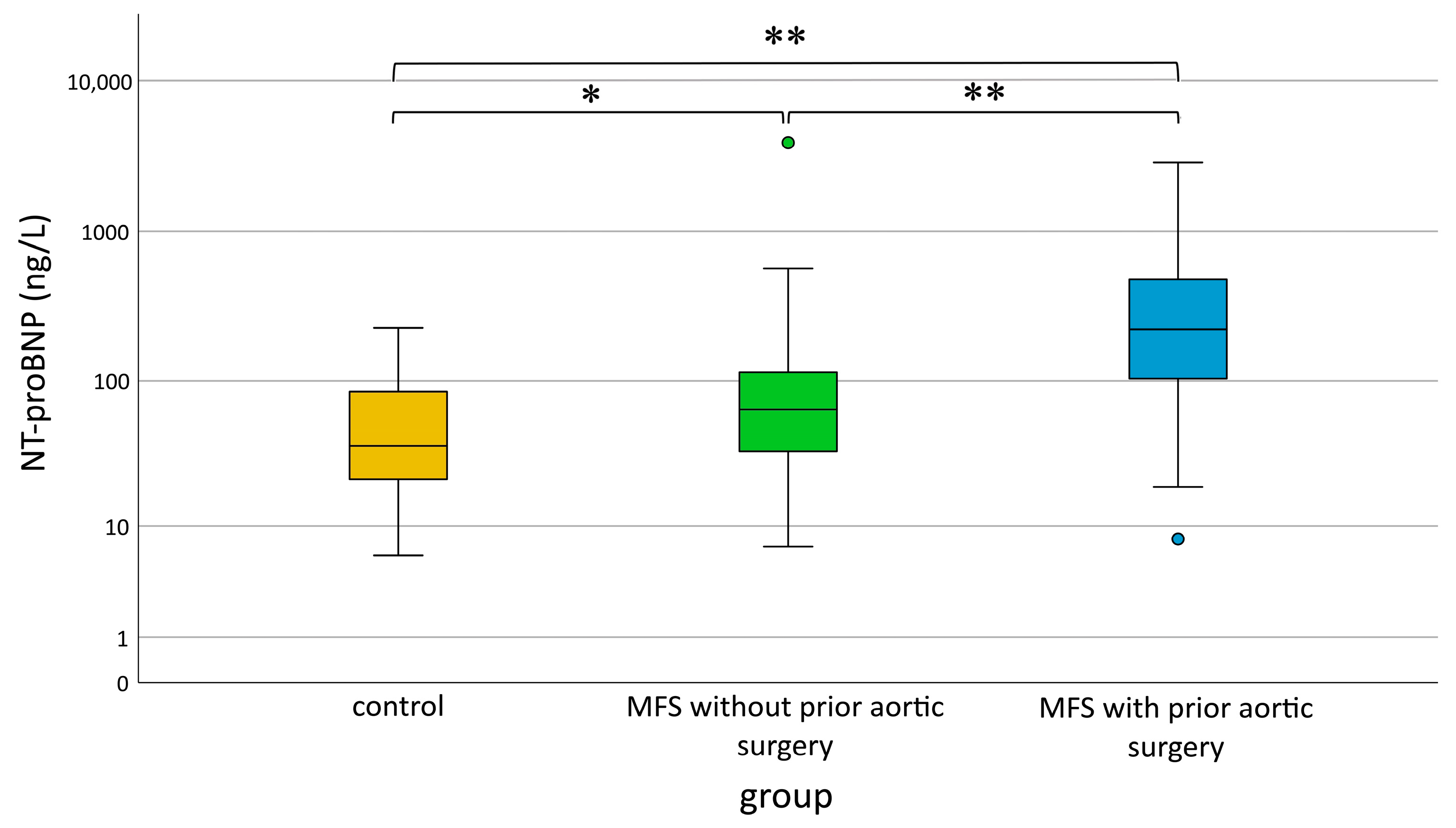
3.5. Increase in NT-proBNP with Normal Filling Pressures
3.6. Influence of MFS Mutation on LA Size and Function
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Judge, D.P.; Dietz, H.C. Marfan’s syndrome. Lancet 2005, 366, 1965–1976. [Google Scholar] [CrossRef] [PubMed]
- Dean, J.C. Marfan syndrome: Clinical diagnosis and management. Eur. J. Hum. Genet. 2007, 15, 724–733. [Google Scholar] [CrossRef] [PubMed]
- Von Kodolitsch, Y.; Demolder, A.; Girdauskas, E.; Kaemmerer, H.; Kornhuber, K.; Muino Mosquera, L.; Morris, S.; Neptune, E.; Pyeritz, R.; Rand-Hendriksen, S.; et al. Features of Marfan syndrome not listed in the Ghent nosology—The dark side of the disease. Expert. Rev. Cardiovasc. Ther. 2019, 17, 883–915. [Google Scholar] [CrossRef] [PubMed]
- Loeys, B.L.; Dietz, H.C.; Braverman, A.C.; Callewaert, B.L.; De Backer, J.; Devereux, R.B.; Hilhorst-Hofstee, Y.; Jondeau, G.; Faivre, L.; Milewicz, D.M.; et al. The revised Ghent nosology for the Marfan syndrome. J. Med. Genet. 2010, 47, 476–485. [Google Scholar] [CrossRef] [PubMed]
- Milewicz, D.M.; Dietz, H.C.; Miller, D.C. Treatment of aortic disease in patients with Marfan syndrome. Circulation 2005, 111, e150–e157. [Google Scholar] [CrossRef]
- Dietz, H.C.; Cutting, G.R.; Pyeritz, R.E.; Maslen, C.L.; Sakai, L.Y.; Corson, G.M.; Puffenberger, E.G.; Hamosh, A.; Nanthakumar, E.J.; Curristin, S.M.; et al. Marfan syndrome caused by a recurrent de novo missense mutation in the fibrillin gene. Nature 1991, 352, 337–339. [Google Scholar] [CrossRef]
- Kainulainen, K.; Pulkkinen, L.; Savolainen, A.; Kaitila, I.; Peltonen, L. Location on chromosome 15 of the gene defect causing Marfan syndrome. N. Engl. J. Med. 1990, 323, 935–939. [Google Scholar] [CrossRef]
- Sakai, L.Y.; Keene, D.R.; Engvall, E. Fibrillin, a new 350-kD glycoprotein, is a component of extracellular microfibrils. J. Cell Biol. 1986, 103, 2499–2509. [Google Scholar] [CrossRef]
- Ramirez, F.; Caescu, C.; Wondimu, E.; Galatioto, J. Marfan syndrome; A connective tissue disease at the crossroads of mechanotransduction, TGFbeta signaling and cell stemness. Matrix Biol. 2018, 71–72, 82–89. [Google Scholar] [CrossRef]
- Takeda, N.; Hara, H.; Fujiwara, T.; Kanaya, T.; Maemura, S.; Komuro, I. TGF-beta Signaling-Related Genes and Thoracic Aortic Aneurysms and Dissections. Int. J. Mol. Sci. 2018, 19, 2125. [Google Scholar] [CrossRef]
- Diller, G.P.; Enders, D.; Lammers, A.E.; Orwat, S.; Schmidt, R.; Radke, R.M.; Gerss, J.; De Torres Alba, F.; Kaleschke, G.; Bauer, U.M.; et al. Mortality and morbidity in patients with congenital heart disease hospitalised for viral pneumonia. Heart 2021, 107, 1069–1076. [Google Scholar] [CrossRef] [PubMed]
- Yetman, A.T.; Bornemeier, R.A.; McCrindle, B.W. Long-term outcome in patients with Marfan syndrome: Is aortic dissection the only cause of sudden death? J. Am. Coll. Cardiol. 2003, 41, 329–332. [Google Scholar] [CrossRef] [PubMed]
- Demolder, A.; von Kodolitsch, Y.; Muino-Mosquera, L.; De Backer, J. Myocardial Function, Heart Failure and Arrhythmia in Marfan Syndrome: A Systematic Literature Review. Diagnostics 2020, 10, 751. [Google Scholar] [CrossRef]
- Muino-Mosquera, L.; De Wilde, H.; Devos, D.; Babin, D.; Jordaens, L.; Demolder, A.; De Groote, K.; De Wolf, D.; De Backer, J. Myocardial disease and ventricular arrhythmia in Marfan syndrome: A prospective study. Orphanet J. Rare Dis. 2020, 15, 300. [Google Scholar] [CrossRef] [PubMed]
- De Backer, J.F.; Devos, D.; Segers, P.; Matthys, D.; Francois, K.; Gillebert, T.C.; De Paepe, A.M.; De Sutter, J. Primary impairment of left ventricular function in Marfan syndrome. Int. J. Cardiol. 2006, 112, 353–358. [Google Scholar] [CrossRef] [PubMed]
- Gehle, P.; Robinson, P.N.; Heinzel, F.; Edelmann, F.; Yigitbasi, M.; Berger, F.; Falk, V.; Pieske, B.; Wellnhofer, E. NT-proBNP and diastolic left ventricular function in patients with Marfan syndrome. Int. J. Cardiol. Heart Vasc. 2016, 12, 15–20. [Google Scholar] [CrossRef]
- Kiotsekoglou, A.; Moggridge, J.C.; Bijnens, B.H.; Kapetanakis, V.; Alpendurada, F.; Mullen, M.J.; Saha, S.; Nassiri, D.K.; Camm, J.; Sutherland, G.R.; et al. Biventricular and atrial diastolic function assessment using conventional echocardiography and tissue-Doppler imaging in adults with Marfan syndrome. Eur. J. Echocardiogr. 2009, 10, 947–955. [Google Scholar] [CrossRef][Green Version]
- Steijns, F.; van Hengel, J.; Sips, P.; De Backer, J.; Renard, M. A heart for fibrillin: Spatial arrangement in adult wild-type murine myocardial tissue. Histochem. Cell Biol. 2018, 150, 271–280. [Google Scholar] [CrossRef] [PubMed]
- Nakajima, H.; Nakajima, H.O.; Salcher, O.; Dittie, A.S.; Dembowsky, K.; Jing, S.; Field, L.J. Atrial but not ventricular fibrosis in mice expressing a mutant transforming growth factor-beta(1) transgene in the heart. Circ. Res. 2000, 86, 571–579. [Google Scholar] [CrossRef]
- Alpendurada, F.; Wong, J.; Kiotsekoglou, A.; Banya, W.; Child, A.; Prasad, S.K.; Pennell, D.J.; Mohiaddin, R.H. Evidence for Marfan cardiomyopathy. Eur. J. Heart Fail. 2010, 12, 1085–1091. [Google Scholar] [CrossRef]
- de Witte, P.; Aalberts, J.J.; Radonic, T.; Timmermans, J.; Scholte, A.J.; Zwinderman, A.H.; Mulder, B.J.; Groenink, M.; van den Berg, M.P. Intrinsic biventricular dysfunction in Marfan syndrome. Heart 2011, 97, 2063–2068. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kiotsekoglou, A.; Saha, S.; Moggridge, J.C.; Kapetanakis, V.; Govindan, M.; Alpendurada, F.; Mullen, M.J.; Nassiri, D.K.; Camm, J.; Sutherland, G.R.; et al. Impaired biventricular deformation in Marfan syndrome: A strain and strain rate study in adult unoperated patients. Echocardiography 2011, 28, 416–430. [Google Scholar] [CrossRef]
- Scherptong, R.W.; Vliegen, H.W.; van der Wall, E.E.; Hilhorst-Hofstee, Y.; Bax, J.J.; Scholte, A.J.; Delgado, V. Biventricular performance in patients with marfan syndrome without significant valvular disease: Comparison to normal subjects and longitudinal follow-up. J. Am. Soc. Echocardiogr. 2011, 24, 1392–1399.e1391. [Google Scholar] [CrossRef] [PubMed]
- Mandoli, G.E.; Cameli, M.; Novo, G.; Agricola, E.; Righini, F.M.; Santoro, C.; D′Ascenzi, F.; Ancona, F.; Sorrentino, R.; D’Andrea, A.; et al. Right ventricular function after cardiac surgery: The diagnostic and prognostic role of echocardiography. Heart Fail. Rev. 2019, 24, 625–635. [Google Scholar] [CrossRef] [PubMed]
- Rossi, A.; Temporelli, P.L.; Quintana, M.; Dini, F.L.; Ghio, S.; Hillis, G.S.; Klein, A.L.; Marsan, N.A.; Prior, D.L.; Yu, C.M.; et al. Independent relationship of left atrial size and mortality in patients with heart failure: An individual patient meta-analysis of longitudinal data (MeRGE Heart Failure). Eur. J. Heart Fail. 2009, 11, 929–936. [Google Scholar] [CrossRef]
- Thomas, L.; Abhayaratna, W.P. Left Atrial Reverse Remodeling: Mechanisms, Evaluation, and Clinical Significance. JACC Cardiovasc. Imaging 2017, 10, 65–77. [Google Scholar] [CrossRef]
- Abd El Rahman, M.; Haase, D.; Rentzsch, A.; Olchvary, J.; Schafers, H.J.; Henn, W.; Wagenpfeil, S.; Abdul-Khaliq, H. Left ventricular systolic dysfunction in asymptomatic Marfan syndrome patients is related to the severity of gene mutation: Insights from the novel three dimensional speckle tracking echocardiography. PLoS ONE 2015, 10, e0124112. [Google Scholar] [CrossRef]
- Ommen, S.R.; Nishimura, R.A.; Appleton, C.P.; Miller, F.A.; Oh, J.K.; Redfield, M.M.; Tajik, A.J. Clinical utility of Doppler echocardiography and tissue Doppler imaging in the estimation of left ventricular filling pressures: A comparative simultaneous Doppler-catheterization study. Circulation 2000, 102, 1788–1794. [Google Scholar] [CrossRef]
- Neptune, E.R.; Frischmeyer, P.A.; Arking, D.E.; Myers, L.; Bunton, T.E.; Gayraud, B.; Ramirez, F.; Sakai, L.Y.; Dietz, H.C. Dysregulation of TGF-beta activation contributes to pathogenesis in Marfan syndrome. Nat. Genet. 2003, 33, 407–411. [Google Scholar] [CrossRef]
- Arslan-Kirchner, M.; Arbustini, E.; Boileau, C.; Child, A.; Collod-Beroud, G.; De Paepe, A.; Epplen, J.; Jondeau, G.; Loeys, B.; Faivre, L. Clinical utility gene card for: Marfan syndrome type 1 and related phenotypes [FBN1]. Eur. J. Hum. Genet. 2010, 18, 1071. [Google Scholar] [CrossRef]
- Loeys, B. The search for genotype/phenotype correlation in Marfan syndrome: To be or not to be? Eur. Heart J. 2016, 37, 3291–3293. [Google Scholar] [CrossRef] [PubMed]
- Goetze, J.P.; Bruneau, B.G.; Ramos, H.R.; Ogawa, T.; de Bold, M.K.; de Bold, A.J. Cardiac natriuretic peptides. Nat. Rev. Cardiol. 2020, 17, 698–717. [Google Scholar] [CrossRef] [PubMed]
- Ramos, H.; de Bold, A.J. Gene expression, processing, and secretion of natriuretic peptides: Physiologic and diagnostic implications. Heart Fail. Clin. 2006, 2, 255–268. [Google Scholar] [CrossRef] [PubMed]


| MFS without Aortic Surgery | MFS with Prior Aortic Surgery | Control | |
|---|---|---|---|
| n | 122 | 85 | 106 |
| Female | 74 (60.7%) | 37 (43.5%) | 52 (49.1%) |
| Age (years) | 37.5 ± 15.0 | 47.4 ± 12.9 | 38.5 ± 14.3 |
| Height (cm) | 185 ± 10 | 188 ± 11 | 181 ± 11 |
| Weight (kg) | 75.5 ± 25.0 | 85.0 ± 29.0 | 69.5 ± 21.5 |
| BMI (kg/m2) | 22.1 ± 5.4 | 25.4 ± 6.5 | 21.2 ± 5.8 |
| BSA (m2) | 2.0 ± 0.3 | 2.1 ± 0.3 | 1.9 ± 0.2 |
| HR (1/min) | 69 ± 10 | 70 ± 12 | 75 ± 14 |
| Medication | |||
| ARB | 82 (67.2%) | 60 (70.6%) | 21 (19.8%) |
| Losartan | 73 | 41 | 16 |
| Candesartan | 7 | 7 | 4 |
| Valsartan | 2 | 12 | 1 |
| Beta blocker | 25 (20.7%) | 60 (70.6%) | 11 (10.4%) |
| ACE inhibitor | 7 (5.7%) | 14 (16.5%) | 2 (1.9%) |
| Aortic diameter [mm] | |||
| Anulus | 23.6 ± 3.7 | 23.6 ± 4.2 | 22.0 ± 3.5 |
| Bulbus | 39.1 ± 7.0 | 34.3 ± 9.8 | 34.0 ± 6.4 |
| ST-junction | 30.8 ± 5.3 | 28.6 ± 6.5 | 28.3 ± 6.4 |
| Ascending aorta | 31.0 ± 5.5 | 29.6 ± 7.6 | 30.6 ± 5.7 |
| n | MFS without Aortic Surgery (1) | n | MFS with Prior Aortic Surgery (2) | n | Control (3) | p 1 vs. 2 | p 1 vs. 3 | p 2 vs. 3 | |
|---|---|---|---|---|---|---|---|---|---|
| Systolic function | |||||||||
| iLVIDd (mm/m2) | 122 | 26.4 ± 4.2 | 85 | 24.7 ± 5.6 | 106 | 26.2 ± 3.8 | ns | ns | ns |
| iLVIDs (mm/m2) | 122 | 16.0 ± 2.7 | 85 | 16.7 ± 4.1 | 106 | 15.7 ± 2.6 | ns | ns | ns |
| iEDV (mL/m2) | 122 | 66.1 ± 18.6 | 85 | 63.6 ± 33.8 | 106 | 61.3 ± 17.5 | ns | <0.05 | ns |
| iESV (mL/m2) | 122 | 19.9 ± 8.7 | 85 | 21.5 ± 14.7 | 106 | 17.8 ± 8.8 | ns | ns | <0.001 |
| FS (%) | 122 | 39.8 ± 7.6 | 85 | 37.0 ± 10.2 | 106 | 40.1 ± 8.6 | <0.01 | ns | <0.01 |
| EF (%) | 122 | 69.7 ± 9.6 | 85 | 66.0 ± 13.6 | 106 | 70.6 ± 11.0 | <0.01 | ns | <0.001 |
| Diastolic function | |||||||||
| E (cm/s) | 121 | 75.0 ± 17.9 | 82 | 74.9 ± 25.0 | 103 | 74.7 ± 17.6 | ns | ns | ns |
| A (cm/s) | 121 | 55.4 ± 20.3 | 81 | 57.4 ± 22.4 | 103 | 52.4 ± 22.6 | ns | ns | ns |
| E/A | 121 | 1.3 ± 0.7 | 81 | 1.3 ± 0.6 | 103 | 1.4 ± 0.7 | ns | ns | ns |
| e′ (cm/s) | 122 | 10.7 ± 2.3 | 84 | 8.9 ± 2.2 | 98 | 11.8 ± 3.3 | <0.001 | <0.01 | <0.001 |
| IVRT (ms) | 104 | 87.5 ± 25.8 | 64 | 82.8 ± 29.9 | 72 | 77.3 ± 29.0 | ns | <0.001 | <0.05 |
| DT (ms) | 121 | 176 ± 35 | 82 | 183 ± 53 | 102 | 170 ± 48 | ns | ns | ns |
| E/e′ | 121 | 7.0 ± 2.0 | 82 | 7.7 ± 4.0 | 95 | 6.3 ± 3.0 | <0.05 | ns | <0.001 |
| iIVSd (mm/m2) | 121 | 5.2 ± 1.4 | 85 | 5.7 ± 1.5 | 106 | 4.8 ± 1.2 | <0.05 | ns | <0.001 |
| iIVSs (mm/m2) | 122 | 7.2 ± 1.7 | 85 | 8.0 ± 1.9 | 106 | 7.2 ± 1.7 | <0.01 | ns | <0.01 |
| iPWd (mm/m2) | 122 | 4.5 ± 0.7 | 85 | 4.7 ± 0.8 | 106 | 4.5 ± 0.8 | ns | ns | ns |
| LV mass (g) | 122 | 186.0 ± 87.1 | 85 | 234.0 ± 129.3 | 106 | 155.0 ± 69.3 | <0.001 | <0.001 | <0.001 |
| iRVIDd (mm/m2) | 119 | 13.2 ± 2.6 | 79 | 13.4 ± 2.6 | 103 | 13.1 ± 2.5 | ns | ns | ns |
| TAPSE (mm) | 99 | 23.8 ± 6.7 | 65 | 17.6 ± 5.0 | 64 | 24.2 ± 6.7 | <0.001 | ns | <0.001 |
| n | MFS without Aortic Surgery (1) | n | MFS with Prior Aortic Surgery (2) | n | Control (3) | p 1 vs. 2 | p 1 vs. 3 | p 2 vs. 3 | |
|---|---|---|---|---|---|---|---|---|---|
| LA area (cm2) + | 111 | 17.1 ± 4.9 | 79 | 19.5 ± 8.2 | 93 | 14.4 ± 6.3 | <0.001 | 0.003 | <0.001 |
| LA volume (mL) + | 62 | 47.3 ± 28.4 | 47 | 57.4 ± 60.7 | 30 | 35.3 ± 21.1 | 0.12 | 0.05 | <0.001 |
| LAVi (mL/BSA) + | 62 | 23.7 ± 16.9 | 47 | 27.0 ± 28.5 | 30 | 19.0 ± 10.3 | 0.42 | 0.07 | 0.002 |
| RA area (cm2) + | 76 | 13.8 ± 4.0 | 59 | 16.7 ± 7.6 | 47 | 13.8 ± 4.0 | 0.002 | 1 | 0.005 |
| Types of Mutation | Patients with MFS |
|---|---|
| n | 231 |
| Missense mutation | 107 (46.3%) |
| Nonsense mutation | 34 (14.7%) |
| Splicing mutation | 19 (8.2%) |
| Frameshift mutation | 31 (13.4%) |
| In-frame deletion/insertion | 5 (2.2%) |
| Polymorphism | 2 (0.9%) |
| Silent variants | 8 (3.5%) |
| No mutation found | 13 (5.6%) |
| Without genetic testing/missing data | 12 (5.2%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, K.; Ernst, L.; Schobert, I.; Philipp, K.; Böning, G.; Heinzel, F.R.; Boldt, L.-H.; Gehle, P. Is Marfan Syndrome Associated with Primary Structural Changes in the Left Atrium? Diagnostics 2023, 13, 3278. https://doi.org/10.3390/diagnostics13203278
Zhang K, Ernst L, Schobert I, Philipp K, Böning G, Heinzel FR, Boldt L-H, Gehle P. Is Marfan Syndrome Associated with Primary Structural Changes in the Left Atrium? Diagnostics. 2023; 13(20):3278. https://doi.org/10.3390/diagnostics13203278
Chicago/Turabian StyleZhang, Kun, Lucas Ernst, Isabel Schobert, Karla Philipp, Georg Böning, Frank R. Heinzel, Leif-Hendrik Boldt, and Petra Gehle. 2023. "Is Marfan Syndrome Associated with Primary Structural Changes in the Left Atrium?" Diagnostics 13, no. 20: 3278. https://doi.org/10.3390/diagnostics13203278
APA StyleZhang, K., Ernst, L., Schobert, I., Philipp, K., Böning, G., Heinzel, F. R., Boldt, L.-H., & Gehle, P. (2023). Is Marfan Syndrome Associated with Primary Structural Changes in the Left Atrium? Diagnostics, 13(20), 3278. https://doi.org/10.3390/diagnostics13203278






