Use of Transradial Access to Install Two Sequential Stents for Pseudoaneurysms along the Celiac Artery and Common Hepatic Artery Axes
Abstract
:Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Ghosh, A.; Zhang, J.; Akhter, N.M. Transradial versus transfemoral arterial access in Yttrium-90 microspheres radioembolization for hepatocellular carcinoma. J. Clin. Imaging Sci. 2022, 12, 27. [Google Scholar] [CrossRef] [PubMed]
- Agostoni, P.; Biondi-Zoccai, G.G.; de Benedictis, M.L.; Rigattieri, S.; Turri, M.; Anselmi, M.; Vassanelli, C.; Zardini, P.; Louvard, Y.; Hamon, M. Radial versus femoral approach for percutaneous coronary diagnostic and interventional procedures; Systematic overview and meta-analysis of randomized trials. J. Am. Coll. Cardiol. 2004, 44, 349–356. [Google Scholar] [CrossRef] [PubMed]
- Valgimigli, M.; Gagnor, A.; Calabro, P.; Frigoli, E.; Leonardi, S.; Zaro, T.; Rubartelli, P.; Briguori, C.; Andò, G.; Repetto, A.; et al. Radial versus femoral access in patients with acute coronary syndromes undergoing invasive management: A randomised multicentre trial. Lancet 2015, 385, 2465–2476. [Google Scholar] [CrossRef] [PubMed]
- Murdock, J.E.; Jensen, H.A.; Thourani, V.H. Nontransfemoral Approaches to Transcatheter Aortic Valve Replacement. Interv. Cardiol. Clin. 2015, 4, 95–105. [Google Scholar] [CrossRef] [PubMed]
- Thourani, V.H.; Li, C.; Devireddy, C.; Jensen, H.A.; Kilgo, P.; Leshnower, B.G.; Mavromatis, K.; Sarin, E.L.; Nguyen, T.C.; Kanitkar, M.; et al. High-Risk Patients With Inoperative Aortic Stenosis: Use of Transapical, Transaortic, and Transcarotid Techniques. Ann. Thorac. Surg. 2015, 99, 817–825. [Google Scholar] [CrossRef] [PubMed]
- Ferrero, E.; Viazzo, A.; Ferri, M.; Robaldo, A.; Piazza, S.; Berardi, G.; Pecchio, A.; Cumbo, P.; Nessi, F. Management and Urgent Repair of Ruptured Visceral Artery Aneurysms. Ann. Vasc. Surg. 2011, 25, 981.e7–981.e11. [Google Scholar] [CrossRef] [PubMed]
- Etezadi, V.; Gandhi, R.T.; Benenati, J.F.; Rochon, P.; Gordon, M.; Benenati, M.J.; Alehashemi, S.; Katzen, B.T.; Geisbüsch, P. Endovascular Treatment of Visceral and Renal Artery Aneurysms. J. Vasc. Interv. Radiol. 2011, 22, 1246–1253. [Google Scholar] [CrossRef] [PubMed]
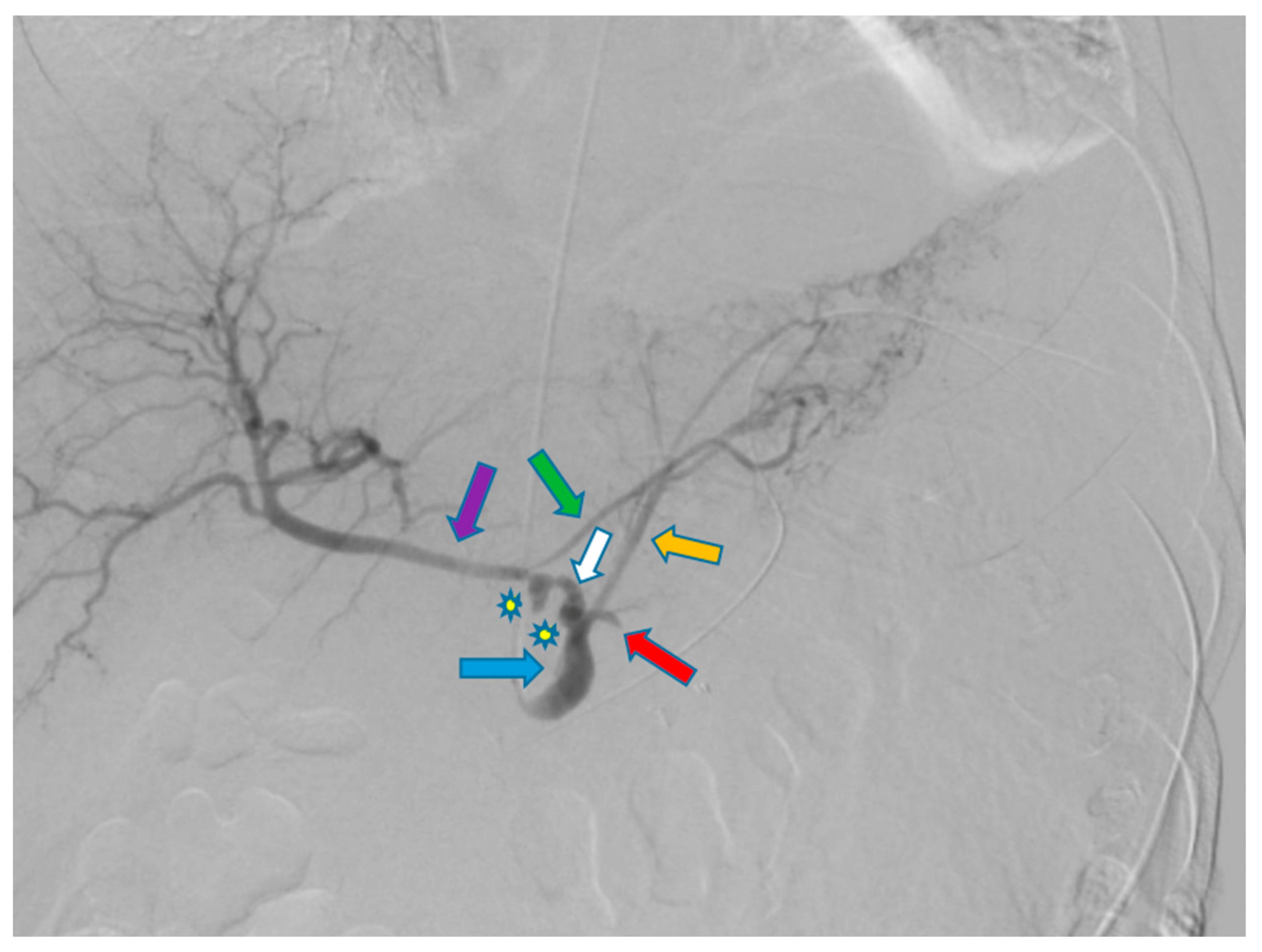
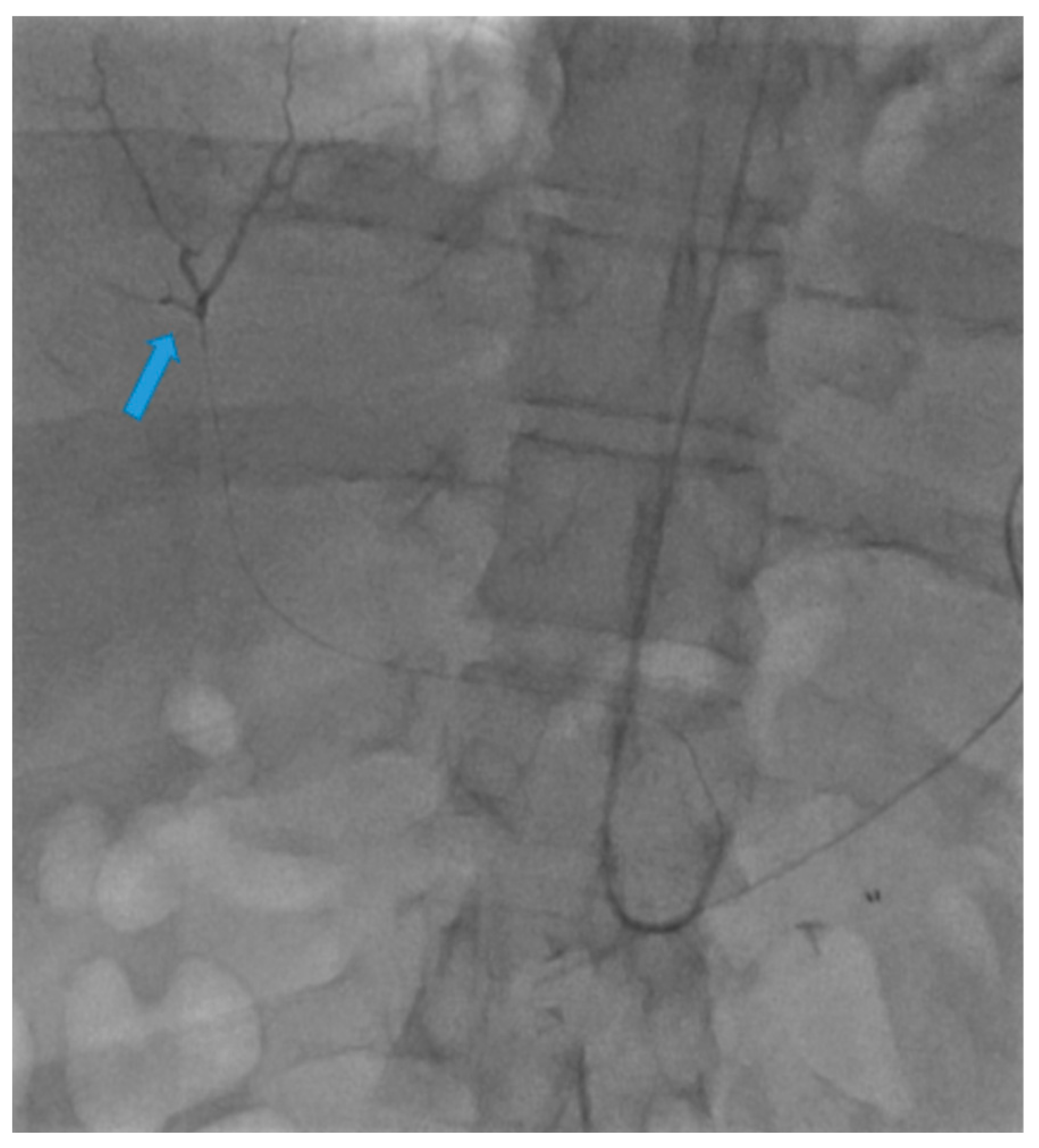
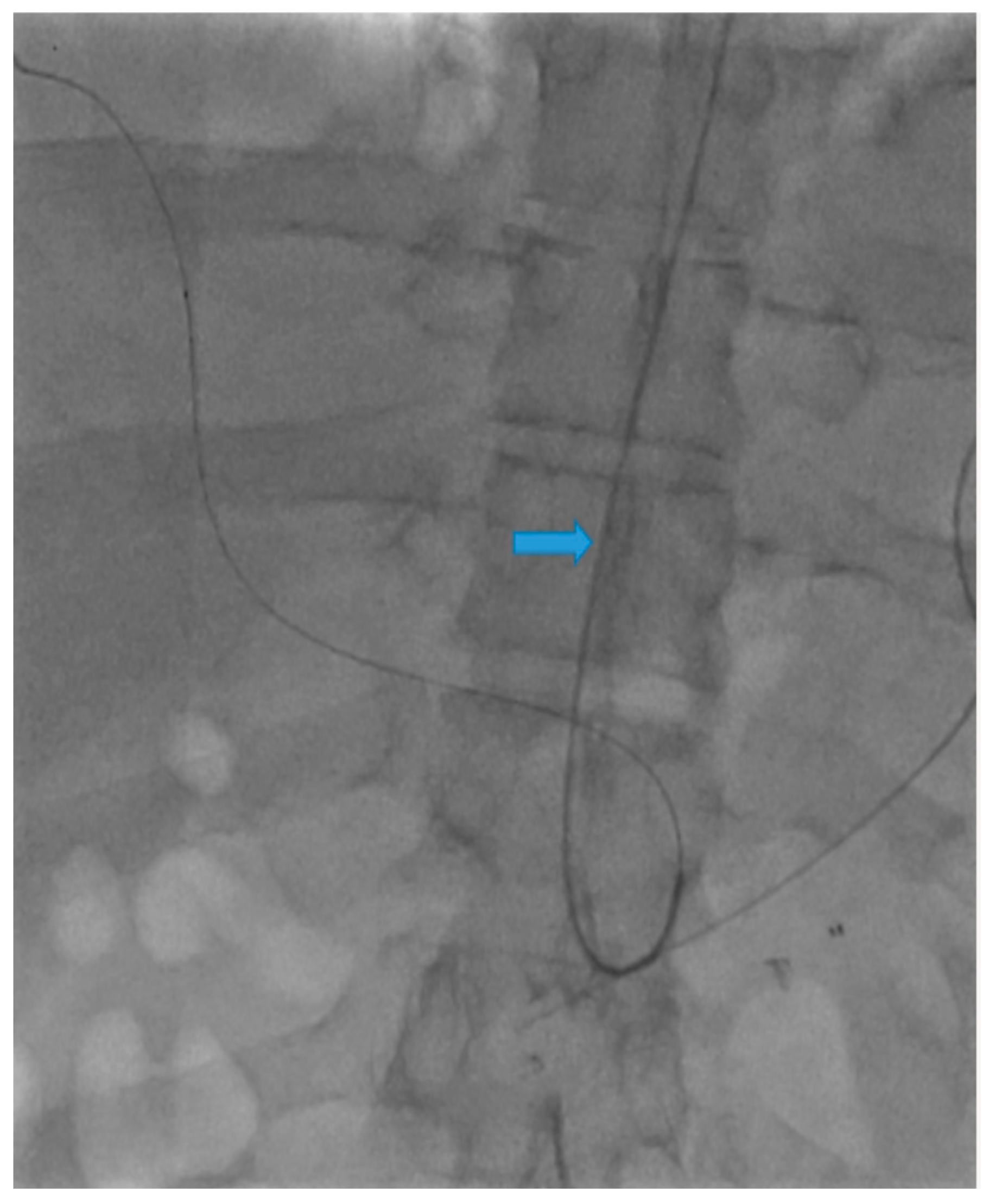
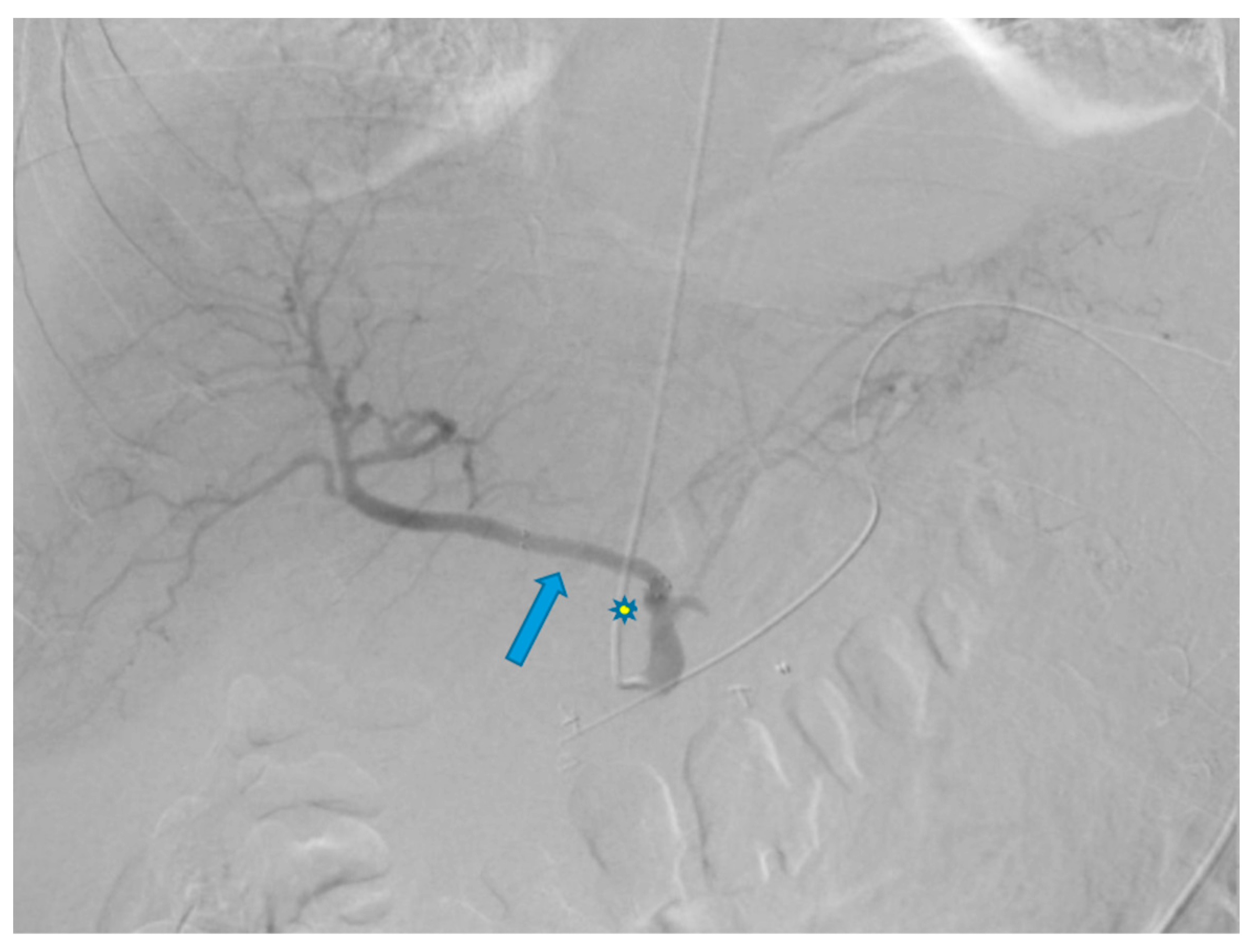
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ghosh, A.; Lee, S.; Lim, C.; Agnihotri, T.; Akhter, N. Use of Transradial Access to Install Two Sequential Stents for Pseudoaneurysms along the Celiac Artery and Common Hepatic Artery Axes. Diagnostics 2023, 13, 3273. https://doi.org/10.3390/diagnostics13203273
Ghosh A, Lee S, Lim C, Agnihotri T, Akhter N. Use of Transradial Access to Install Two Sequential Stents for Pseudoaneurysms along the Celiac Artery and Common Hepatic Artery Axes. Diagnostics. 2023; 13(20):3273. https://doi.org/10.3390/diagnostics13203273
Chicago/Turabian StyleGhosh, Abheek, Sean Lee, Christina Lim, Tanvir Agnihotri, and Nabeel Akhter. 2023. "Use of Transradial Access to Install Two Sequential Stents for Pseudoaneurysms along the Celiac Artery and Common Hepatic Artery Axes" Diagnostics 13, no. 20: 3273. https://doi.org/10.3390/diagnostics13203273
APA StyleGhosh, A., Lee, S., Lim, C., Agnihotri, T., & Akhter, N. (2023). Use of Transradial Access to Install Two Sequential Stents for Pseudoaneurysms along the Celiac Artery and Common Hepatic Artery Axes. Diagnostics, 13(20), 3273. https://doi.org/10.3390/diagnostics13203273



