Updates on HPV Vaccination
Abstract
:1. History of the HPV Vaccine
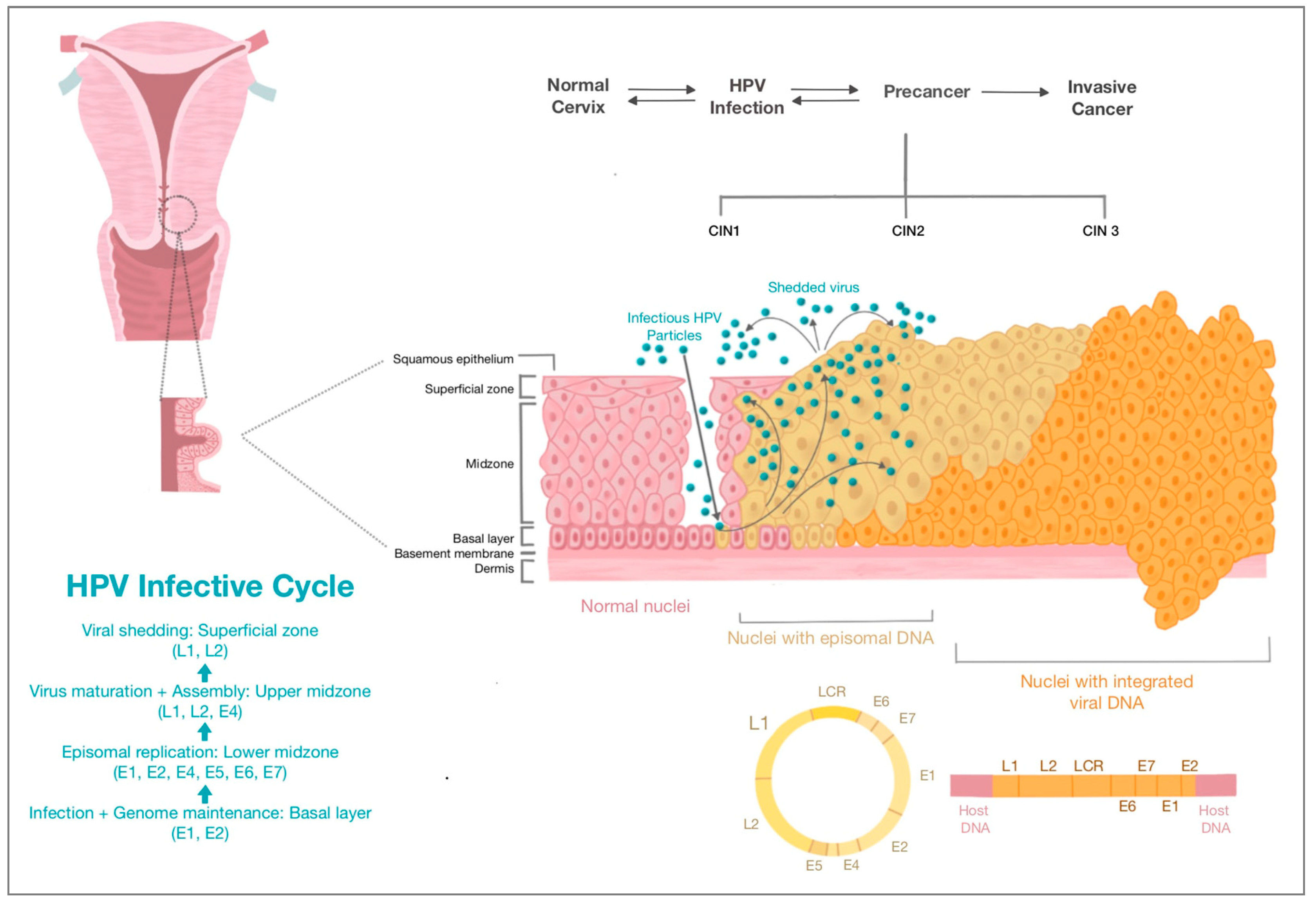
2. HPV and Cervical Carcinogenesis
HPV-Induced Cervical Carcinogenesis
3. Prophylactic HPV Vaccine Types and Mechanism of Action
4. Prophylactic HPV Vaccine Clinical Trials
4.1. Clinical Trials in Younger Females
4.1.1. The Quadrivalent HPV Vaccine—Gardasil®
4.1.2. The Bivalent HPV Vaccine—Cervarix®
4.1.3. The Nonavalent HPV Vaccine—Gardasil 9®
4.1.4. The Bivalent Cecolin® Vaccine
| Trial | Number of Participants | Participant Ages | Efficacy against Vaccine-Type CIN2+ |
|---|---|---|---|
| Gardasil® | |||
| FUTURE I and II [33] NCT00092521 and NCT00092534 | 12,167 | 15–26 | 98% |
| Cervarix® | |||
| PATRICIA [37] NCT00122681 | 18,644 | 15–25 | 92.9% |
| CVT [39] NCT00128661 | 7466 | 15–25 | 89.5% |
| Gardasil 9® | |||
| NCT00543543 [42] | 14,215 | 16–25 | 97.1% |
| Cecolin® | |||
| NCT01735006 [45] | 3723 | 18–26 | 100% |
4.2. Clinical Trials in Older Females
4.3. HPV Vaccine with Previous Known Infection
4.4. HPV Vaccine in HIV Infection
4.5. HPV Vaccination in Males
4.6. Cross-Protection against Nonvaccine HPV Subtypes
5. HPV Vaccine Safety
6. Impact of HPV Vaccination on Populations
7. Number of Doses
8. Prophylactic HPV Vaccine as Adjunct Treatment for CIN
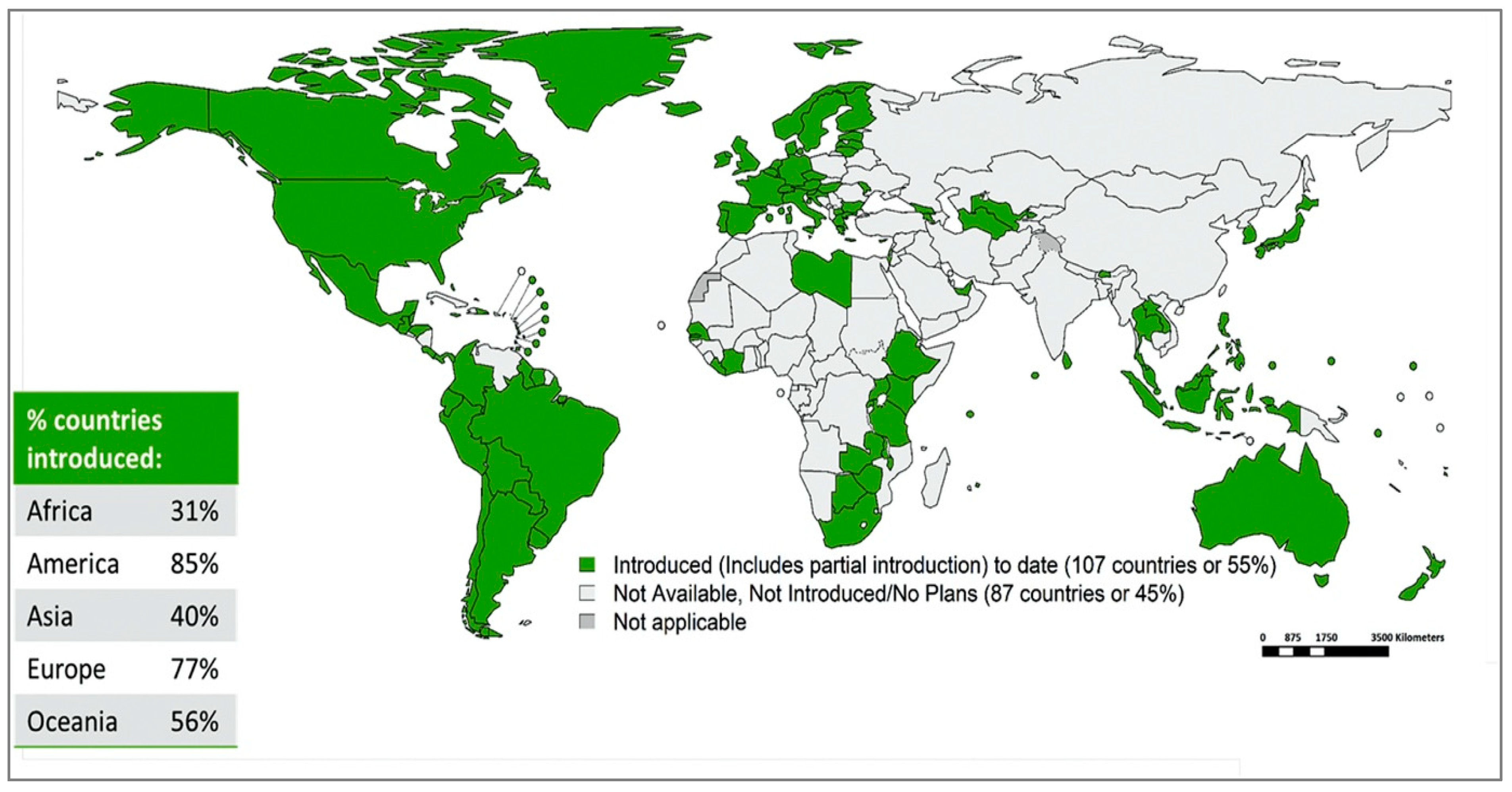
9. HPV Vaccination Implementation, Programmes and Coverage
10. Barriers to HPV Vaccine Uptake
10.1. Cost
10.2. HPV Vaccine Shortage
10.3. Cold-Chain Requirement
10.4. Adolescent Age Group
10.5. COVID-19 Pandemic
10.6. Vaccine Hesitancy
11. Therapeutic HPV Vaccines
12. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gey, G.O.; Coffman, W.D.; Kubicek, M.T. Tissue culture studies of the proliferative capacity of cervical carcinoma and normal epithelium. Cancer Res. 1952, 12, 264–265. [Google Scholar]
- Hausen, H.Z. Human papillomaviruses and their possible role in squamous cell carcinomas. In Current Topics in Microbiology and Immunology; Arber, W., Henle, W., Hofschneider, P.H., Humphrey, J.H., Klein, J., Koldovský, P., Koprowski, H., Maaløe, O., Melchers, F., Rott, R., et al., Eds.; Springer: Berlin/Heidelberg, Germany, 1977; pp. 1–30. [Google Scholar] [CrossRef]
- Schwarz, E.; Freese, U.K.; Gissmann, L.; Mayer, W.; Roggenbuck, B.; Stremlau, A.; Hausen, H.Z. Structure and transcription of human papillomavirus sequences in cervical carcinoma cells. Nature 1985, 314, 111–114. [Google Scholar] [CrossRef] [PubMed]
- Wallin, K.-L.; Wiklund, F.; Ångström, T.; Bergman, F.; Stendahl, U.; Wadell, G.; Hallmans, G.; Dillner, J. Type-specific persistence of human papillomavirus DNA before the development of invasive cervical cancer. N. Engl. J. Med. 1999, 341, 1633–1638. [Google Scholar] [CrossRef]
- Walboomers, J.M.M.; Jacobs, M.V.; Manos, M.M.; Bosch, F.X.; Kummer, J.A.; Shah, K.V.; Snijders, P.J.; Peto, J.; Meijer, C.J.; Muñoz, N. Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. J. Pathol. 1999, 189, 12–19. [Google Scholar] [CrossRef]
- World Health Organization. WHO Guideline for Screening and Treatment of Cervical Pre-Cancer Lesions for Cervical Cancer Prevention, 2nd ed.; World Health Organization: Geneva, Switzerland, 2021; Available online: https://apps.who.int/iris/handle/10665/342365 (accessed on 2 December 2021).
- Frazer, I.H. The HPV vaccine story. ACS Pharmacol. Transl. Sci. 2019, 2, 210–212. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- World Health Organization. Comprehensive Cervical Cancer Control: A Guide to Essential Practice, 2nd ed.; World Health Organization: Geneva, Switzerland, 2014; Available online: https://apps.who.int/iris/handle/10665/144785 (accessed on 3 December 2021).
- Crosbie, E.J.; Einstein, M.H.; Franceschi, S.; Kitchener, H.C. Human papillomavirus and cervical cancer. Lancet 2013, 382, 889–899. [Google Scholar] [CrossRef] [PubMed]
- Human Papillomavirus (HPV) and Cervical Cancer. Available online: https://www.who.int/news-room/fact-sheets/detail/human-papillomavirus-(hpv)-and-cervical-cancer (accessed on 10 December 2021).
- De Sanjosé, S.; Diaz, M.; Castellsagué, X.; Clifford, G.; Bruni, L.; Muñoz, N.; Bosch, F.X. Worldwide prevalence and genotype distribution of cervical human papillomavirus DNA in women with normal cytology: A meta-analysis. Lancet Infect. Dis. 2007, 7, 453–459. [Google Scholar] [CrossRef]
- Okunade, K.S. Human papillomavirus and cervical cancer. J. Obstet. Gynaecol. 2020, 40, 602–608. [Google Scholar] [CrossRef]
- Wiley, D.J.; Douglas, J.; Beutner, K.; Cox, T.; Fife, K.; Moscicki, A.; Fukumoto, L. External genital warts: Diagnosis, treatment, and prevention. Clin. Infect. Dis. 2002, 35, S210–S224. [Google Scholar] [CrossRef] [Green Version]
- Human Papillomaviruses. Available online: https://monographs.iarc.who.int/wp-content/uploads/2018/06/mono100B-11.pdf (accessed on 11 December 2021).
- Bruni, L.; Albero, G.; Serrano, B.; Mena, M.; Collado, J.J.; Gómez, D.; Muñoz, J.; Bosch, F.X.; de Sanjosé, S. Human Papillomavirus and Related Diseases in the World. Summary Report. October 2021. Available online: https://hpvcentre.net/statistics/reports/XWX.pdf (accessed on 30 September 2022).
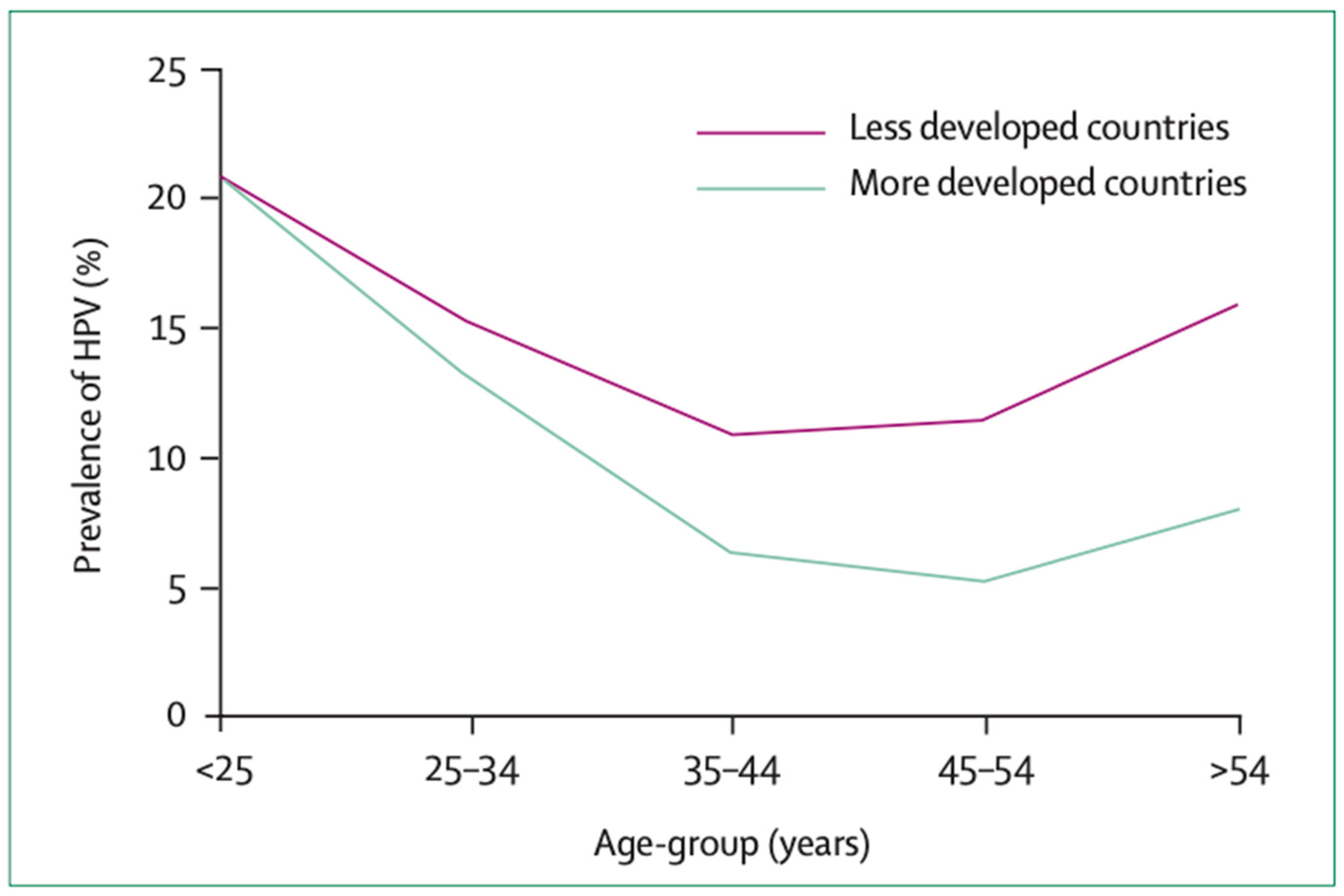
- Franceschi, S.; Herrero, R.; Clifford, G.M.; Snijders, P.J.; Arslan, A.; Anh, P.T.H.; Bosch, F.X.; Ferreccio, C.; Hieu, N.T.; Lazcano-Ponce, E.; et al. Variations in the age-specific curves of human papillomavirus prevalence in women worldwide. Int. J. Cancer 2006, 119, 2677–2684. [Google Scholar] [CrossRef]
- Williams, E.A.; Newberg, J.; Williams, K.J.; Montesion, M.; Alexander, B.M.; Lin, D.I.; Elvin, J.A. Prevalence of high-risk nonvaccine human papillomavirus types in advanced squamous cell carcinoma among individuals of African vs non-African ancestry. JAMA Netw. Open 2021, 4, e216481. [Google Scholar] [CrossRef] [PubMed]
- Mendoza, R.P.; Haidary, T.; Gabutan, E.; Zhou, Y.Y.; Bukhari, Z.; Connelly, C.; Lee, W.-C.; Lee, Y.-C.; Wadgaonkar, R.; Agrawal, R.; et al. Mixed and nonvaccine high risk HPV types are associated with higher mortality in Black women with cervical cancer. Sci. Rep. 2021, 11, 14064. [Google Scholar] [CrossRef] [PubMed]
- de Sanjose, S.; Quint, W.G.V.; Alemany, L.; Geraets, D.T.; Klaustermeier, J.E.; Lloveras, B.; Tous, S.; Felix, A.; Bravo, L.E.; Shin, H.-R.; et al. Human papillomavirus genotype attribution in invasive cervical cancer: A retrospective cross-sectional worldwide study. Lancet Oncol. 2010, 11, 1048–1056. [Google Scholar] [CrossRef] [PubMed]
- Huber, J.; Mueller, A.; Sailer, M.; Regidor, P.-A. Human papillomavirus persistence or clearance after infection in reproductive age. What is the status? Review of the literature and new data of a vaginal gel containing silicate dioxide, citric acid, and selenite. Womens Health 2021, 17, 17455065211020702. [Google Scholar] [CrossRef]
- Wang, R.; Pan, W.; Jin, L.; Huang, W.; Li, Y.; Wu, D.; Gao, C.; Ma, D.; Liao, S. Human papillomavirus vaccine against cervical cancer: Opportunity and challenge. Cancer Lett. 2020, 471, 88–102. [Google Scholar] [CrossRef]
- Pils, S.; Joura, E.A. From the monovalent to the nine-valent HPV vaccine. Clin. Microbiol. Infect. 2015, 21, 827–833. [Google Scholar] [CrossRef] [Green Version]
- Hu, Y.; Huang, S.-J.; Chu, K.; Wu, T.; Wang, Z.-Z.; Yang, C.-L.; Cai, J.-P.; Jiang, H.-M.; Wang, Y.-J.; Guo, M.; et al. Safety of an Escherichia coli-expressed bivalent human papillomavirus (types 16 and 18) L1 virus-like particle vaccine. Hum. Vaccines Immunother. 2013, 10, 469–475. [Google Scholar] [CrossRef] [Green Version]
- WHO HPV Vaccine Global Market Study April 2022. Available online: https://www.who.int/publications/m/item/who-hpv-vaccine-global-market-study-april-2022 (accessed on 24 April 2022).
- Oncology, T.L. HPV vaccination in south Asia: New progress, old challenges. Lancet Oncol. 2022, 23, 1233. [Google Scholar] [CrossRef]
- Bryan, J.T. Developing an HPV vaccine to prevent cervical cancer and genital warts. Vaccine 2007, 25, 3001–3006. [Google Scholar] [CrossRef]
- Schiller, J.; Lowy, D. Explanations for the high potency of HPV prophylactic vaccines. Vaccine 2018, 36, 4768–4773. [Google Scholar] [CrossRef]
- Frazer, I.H. Prevention of cervical cancer through papillomavirus vaccination. Nat. Rev. Immunol. 2004, 4, 46–55. [Google Scholar] [CrossRef] [PubMed]
- Wheeler, C.M.; Kjaer, S.K.; Sigurdsson, K.; Iversen, O.-E.; Hernandez-Avila, M.; Perez, G.; Brown, D.R.; Koutsky, L.A.; Tay, E.H.; García, P.; et al. The impact of quadrivalent human papillomavirus (HPV. Types 6, 11, 16, and 18) L1 virus-like particle vaccine on infection and disease due to oncogenic nonvaccine HPV types in sexually active women aged 16–26 Years. J. Infect. Dis. 2009, 199, 936–944. [Google Scholar] [CrossRef] [PubMed]
- Pagliusi, S.R.; Aguado, M.T. Efficacy and other milestones for human papillomavirus vaccine introduction. Vaccine 2004, 23, 569–578. [Google Scholar] [CrossRef] [PubMed]
- Arbyn, M.; Xu, L.; Simoens, C.; Martin-Hirsch, P.P. Prophylactic vaccination against human papillomaviruses to prevent cervical cancer and its precursors. Cochrane Database Syst. Rev. 2018, 2020, CD009069. [Google Scholar] [CrossRef]
- FUTURE II Study Group. Quadrivalent vaccine against human papillomavirus to prevent high-grade cervical lesions. N. Engl. J. Med. 2007, 356, 1915–1927. [Google Scholar] [CrossRef] [PubMed]
- Kjaer, S.K.; Nygård, M.; Sundström, K.; Dillner, J.; Tryggvadottir, L.; Munk, C.; Berger, S.; Enerly, E.; Hortlund, M.; Ágústsson, I.; et al. Final analysis of a 14-year long-term follow-up study of the effectiveness and immunogenicity of the quadrivalent human papillomavirus vaccine in women from four Nordic countries. EClinicalMedicine 2020, 23, 100401. [Google Scholar] [CrossRef]
- Yoshikawa, H.; Ebihara, K.; Tanaka, Y.; Noda, K. Efficacy of quadrivalent human papillomavirus (types 6, 11, 16 and 18) vaccine (GARDASIL) in Japanese women aged 18–26 years. Cancer Sci. 2013, 104, 465–472. [Google Scholar] [CrossRef]
- Paavonen, J.; Jenkins, D.; Bosch, F.X.; Naud, P.; Salmerón, J.; Wheeler, C.M.; Chow, S.-N.; Apter, D.L.; Kitchener, H.C.; Castellsague, X.; et al. Efficacy of a prophylactic adjuvanted bivalent L1 virus-like-particle vaccine against infection with human papillomavirus types 16 and 18 in young women: An interim analysis of a phase III double-blind, randomised controlled trial. Lancet 2007, 369, 2161–2170. [Google Scholar] [CrossRef]
- Paavonen, J.; Naud, P.; Salmerón, J.; Wheeler, C.; Chow, S.-N.; Apter, D.; Kitchener, H.; Castellsague, X.; Teixeira, J.; Skinner, S.; et al. Efficacy of human papillomavirus (HPV)-16/18 AS04-adjuvanted vaccine against cervical infection and precancer caused by oncogenic HPV types (PATRICIA): Final analysis of a double-blind, randomised study in young women. Lancet 2009, 374, 301–314. [Google Scholar] [CrossRef]
- Lehtinen, M.; Paavonen, J.; Wheeler, C.M.; Jaisamrarn, U.; Garland, S.M.; Castellsagué, X.; Skinner, S.R.; Apter, D.; Naud, P.; Salmerón, J.; et al. Overall efficacy of HPV-16/18 AS04-adjuvanted vaccine against grade 3 or greater cervical intraepithelial neoplasia: 4-year end-of-study analysis of the randomised, double-blind PATRICIA trial. Lancet Oncol. 2012, 13, 89–99. [Google Scholar] [CrossRef]
- Hildesheim, A.; Wacholder, S.; Catteau, G.; Struyf, F.; Dubin, G.; Herrero, R. Efficacy of the HPV-16/18 Vaccine: Final according to protocol results from the blinded phase of the randomized Costa Rica HPV-16/18 Vaccine Trial. Vaccine 2014, 32, 5087–5097. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Porras, C.; Tsang, S.H.; Herrero, R.; Guillén, D.; Darragh, T.M.; Stoler, M.H.; Hildesheim, A.; Wagner, S.; Boland, J.; Lowy, D.R.; et al. Efficacy of the bivalent HPV vaccine against HPV 16/18-associated precancer: Long-term follow-up results from the Costa Rica vaccine Trial. Lancet Oncol. 2020, 21, 1643–1652. [Google Scholar] [CrossRef] [PubMed]
- Zhu, F.; Hu, S.; Hong, Y.; Hu, Y.; Zhang, X.; Zhang, Y.; Pan, Q.; Zhang, W.; Zhao, F.; Zhang, C.; et al. Efficacy, immunogenicity and safety of the AS04-HPV-16/18 vaccine in Chinese women aged 18–25 years: End-of-study results from a phase II/III, randomised, controlled trial. Cancer Med. 2019, 8, 6195–6211. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huh, W.K.; Joura, E.A.; Giuliano, A.R.; Iversen, O.-E.; De Andrade, R.P.; Ault, K.A.; Bartholomew, D.; Cestero, R.M.; Fedrizzi, E.N.; Hirschberg, A.L.; et al. Final efficacy, immunogenicity, and safety analyses of a nine-valent human papillomavirus vaccine in women aged 16–26 years: A randomised, double-blind trial. Lancet 2017, 390, 2143–2159. [Google Scholar] [CrossRef] [PubMed]
- Guevara, A.; Cabello, R.; Woelber, L.; Moreira, E.D., Jr.; Joura, E.; Reich, O.; Shields, C.; Ellison, M.C.; Joshi, A.; Luxembourg, A. Antibody persistence and evidence of immune memory at 5years following administration of the 9-valent HPV vaccine. Vaccine 2017, 35, 5050–5057. [Google Scholar] [CrossRef]
- Qiao, Y.-L.; Wu, T.; Li, R.-C.; Hu, Y.-M.; Wei, L.-H.; Li, C.-G.; Chen, W.; Huang, S.-J.; Zhao, F.-H.; Li, M.-Q.; et al. Efficacy, safety, and immunogenicity of an Escherichia coli-produced bivalent human papillomavirus vaccine: An interim analysis of a randomized clinical trial. JNCI 2019, 112, 145–153. [Google Scholar] [CrossRef]
- Zhao, F.-H.; Wu, T.; Hu, Y.-M.; Wei, L.-H.; Li, M.-Q.; Huang, W.-J.; Chen, W.; Huang, S.-J.; Pan, Q.-J.; Zhang, X.; et al. Efficacy, safety, and immunogenicity of an Escherichia coli-produced Human Papillomavirus (16 and 18) L1 virus-like-particle vaccine: End-of-study analysis of a phase 3, double-blind, randomised, controlled trial. Lancet Infect. Dis. 2022, 22, 1756–1768. [Google Scholar] [CrossRef]
- Muñoz, N.; Manalastas, R.; Pitisuttithum, P.; Tresukosol, D.; Monsonego, J.; Ault, K.; Clavel, C.; Luna, J.; Myers, E.; Hood, S.; et al. Safety, immunogenicity, and efficacy of quadrivalent human papillomavirus (types 6, 11, 16, 18) recombinant vaccine in women aged 24–45 years: A randomised, double-blind trial. Lancet 2009, 373, 1949–1957. [Google Scholar] [CrossRef]
- Castellsagué, X.; Muñoz, N.; Pitisuttithum, P.; Ferris, D.; Monsonego, J.; Ault, K.; Luna, J.; Myers, E.; Mallary, S.; Bautista, O.M.; et al. End-of-study safety, immunogenicity, and efficacy of quadrivalent HPV (types 6, 11, 16, 18) recombinant vaccine in adult women 24–45 years of age. Br. J. Cancer 2011, 105, 28–37. [Google Scholar] [CrossRef]
- Wei, L.; Xie, X.; Liu, J.; Zhao, Y.; Chen, W.; Zhao, C.; Wang, S.; Liao, X.; Shou, Q.; Qiu, Y.; et al. Efficacy of quadrivalent human papillomavirus vaccine against persistent infection and genital disease in Chinese women: A randomized, placebo-controlled trial with 78-month follow-up. Vaccine 2019, 37, 3617–3624. [Google Scholar] [CrossRef]
- Wheeler, C.M.; Skinner, S.R.; Del Rosario-Raymundo, M.R.; Garland, S.M.; Chatterjee, A.; Lazcano-Ponce, E.; Salmerón, J.; McNeil, S.; Stapleton, J.T.; Bouchard, C.; et al. Efficacy, safety, and immunogenicity of the human papillomavirus 16/18 AS04-adjuvanted vaccine in women older than 25 years: 7-year follow-up of the phase 3, double-blind, randomised controlled VIVIANE study. Lancet Infect. Dis. 2016, 16, 1154–1168. [Google Scholar] [CrossRef] [PubMed]
- GOV.UK. Information on HPV Vaccination. Available online: https://www.gov.uk/government/publications/hpv-vaccine-vaccination-guide-leaflet/information-on-hpv-vaccination (accessed on 19 September 2022).
- Meites, E.; Szilagyi, P.G.; Chesson, H.W.; Unger, E.R.; Romero, J.R.; Markowitz, L.E. human papillomavirus vaccination for adults: Updated recommendations of the advisory committee on immunization practices. Morb. Mortal. Wkly. Rep. 2019, 68, 698–702. [Google Scholar] [CrossRef] [Green Version]
- Eochagain, C.M.; Power, R.; Parker, I.; Brennan, D. HPV vaccination among seropositive, DNA negative cohorts: A systematic review & meta-analysis. J. Gynecol. Oncol. 2022, 33, e24. [Google Scholar] [CrossRef]
- Zizza, A.; Banchelli, F.; Guido, M.; Marotta, C.; Di Gennaro, F.; Mazzucco, W.; Pistotti, V.; D’Amico, R. Efficacy and safety of human papillomavirus vaccination in HIV-infected patients: A systematic review and meta-analysis. Sci. Rep. 2021, 11, 4954. [Google Scholar] [CrossRef] [PubMed]
- Rosalik, K.; Tarney, C.; Han, J. Human papilloma virus vaccination. Viruses 2021, 13, 1091. [Google Scholar] [CrossRef] [PubMed]
- Giuliano, A.R.; Palefsky, J.M.; Goldstone, S.; Moreira, E.D.; Penny, M.E.; Aranda, C.; Vardas, E.; Moi, H.; Jessen, H.; Hillman, R.; et al. Efficacy of quadrivalent HPV vaccine against HPV infection and disease in males. N. Engl. J. Med. 2011, 364, 401–411. [Google Scholar] [CrossRef] [Green Version]
- Goldstone, S.E.; Giuliano, A.R.; Palefsky, J.M.; Lazcano-Ponce, E.; Penny, M.E.; Cabello, R.E.; Moreira, E.D.; Baraldi, E.; Jessen, H.; Ferenczy, A.; et al. Efficacy, immunogenicity, and safety of a quadrivalent HPV vaccine in men: Results of an open-label, long-term extension of a randomised, placebo-controlled, phase 3 trial. Lancet Infect. Dis. 2021, 22, 413–425. [Google Scholar] [CrossRef]
- Goldstone, S.E.; Jessen, H.; Palefsky, J.M.; Giuliano, A.R.; Moreira, E.D.; Vardas, E.; Aranda, C.; Hillman, R.J.; Ferris, D.G.; Coutlee, F.; et al. Quadrivalent HPV vaccine efficacy against disease related to vaccine and non-vaccine HPV types in males. Vaccine 2013, 31, 3849–3855. [Google Scholar] [CrossRef]
- Van Damme, P.; Meijer, C.J.; Kieninger, D.; Schuyleman, A.; Thomas, S.; Luxembourg, A.; Baudin, M. A phase III clinical study to compare the immunogenicity and safety of the 9-valent and quadrivalent HPV vaccines in men. Vaccine 2016, 34, 4205–4212. [Google Scholar] [CrossRef] [Green Version]
- Castellsagué, X.; Giuliano, A.; Goldstone, S.; Guevara, A.; Mogensen, O.; Palefsky, J.; Group, T.; Shields, C.; Liu, K.; Maansson, R.; et al. Immunogenicity and safety of the 9-valent HPV vaccine in men. Vaccine 2015, 33, 6892–6901. [Google Scholar] [CrossRef]
- Giuliano, A.R.; Wilkin, T.; Bautista, O.M.; Cheon, K.; Connor, L.; Dubey, S.; Luxembourg, A.; Rawat, S.; Shaw, A.; Velicer, C.; et al. Design of a phase III efficacy, immunogenicity, and safety study of 9-valent human papillomavirus vaccine in prevention of oral persistent infection in men. Contemp. Clin. Trials 2021, 115, 106592. [Google Scholar] [CrossRef] [PubMed]
- Wheeler, C.M.; Castellsagué, X.; Garland, S.M.; Szarewski, A.; Paavonen, J.; Naud, P.; Salmerón, J.; Chow, S.N.; Apter, D.; Kitchener, H.; et al. Cross-protective efficacy of HPV-16/18 AS04-adjuvanted vaccine against cervical infection and precancer caused by non-vaccine oncogenic HPV types: 4-year end-of-study analysis of the randomised, double-blind PATRICIA trial. Lancet Oncol. 2012, 13, 100–110. [Google Scholar] [CrossRef] [PubMed]
- Herrero, R.; Wacholder, S.; Rodríguez, A.C.; Solomon, D.; González, P.; Kreimer, A.R.; Porras, C.; Schussler, J.; Jiménez, S.; Sherman, M.E.; et al. Prevention of persistent human papillomavirus infection by an HPV16/18 vaccine: A community-based randomized clinical trial in Guanacaste, Costa Rica. Cancer Discov. 2011, 1, 408–419. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsang, S.H.; Sampson, J.N.; Schussler, J.; Porras, C.; Wagner, S.; Boland, J.; Cortes, B.; Lowy, D.R.; Schiller, J.T.; Schiffman, M.; et al. Durability of cross-protection by different schedules of the bivalent HPV vaccine: The CVT trial. JNCI 2020, 112, 1030–1037. [Google Scholar] [CrossRef] [Green Version]
- Schwarz, T.F.; Huang, L.-M.; Valencia, A.; Panzer, F.; Chiu, C.-H.; Decreux, A.; Poncelet, S.; Karkada, N.; Folschweiller, N.; Lin, L.; et al. A ten-year study of immunogenicity and safety of the AS04-HPV-16/18 vaccine in adolescent girls aged 10–14 years. Hum. Vaccines Immunother. 2019, 15, 1970–1979. [Google Scholar] [CrossRef] [Green Version]
- Phillips, A.; Patel, C.; Pillsbury, A.; Brotherton, J.; Macartney, K. Safety of human papillomavirus vaccines: An updated review. Drug Saf. 2018, 41, 329–346. [Google Scholar] [CrossRef]
- Gee, J.; Weinbaum, C.; Sukumaran, L.; Markowitz, L.E. Quadrivalent HPV vaccine safety review and safety monitoring plans for nine-valent HPV vaccine in the United States. Hum. Vaccines Immunother. 2016, 12, 1406–1417. [Google Scholar] [CrossRef] [Green Version]
- Descamps, D.; Hardt, K.; Spiessens, B.; Izurieta, P.; Verstraeten, T.; Breuer, T.; Dubin, G. Safety of human papillomavirus (HPV)-16/18 AS04-adjuvanted vaccine for cervical cancer prevention: A pooled analysis of 11 clinical trials. Hum. Vaccines 2009, 5, 332–340. [Google Scholar] [CrossRef]
- Costa, A.P.F.; Cobucci, R.N.O.; Da Silva, J.M.; Lima, P.H.D.C.; Giraldo, P.C.; Gonçalves, A. Safety of human papillomavirus 9-valent vaccine: A meta-analysis of randomized trials. J. Immunol. Res. 2017, 2017, 3736201. [Google Scholar] [CrossRef] [Green Version]
- Shimabukuro, T.T.; Su, J.R.; Marquez, P.L.; Mba-Jonas, A.; Arana, J.E.; Cano, M.V. Safety of the 9-valent human papillomavirus vaccine. Pediatrics 2019, 144, e20191791. [Google Scholar] [CrossRef]
- Human Papillomavirus Vaccines: WHO Position Paper 2017. Available online: https://apps.who.int/iris/bitstream/handle/10665/255354/WER9219-241-268.pdf?sequence=1&isAllowed=y (accessed on 24 May 2022).
- World Health Organization. Meeting of the Global Advisory Committee on Vaccine Safety 12 July 2019. Available online: https://apps.who.int/iris/bitstream/handle/10665/325849/WER9428-en-fr.pdf (accessed on 29 September 2022).
- Simms, K.T.; Hanley, S.J.B.; Smith, M.A.; Keane, A.; Canfell, K. Impact of HPV vaccine hesitancy on cervical cancer in Japan: A modelling study. Lancet Public Health 2020, 5, e223–e234. [Google Scholar] [CrossRef] [PubMed]
- Sekine, M.; Yamaguchi, M.; Kudo, R.; Hanley, S.J.; Ueda, Y.; Adachi, S.; Kurosawa, M.; Miyagi, E.; Hara, M.; Enomoto, T. Suspension of proactive recommendations for HPV vaccination has led to a significant increase in HPV infection rates in young Japanese women: Real-world data. Lancet Reg. Health West. Pac. 2021, 16, 100300. [Google Scholar] [CrossRef] [PubMed]
- Haruyama, R.; Obara, H.; Fujita, N. Japan resumes active recommendations of HPV vaccine after 8.5 years of suspension. Lancet Oncol. 2022, 23, 197–198. [Google Scholar] [CrossRef] [PubMed]
- Drolet, M.; Bénard, É.; Pérez, N.; Brisson, M. Population-level impact and herd effects following the introduction of human papillomavirus vaccination programmes: Updated systematic review and meta-analysis. Lancet Lond. Engl. 2019, 394, 497–509. [Google Scholar] [CrossRef] [PubMed] [Green Version]
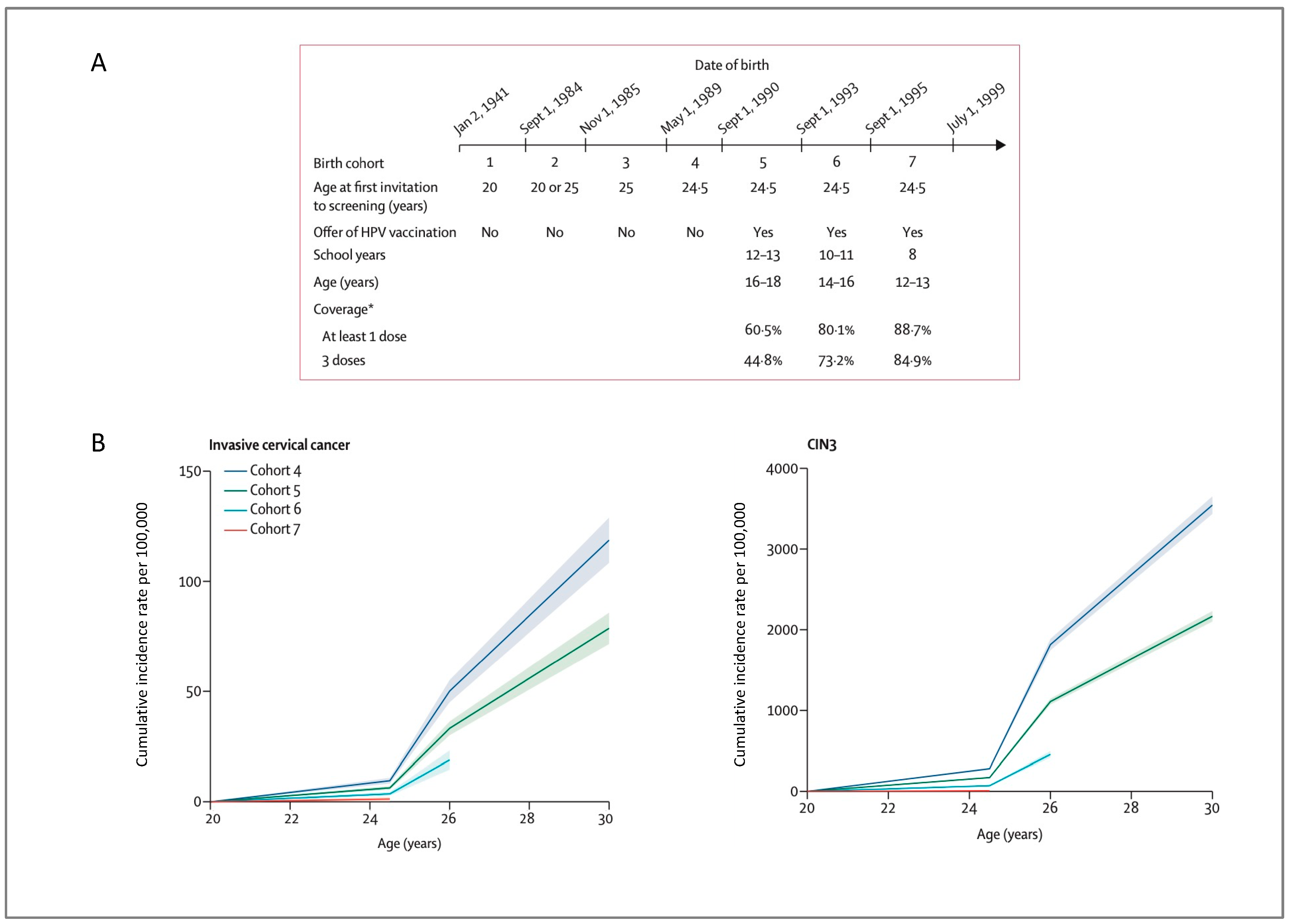
- Lei, J.; Ploner, A.; Elfström, K.M.; Wang, J.; Roth, A.; Fang, F.; Sundström, K.; Dillner, J.; Sparén, P. HPV vaccination and the risk of invasive cervical cancer. N. Engl. J. Med. 2020, 383, 1340–1348. [Google Scholar] [CrossRef]
- Falcaro, M.; Castañon, A.; Ndlela, B.; Checchi, M.; Soldan, K.; Lopez-Bernal, J.; Elliss-Brookes, L.; Sasieni, P. The effects of the national HPV vaccination programme in England, UK, on cervical cancer and grade 3 cervical intraepithelial neoplasia incidence: A register-based observational study. Lancet 2021, 398, 2084–2092. [Google Scholar] [CrossRef]
- Kjaer, S.K.; Dehlendorff, C.; Belmonte, F.; Baandrup, L. Real-world effectiveness of human papillomavirus vaccination against cervical cancer. J. Natl. Cancer Inst. 2021, 113, 1329–1335. [Google Scholar] [CrossRef]
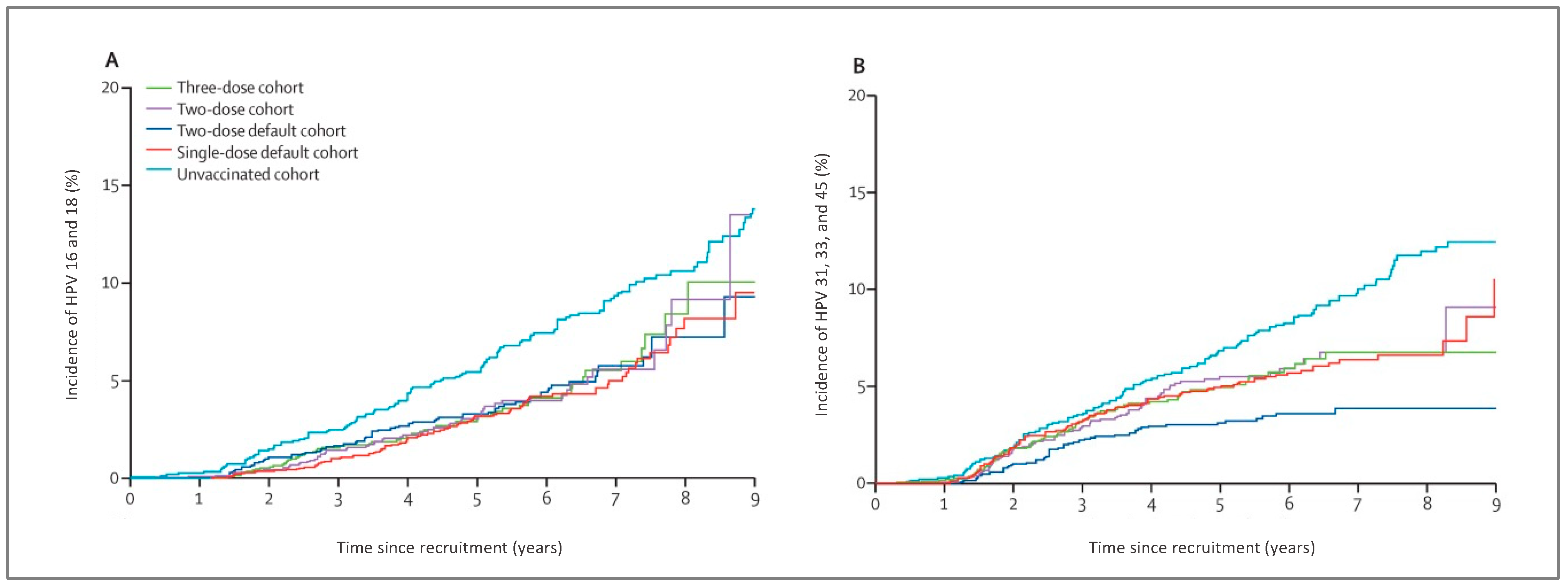
- Kreimer, A.R.; Struyf, F.; Del Rosario-Raymundo, M.R.; Hildesheim, A.; Skinner, R.; Wacholder, S.; Garland, S.M.; Herrero, R.; David, M.-P.; Wheeler, C.M. Efficacy of fewer than three doses of an HPV-16/18 AS04-adjuvanted vaccine: Combined analysis of data from the Costa Rica Vaccine and PATRICIA trials. Lancet Oncol. 2015, 16, 775–786. [Google Scholar] [CrossRef] [Green Version]
- Kreimer, A.R.; Rodriguez, A.C.; Hildesheim, A.; Herrero, R.; Porras, C.; Schiffman, M.; González, P.; Solomon, D.; Jiménez, S.; Schiller, J.T.; et al. Proof-of-principle evaluation of the efficacy of fewer than three doses of a bivalent HPV16/18 vaccine. J. Natl. Cancer Inst. 2011, 103, 1444–1451. [Google Scholar] [CrossRef]
- Kreimer, A.R.; Sampson, J.N.; Porras, C.; Schiller, J.T.; Kemp, T.; Herrero, R.; Wagner, S.; Boland, J.; Schussler, J.; Lowy, D.R.; et al. Evaluation of durability of a single dose of the bivalent HPV vaccine: The CVT trial. J. Natl. Cancer Inst. 2020, 112, 1038–1046. [Google Scholar] [CrossRef]
- Safaeian, M.; Sampson, J.N.; Pan, Y.; Porras, C.; Kemp, T.J.; Herrero, R.; Quint, W.; van Doorn, L.J.; Schussler, J.; Lowy, D.R.; et al. Durability of protection afforded by fewer doses of the HPV16/18 vaccine: The CVT trial. JNCI 2018, 110, 205–212. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prem, K.; Choi, Y.H.; Bénard, É.; Burger, E.A.; Mmath, L.H.; Laprise, J.F.; Regan, C.; Drolet, M.; Sy, S.; Abbas, K.; et al. Global impact and cost-effectiveness of one-dose versus two-dose human papillomavirus vaccination schedules: A comparative modelling analysis. medRxiv 2021. [Google Scholar] [CrossRef]
- Sankaranarayanan, R.; Prabhu, P.R.; Pawlita, M.; Gheit, T.; Bhatla, N.; Muwonge, R.; Nene, B.M.; Esmy, P.O.; Joshi, S.; Poli, U.R.R.; et al. Immunogenicity and HPV infection after one, two, and three doses of quadrivalent HPV vaccine in girls in India: A multicentre prospective cohort study. Lancet Oncol. 2016, 17, 67–77. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sankaranarayanan, R.; Joshi, S.; Muwonge, R.; Esmy, P.O.; Basu, P.; Prabhu, P.; Bhatla, N.; Nene, B.M.; Shaw, J.; Poli, U.R.R.; et al. Can a single dose of human papillomavirus (HPV) vaccine prevent cervical cancer? Early findings from an Indian study. Vaccine 2018, 36, 4783–4791. [Google Scholar] [CrossRef]
- Basu, P.; Malvi, S.G.; Joshi, S.; Bhatla, N.; Muwonge, R.; Lucas, E.; Verma, Y.; Esmy, P.O.; Poli, U.R.R.; Shah, A.; et al. Vaccine efficacy against persistent human papillomavirus (HPV) 16/18 infection at 10 years after one, two, and three doses of quadrivalent HPV vaccine in girls in India: A multicentre, prospective, cohort study. Lancet Oncol. 2021, 22, 1518–1529. [Google Scholar] [CrossRef]
- Porras, C.; Sampson, J.N.; Herrero, R.; Gail, M.H.; Cortés, B.; Hildesheim, A.; Cyr, J.; Romero, B.; Schiller, J.T.; Montero, C.; et al. Rationale and design of a double-blind randomized non-inferiority clinical trial to evaluate one or two doses of vaccine against human papillomavirus including an epidemiologic survey to estimate vaccine efficacy: The Costa Rica ESCUDDO trial. Vaccine 2022, 40, 76–88. [Google Scholar] [CrossRef]
- Barnabas, R.V.; Brown, E.R.; Onono, M.; Bukusi, E.A.; Njoroge, B.; Winer, R.L.; Donnell, D.; Galloway, D.; Cherne, S.; Heller, K.; et al. Single-dose HPV vaccination efficacy among adolescent girls and young women in Kenya (the KEN SHE Study): Study protocol for a randomized controlled trial. Trials 2021, 22, 661. [Google Scholar] [CrossRef]
- Barnabas, R.V.; Brown, E.R.; Onono, M.A.; Bukusi, E.A.; Njoroge, B.; Winer, R.L.; Galloway, D.A.; Pinder, L.F.; Donnell, D.; Wakhungu, I.; et al. Efficacy of single-dose HPV vaccination among young African women. NEJM Evid. 2022, 1, EVIDoa2100056. [Google Scholar] [CrossRef]
- E Markowitz, L.; Naleway, A.L.; Klein, N.P.; Lewis, R.M.; Crane, B.; Querec, T.D.; Hsiao, A.; Aukes, L.; Timbol, J.; Weinmann, S.; et al. Human papillomavirus vaccine effectiveness against HPV infection: Evaluation of one, two, and three doses. J. Infect. Dis. 2020, 221, 910–918. [Google Scholar] [CrossRef]
- Verdoodt, F.; Dehlendorff, C.; Kjaer, S.K. Dose-related effectiveness of quadrivalent human papillomavirus vaccine against cervical intraepithelial neoplasia: A Danish nationwide cohort study. Clin. Infect. Dis. 2020, 70, 608–614. [Google Scholar] [CrossRef]
- Rodriguez, A.M.; Zeybek, B.; Vaughn, M.; Westra, J.; Kaul, S.; Montealegre, J.R.; Lin, Y.; Kuo, Y. Comparison of the long-term impact and clinical outcomes of fewer doses and standard doses of human papillomavirus vaccine in the United States: A database study. Cancer 2020, 126, 1656–1667. [Google Scholar] [CrossRef] [PubMed]
- GOV.UK. JCVI Statement on a One-Dose Schedule for the Routine HPV Immunisation Programme. Available online: https://www.gov.uk/government/publications/single-dose-of-hpv-vaccine-jcvi-concluding-advice/jcvi-statement-on-a-one-dose-schedule-for-the-routine-hpv-immunisation-programme (accessed on 20 September 2022).
- One-Dose Human Papillomavirus (HPV) Vaccine Offers Solid Protection against Cervical Cancer. Available online: https://www.who.int/news/item/11-04-2022-one-dose-human-papillomavirus-(hpv)-vaccine-offers-solid-protection-against-cervical-cancer (accessed on 24 April 2022).
- Kalliala, I.; Athanasiou, A.; Veroniki, A.; Salanti, G.; Efthimiou, O.; Raftis, N.; Bowden, S.; Paraskevaidi, M.; Aro, K.; Arbyn, M.; et al. Incidence and mortality from cervical cancer and other malignancies after treatment of cervical intraepithelial neoplasia: A systematic review and meta-analysis of the literature. Ann. Oncol. 2020, 31, 213–227. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Soutter, W.P.; Sasieni, P.; Panoskaltsis, T. Long-term risk of invasive cervical cancer after treatment of squamous cervical intraepithelial neoplasia. Int. J. Cancer 2006, 118, 2048–2055. [Google Scholar] [CrossRef] [PubMed]
- Ghelardi, A.; Parazzini, F.; Martella, F.; Pieralli, A.; Bay, P.; Tonetti, A.; Svelato, A.; Bertacca, G.; Lombardi, S.; Joura, E.A. SPERANZA project: HPV vaccination after treatment for CIN2+. Gynecol. Oncol. 2018, 151, 229–234. [Google Scholar] [CrossRef] [PubMed]
- Casajuana-Pérez, A.; Ramírez-Mena, M.; Ruipérez-Pacheco, E.; Gil-Prados, I.; García-Santos, J.; Amo, M.B.-D.; Hernández-Aguado, J.J.; de la Fuente-Valero, J.; Zapardiel, I.; Coronado-Martín, P.J. Effectiveness of prophylactic human papillomavirus vaccine in the prevention of recurrence in women conized for HSIL/CIN 2-3: The VENUS study. Vaccines 2022, 10, 288. [Google Scholar] [CrossRef]
- Karimi-Zarchi, M.; Allahqoli, L.; Nehmati, A.; Kashi, A.M.; Taghipour-Zahir, S.; Alkatout, I. Can the prophylactic quadrivalent HPV vaccine be used as a therapeutic agent in women with CIN? A randomized trial. BMC Public Health 2020, 20, 274. [Google Scholar] [CrossRef]
- Pieralli, A.; Bianchi, C.; Auzzi, N.; Fallani, M.G.; Bussani, C.; Fambrini, M.; Cariti, G.; Scarselli, G.; Petraglia, F.; Ghelardi, A. Indication of prophylactic vaccines as a tool for secondary prevention in HPV-linked disease. Arch. Gynecol. Obstet. 2018, 298, 1205–1210. [Google Scholar] [CrossRef]
- Kechagias, K.S.; Kalliala, I.; Bowden, S.J.; Athanasiou, A.; Paraskevaidi, M.; Paraskevaidis, E.; Dillner, J.; Nieminen, P.; Strander, B.; Sasieni, P.; et al. Role of human papillomavirus (HPV) vaccination on HPV infection and recurrence of HPV related disease after local surgical treatment: Systematic review and meta-analysis. BMJ 2022, 378, e070135. [Google Scholar] [CrossRef]
- Human Papillomaviruses Vaccines: WHO Position Paper 2009. Available online: https://apps.who.int/iris/bitstream/handle/10665/241310/WER8415_118-131.PDF?sequence=1&isAllowed=y (accessed on 24 May 2022).
- Human Papillomavirus Vaccines: WHO Position Paper 2014. Available online: https://apps.who.int/iris/bitstream/handle/10665/242277/WER8943_465-491.PDF?sequence=1&isAllowed=y (accessed on 24 May 2022).
- Human Papillomavirus (HPV) Vaccination Coverage. Available online: https://immunizationdata.who.int/pages/coverage/hpv.html?CODE=Global&ANTIGEN=PRHPVC_F&YEAR= (accessed on 24 May 2022).
- Patel, C.; Brotherton, J.M.; Pillsbury, A.; Jayasinghe, S.; Donovan, B.; Macartney, K.; Marshall, H. The impact of 10 years of human papillomavirus (HPV) vaccination in Australia: What additional disease burden will a nonavalent vaccine prevent? Eurosurveillance 2018, 23, 1700737. [Google Scholar] [CrossRef] [Green Version]
- Hall, M.T.; Simms, K.T.; Lew, J.-B.; A Smith, M.; Brotherton, J.M.; Saville, M.; Frazer, I.H.; Canfell, K. The projected timeframe until cervical cancer elimination in Australia: A modelling study. Lancet Public Health 2019, 4, e19–e27. [Google Scholar] [CrossRef]
- UK Health Security Agency. Human papillomavirus (HPV) vaccination coverage in adolescent females and males in England: 2020 to 2021. Health Prot. Rep. 2021, 15, 1–16. [Google Scholar]
- Meites, E. Use of a 2-dose schedule for human papillomavirus vaccination—Updated recommendations of the advisory committee on immunization practices. MMWR 2016, 65, 1405–1408. [Google Scholar] [CrossRef] [PubMed]
- Pingali, C.; Yankey, D.; Elam-Evans, L.D.; Markowitz, L.E.; Williams, C.L.; Fredua, B.; McNamara, L.A.; Stokley, S.; Singleton, J.A. National, regional, state, and selected local area vaccination coverage among adolescents aged 13–17 years—United States, 2020. MMWR 2021, 70, 1183–1190. [Google Scholar] [CrossRef] [PubMed]
- Lewis, R.M.; Markowitz, L.E. Disparities in human papillomavirus vaccination coverage in the United States, National Health and Nutrition Examination Survey, January 2017–March 2020. Vaccine 2022, 40, 2828–2832. [Google Scholar] [CrossRef]
- Global HPV Vaccine Introduction Overview. Available online: https://path.azureedge.net/media/documents/Global_Vaccine_Intro_Overview_Slides_Final_PATHwebsite_MAR_2022_qT92Wwh.pdf (accessed on 24 May 2022).
- Bruni, L.; Saura-Lázaro, A.; Montoliu, A.; Brotons, M.; Alemany, L.; Diallo, M.S.; Afsar, O.Z.; LaMontagne, D.S.; Mosina, L.; Contreras, M.; et al. HPV vaccination introduction worldwide and WHO and UNICEF estimates of national HPV immunization coverage 2010–2019. Prev. Med. 2021, 144, 106399. [Google Scholar] [CrossRef]
- Torres-Rueda, S.; Rulisa, S.; Burchett, H.E.; Mivumbi, N.V.; Mounier-Jack, S. HPV vaccine introduction in Rwanda: Impacts on the broader health system. Sex. Reprod. Healthc. 2016, 7, 46–51. [Google Scholar] [CrossRef] [Green Version]
- Kramer, J. Eradicating cervical cancer: Lessons learned from Rwanda and Australia. Int. J. Gynecol. Obstet. 2021, 154, 270–276. [Google Scholar] [CrossRef]
- Colzani, E.; Johansen, K.; Johnson, H.; Celentano, L.P. Human papillomavirus vaccination in the European Union/European Economic Area and globally: A moral dilemma. Eurosurveillance 2021, 26, 2001659. [Google Scholar] [CrossRef]
- World Health Organization. Meeting of the strategic advisory group of experts on immunization, October 2009–Conclusions and recommendations. Biologicals 2010, 38, 170–177. [Google Scholar] [CrossRef]
- Gallagher, K.E.; LaMontagne, D.S.; Watson-Jones, D. Status of HPV vaccine introduction and barriers to country uptake. Vaccine 2018, 36, 4761–4767. [Google Scholar] [CrossRef]
- LaMontagne, D.S.; Gallagher, K.E.; Watson-Jones, D. Why has global HPV vaccine uptake lagged? A contextual reframing of vaccine introduction. HPV World 2017, 1, 10–12. [Google Scholar]
- HPV-Supply-And-Procurement--Roadmappdf.pdf. Available online: https://www.gavi.org/sites/default/files/document/hpv-supply-and-procurement--roadmappdf.pdf (accessed on 25 September 2022).
- Hassett, K.J.; Meinerz, N.M.; Semmelmann, F.; Cousins, M.C.; Garcea, R.L.; Randolph, T.W. Development of a highly thermostable, adjuvanted human papillomavirus vaccine. Eur. J. Pharm. Biopharm. 2015, 94, 220–228. [Google Scholar] [CrossRef]
- Toh, Z.Q.; Russell, F.M.; Garland, S.M.; Mulholland, E.K.; Patton, G.; Licciardi, P.V. Human papillomavirus vaccination after COVID-19. JNCI Cancer Spectr. 2021, 5, pkab011. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhu, P.; Wu, M.; Zhang, Y.; Li, L. Immunogenicity and safety of human papillomavirus vaccine coadministered with other vaccines in individuals aged 9–25 years: A systematic review and meta-analysis. Vaccine 2020, 38, 119–134. [Google Scholar] [CrossRef] [PubMed]
- Hancock, G.; Hellner, K.; Dorrell, L. Therapeutic HPV vaccines. Best Pract. Res. Clin. Obstet. Gynaecol. 2018, 47, 59–72. [Google Scholar] [CrossRef]
- Garbuglia, A.R.; Lapa, D.; Sias, C.; Capobianchi, M.R.; Del Porto, P. The use of both therapeutic and prophylactic vaccines in the therapy of papillomavirus disease. Front. Immunol. 2020, 11, 188. [Google Scholar] [CrossRef] [Green Version]
- Trimble, C.L.; Morrow, M.P.; Kraynyak, K.A.; Shen, X.; Dallas, M.; Yan, J.; Edwards, L.; Parker, R.L.; Denny, L.; Giffear, M.; et al. Safety, efficacy, and immunogenicity of VGX-3100, a therapeutic synthetic DNA vaccine targeting human papillomavirus 16 and 18 E6 and E7 proteins for cervical intraepithelial neoplasia 2/3: A randomised, double-blind, placebo-controlled phase 2b trial. Lancet 2015, 386, 2078–2088. [Google Scholar] [CrossRef] [Green Version]
- Bhuyan, P.K.; Dallas, M.; Kraynyak, K.; Herring, T.; Morrow, M.; Boyer, J.; Duff, S.; Kim, J.; Weiner, D.B. Durability of response to VGX-3100 treatment of HPV16/18 positive cervical HSIL. Hum. Vaccines Immunother. 2021, 17, 1288–1293. [Google Scholar] [CrossRef]
- Ikeda, Y.; Uemura, Y.; Asai-Sato, M.; Nakao, T.; Nakajima, T.; Iwata, T.; Akiyama, A.; Satoh, T.; Yahata, H.; Kato, K.; et al. Safety and efficacy of mucosal immunotherapy using human papillomavirus (HPV) type 16 E7-expressing Lactobacillus-based vaccine for the treatment of high-grade squamous intraepithelial lesion (HSIL): The study protocol of a randomized placebo-controlled clinical trial (MILACLE study). Jpn. J. Clin. Oncol. 2019, 49, 877–880. [Google Scholar] [CrossRef]
- Basu, P.; Mehta, A.; Jain, M.; Gupta, S.; Nagarkar, R.V.; John, S.; Petit, R. A randomized phase 2 study of ADXS11-001 Listeria monocytogenes–Listeriolysin O Immunotherapy with or without cisplatin in treatment of advanced cervical cancer. Int. J. Gynecol. Cancer 2018, 28, 764–772. [Google Scholar] [CrossRef] [Green Version]
- Hudson, K.; Cross, N.; Jordan-Mahy, N.; Leyland, R. The extrinsic and intrinsic roles of PD-L1 and its receptor PD-1: Implications for immunotherapy treatment. Front. Immunol. 2020, 11, 568931. [Google Scholar] [CrossRef] [PubMed]
- de Sousa, L.G.; Rajapakshe, K.; Canales, J.R.; Chin, R.L.; Feng, L.; Wang, Q.; Barrese, T.Z.; Massarelli, E.; William, W.; Johnson, F.M.; et al. ISA101 and nivolumab for HPV-16+ cancer: Updated clinical efficacy and immune correlates of response. J. Immunother. Cancer 2022, 10, e004232. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, C.; Saba, N.; Algazi, A.; Sukari, A.; Seiwert, T.; Haigentz, M.; Porosnicu, M.; Bonomi, M.; Boyer, J.; Durham, N.; et al. 916MO Safety and efficacy of MEDI0457 plus durvalumab in patients (pts) with human papillomavirus-associated recurrent/metastatic head and neck squamous cell carcinoma (HPV+ R/M HNSCC). Ann. Oncol. 2020, 31, S661–S662. [Google Scholar] [CrossRef]
- Rumfield, C.S.; Pellom, S.T.; Ii, Y.M.M.; Schlom, J.; Jochems, C. Immunomodulation to enhance the efficacy of an HPV therapeutic vaccine. J. Immunother. Cancer 2020, 8, e000612. [Google Scholar] [CrossRef]
- Boilesen, D.R.; Nielsen, K.N.; Holst, P.J. Novel Antigenic Targets of HPV Therapeutic Vaccines. Vaccines 2021, 9, 1262. [Google Scholar] [CrossRef]
- Hancock, G.; Blight, J.; Lopez-Camacho, C.; Kopycinski, J.; Pocock, M.; Byrne, W.; Price, M.J.; Kemlo, P.; Evans, R.I.; Bloss, A.; et al. A multi-genotype therapeutic human papillomavirus vaccine elicits potent T cell responses to conserved regions of early proteins. Sci. Rep. 2019, 9, 18713. [Google Scholar] [CrossRef] [Green Version]
- Roden, R.B.S.; Stern, P.L. Opportunities and challenges for human papillomavirus vaccination in cancer. Nat. Rev. Cancer 2018, 18, 240–254. [Google Scholar] [CrossRef]
- Stern, P.L.; Roden, R.B. Opportunities to improve immune-based prevention of HPV-associated cancers. Papillomavirus Res. 2019, 7, 150–153. [Google Scholar] [CrossRef]
- Bosch, F.X.; Robles, C.; Díaz, M.; Arbyn, M.; Baussano, I.; Clavel, C.; Ronco, G.; Dillner, J.; Lehtinen, M.; Petry, K.-U.; et al. HPV-FASTER: Broadening the scope for prevention of HPV-related cancer. Nat. Rev. Clin. Oncol. 2016, 13, 119–132. [Google Scholar] [CrossRef]
- León-Maldonado, L.; Cabral, A.; Brown, B.; Ryan, G.W.; Maldonado, A.; Salmerón, J.; Allen-Leigh, B.; Lazcano-Ponce, E. Feasibility of a combined strategy of HPV vaccination and screening in Mexico: The FASTER-Tlalpan study experience. Hum. Vaccines Immunother. 2019, 15, 1986–1994. [Google Scholar] [CrossRef]
- Huepenbecker, S.P.; A Meyer, L. How can we pursue equity in cervical cancer prevention with existing HPV genotype differences? JNCI 2022, 114, 787–789. [Google Scholar] [CrossRef] [PubMed]
- Lewis, R.M.; Naleway, A.L.; Klein, N.P.; Crane, B.; Hsiao, A.; Aukes, L.; Timbol, J.; Querec, T.D.; Steinau, M.; Weinmann, S.; et al. Changes in cervical cytology results and human papillomavirus types among persons screened for cervical cancer, 2007 and 2015–2017. J. Low. Genit. Tract Dis. 2022, 26, 135–139. [Google Scholar] [CrossRef] [PubMed]
| Vaccine Brand Name | Valency and VLP Types | Manufacturer and Licensure Date | Adjuvant | Expression System |
|---|---|---|---|---|
| Gardasil® | Quadrivalent HPV-6, HPV-11, HPV-16, HPV-18 | Merck & Co. 2006 | Amorphous aluminium hydroxyphosphate sulphate 225 µg | Yeast Saccharomyces cerevisiae expressing L1 |
| Cervarix® | Bivalent HPV-16, HPV-18 | GlaxoSmithKline 2007 | AS04 0.5 mg aluminium hydroxide and 50 µg 3-0-desacyl-4’ monophosphoryl lipid A | Insect cell line infected with recombinant baculovirus encoding L1 |
| Gardasil 9® | Nonavalent HPV-6, HPV-11, HPV-16, HPV-18, HPV-31, HPV-33, HPV-45, HPV-52, HPV-58 | Merck & Co. 2014 | Amorphous aluminium hydroxyphosphate sulphate 500 µg | Yeast Saccharomyces cerevisiae expressing L1 |
| Cecolin® | Bivalent HPV-16, HPV-18 | Xiamen Innovax Biotechnology 2020 | Aluminium hydroxide 208 µg | Escherichia coli expressing L1 |
| Walvax recombinant HPV vaccine | Bivalent HPV-16, HPV-18 | Shanghai Zerun Biotechnology (Subsidiary of Walvax Biotechnology) 2022 | Aluminium phosphate | Yeast Pichia Pastoris expressing L1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Illah, O.; Olaitan, A. Updates on HPV Vaccination. Diagnostics 2023, 13, 243. https://doi.org/10.3390/diagnostics13020243
Illah O, Olaitan A. Updates on HPV Vaccination. Diagnostics. 2023; 13(2):243. https://doi.org/10.3390/diagnostics13020243
Chicago/Turabian StyleIllah, Ojone, and Adeola Olaitan. 2023. "Updates on HPV Vaccination" Diagnostics 13, no. 2: 243. https://doi.org/10.3390/diagnostics13020243




