Protein Glycosylation as Biomarkers in Gynecologic Cancers
Abstract
:1. Introduction
2. Method
3. Definition of Glycosylation
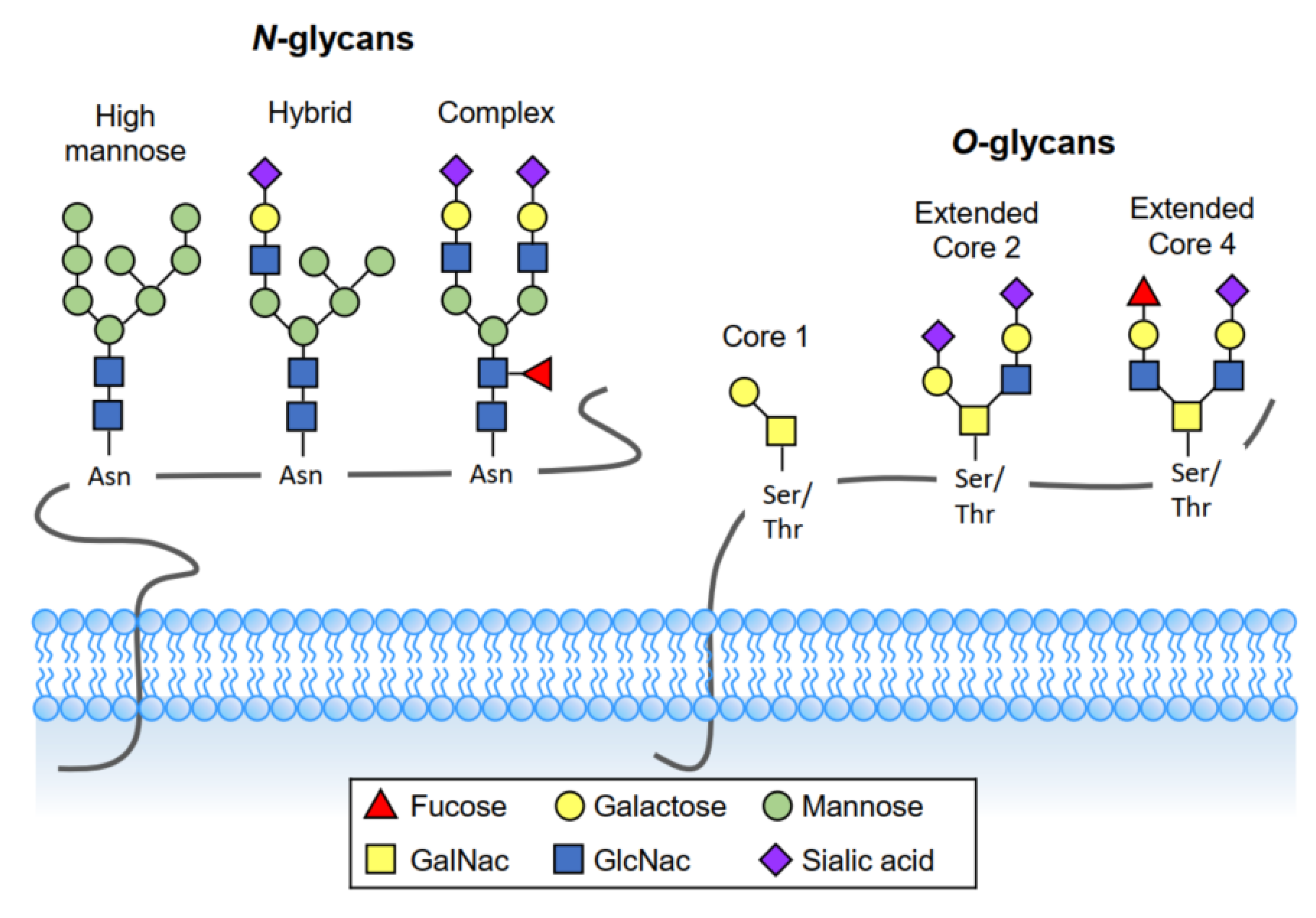
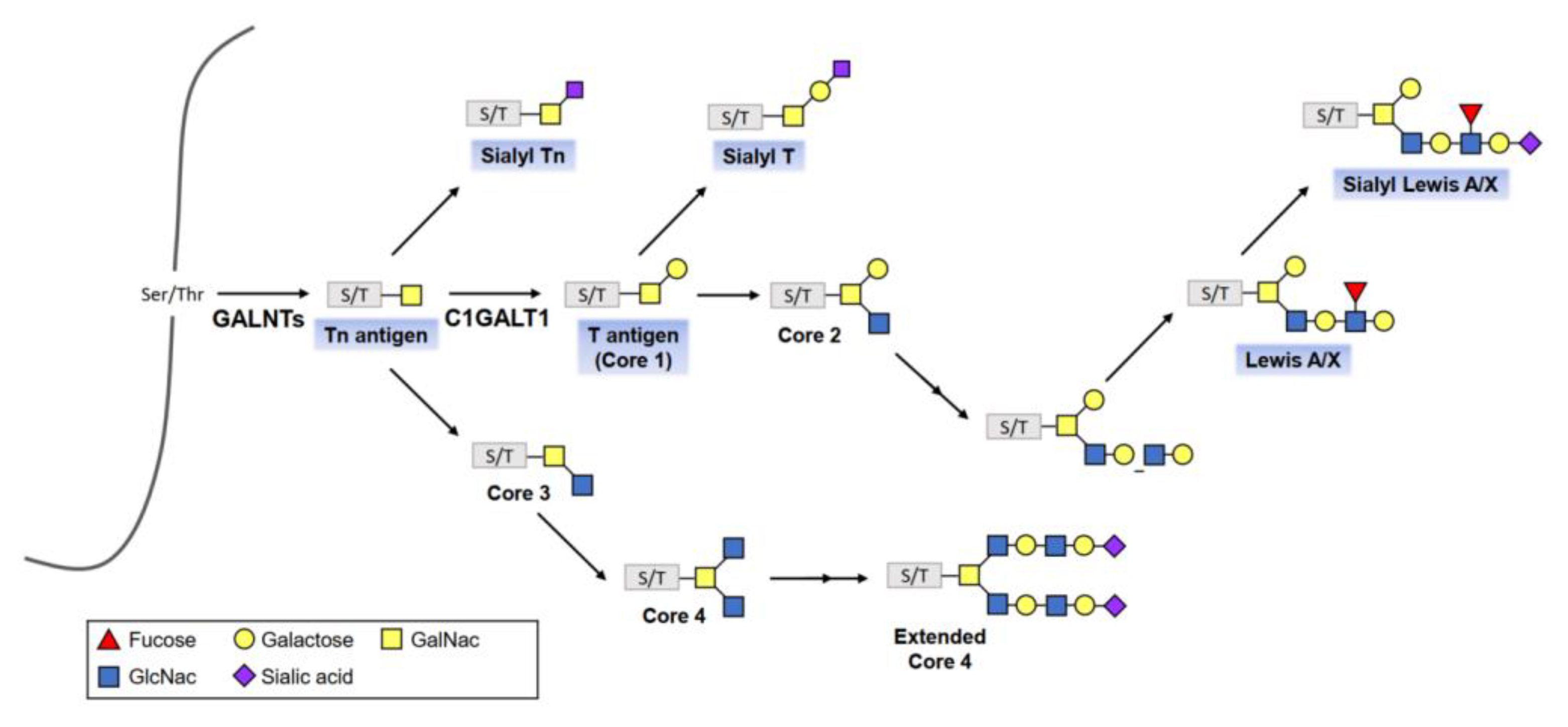
3.1. O-Linked Glycosylation
3.2. N-Linked Glycosylation
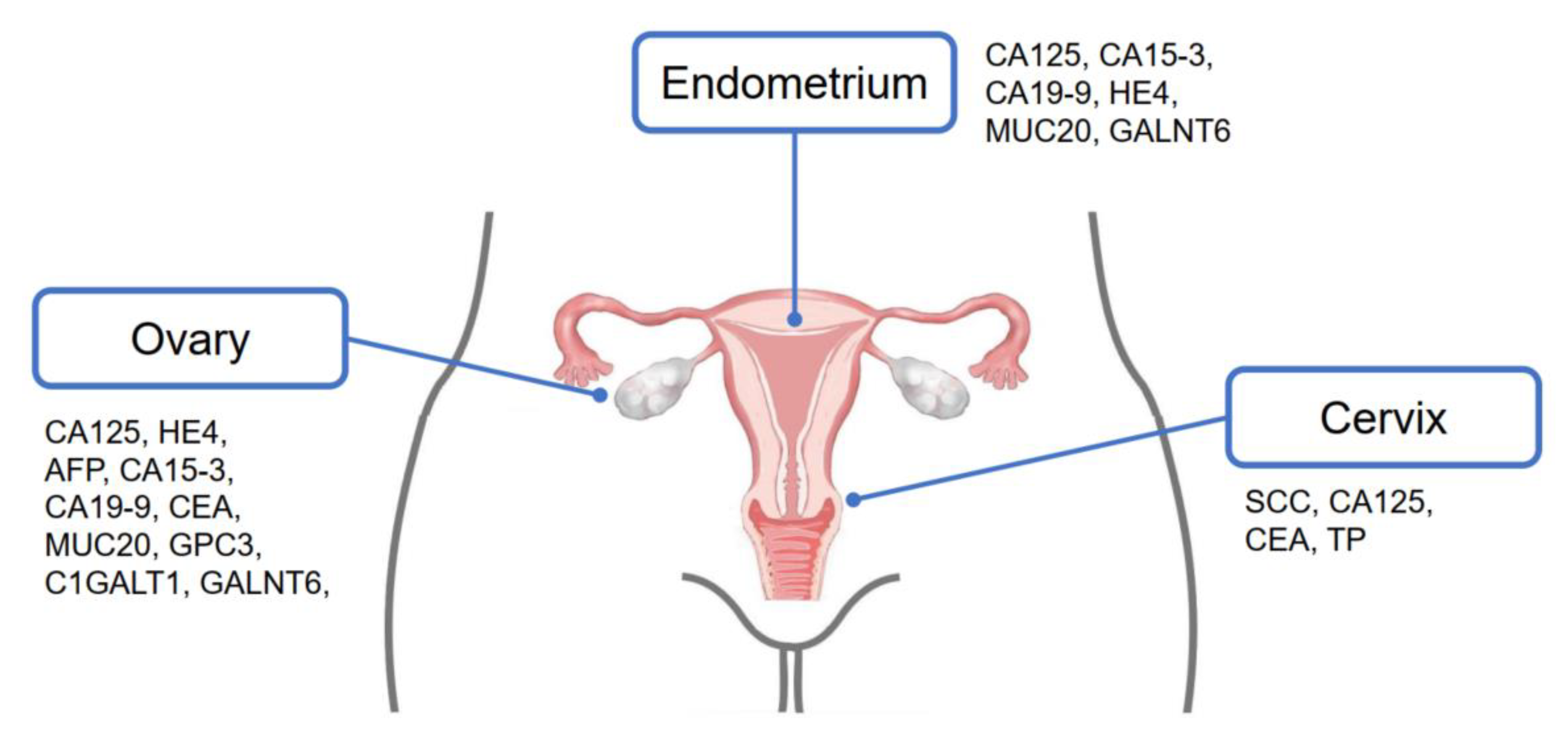
4. Protein Glycosylation Markers for Gynecologic Cancers
4.1. Glycoprotein Markers
4.1.1. Cancer Antigen 125 (CA125)
4.1.2. Cancer Antigen 15-3 (CA15-3)
4.1.3. Mucin 20 (MUC20)
4.1.4. Alpha-Fetoprotein (AFP)
4.1.5. Carcinoembryonic Antigen (CEA)
4.1.6. Human Epididymis Protein 4 (HE4)
4.1.7. Squamous Cell Carcinoma Antigen (SCC-Ag)
4.1.8. Glypican-3 (GPC3)
4.1.9. Tissue Factor (TF)
4.2. Glycan Markers
Cancer Antigen 19-9 (CA19-9)
4.3. Enzyme Markers
4.3.1. N-acetylgalactosaminyltransferase 6 (GALNT6)
4.3.2. Core 1 β1, 3 Galactosyltransferase 1 (C1GALT1)
5. Summary
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Morice, P.; Leary, A.; Creutzberg, C.; Abu-Rustum, N.; Darai, E. Endometrial cancer. Lancet 2016, 387, 1094–1108. [Google Scholar] [CrossRef] [PubMed]
- Hutt, S.; Tailor, A.; Ellis, P.; Michael, A.; Butler-Manuel, S.; Chatterjee, J. The role of biomarkers in endometrial cancer and hyperplasia: A literature review. Acta. Oncol. 2019, 58, 342–352. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Menon, U.; Gentry-Maharaj, A.; Burnell, M.; Singh, N.; Ryan, A.; Karpinskyj, C.; Carlino, G.; Taylor, J.; Massingham, S.K.; Raikou, M.; et al. Ovarian cancer population screening and mortality after long-term follow-up in the UK Collaborative Trial of Ovarian Cancer Screening (UKCTOCS): A randomised controlled trial. Lancet 2021, 397, 2182–2193. [Google Scholar] [CrossRef]
- Henderson, J.T.; Webber, E.M.; Sawaya, G.F. Screening for Ovarian Cancer: Updated Evidence Report and Systematic Review for the US Preventive Services Task Force. Jama 2018, 319, 595–606. [Google Scholar] [CrossRef] [Green Version]
- Ludwig, J.A.; Weinstein, J.N. Biomarkers in Cancer Staging, Prognosis and Treatment Selection. Nat. Rev. Cancer 2005, 5, 845–856. [Google Scholar] [CrossRef]
- Pinho, S.S.; Reis, C.A. Glycosylation in cancer: Mechanisms and clinical implications. Nat. Rev. Cancer 2015, 15, 540–555. [Google Scholar] [CrossRef]
- Gupta, R.; Leon, F.; Rauth, S.; Batra, S.K.; Ponnusamy, M.P. A Systematic Review on the Implications of O-linked Glycan Branching and Truncating Enzymes on Cancer Progression and Metastasis. Cells 2020, 9, 446. [Google Scholar] [CrossRef] [Green Version]
- Gadducci, A.; Tana, R.; Cosio, S.; Genazzani, A.R. The serum assay of tumour markers in the prognostic evaluation, treatment monitoring and follow-up of patients with cervical cancer: A review of the literature. Crit. Rev. Oncol. 2008, 66, 10–20. [Google Scholar] [CrossRef]
- Hanash, S.M.; Pitteri, S.J.; Faca, V.M. Mining the plasma proteome for cancer biomarkers. Nature 2008, 452, 571–579. [Google Scholar] [CrossRef]
- Reily, C.; Stewart, T.J.; Renfrow, M.B.; Novak, J. Glycosylation in health and disease. Nat. Rev. Nephrol. 2019, 15, 346–366. [Google Scholar] [CrossRef] [PubMed]
- Marin, F.; Luquet, G.; Marie, B.; Medakovic, D. Molluscan Shell Proteins: Primary Structure, Origin, and Evolution. Curr. Top. Dev. Biol. 2007, 80, 209–227. [Google Scholar]
- Kufe, D.W. Mucins in cancer: Function, prognosis and therapy. Nat. Rev. Cancer 2009, 9, 874–885. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hijikata, M.; Matsushita, I.; Tanaka, G.; Tsuchiya, T.; Ito, H.; Tokunaga, K.; Ohashi, J.; Homma, S.; Kobashi, Y.; Taguchi, Y.; et al. Molecular cloning of two novel mucin-like genes in the disease-susceptibility locus for diffuse panbronchiolitis. Qual. Life Res. 2010, 129, 117–128. [Google Scholar] [CrossRef]
- Bansil, R.; Turner, B.S. The biology of mucus: Composition, synthesis and organization. Adv. Drug Deliv. Rev. 2018, 124, 3–15. [Google Scholar] [CrossRef]
- Shyu, M.K.; Chen, C.W.; Lin, N.Y.; Liao, W.C.; Chen, C.H.; Lin, C.J.; Huang, H.C.; Lee, J.J.; Huang, M.J.; Tseng, G.F.; et al. MUC1 expression is elevated in severe preeclamptic placentas and suppresses trophoblast cell invasion via beta1-integrin signaling. J. Clin. Endocrinol. Metab. 2011, 96, 3759–3767. [Google Scholar] [CrossRef] [Green Version]
- Singh, A.P.; Senapati, S.; Ponnusamy, M.P.; Jain, M.; Lele, S.M.; Davis, J.S.; Remmenga, S.; Batra, S.K. Clinical potential of mucins in diagnosis, prognosis, and therapy of ovarian cancer. Lancet Oncol. 2008, 9, 1076–1085. [Google Scholar] [CrossRef] [Green Version]
- He, Y.F.; Zhang, M.Y.; Wu, X.; Sun, X.J.; Xu, T.; He, Q.Z.; Di, W. High MUC2 expression in ovarian cancer is inversely associated with the M1/M2 ratio of tumor-associated macrophages and patient survival time. PLoS ONE 2013, 8, e79769. [Google Scholar] [CrossRef] [Green Version]
- Ponnusamy, M.P.; Lakshmanan, I.; Jain, M.; Das, S.; Chakraborty, S.; Dey, P.; Batra, S.K. MUC4 mucin-induced epithelial to mesenchymal transition: A novel mechanism for metastasis of human ovarian cancer cells. Oncogene 2010, 29, 5741–5754. [Google Scholar] [CrossRef] [Green Version]
- Chen, C.H.; Shyu, M.K.; Wang, S.W.; Chou, C.H.; Huang, M.J.; Lin, T.C.; Chen, S.T.; Lin, H.H.; Huang, M.C. MUC20 promotes aggressive phenotypes of epithelial ovarian cancer cells via activation of the integrin beta1 pathway. Gynecol. Oncol. 2016, 140, 131–137. [Google Scholar] [CrossRef]
- Reis, C.A.; Osorio, H.; Silva, L.; Gomes, C.; David, L. Alterations in glycosylation as biomarkers for cancer detection. J. Clin. Pathol. 2010, 63, 322–329. [Google Scholar] [CrossRef] [PubMed]
- Kirwan, A.; Utratna, M.; O’Dwyer, M.E.; Joshi, L.; Kilcoyne, M. Glycosylation-Based Serum Biomarkers for Cancer Diagnostics and Prognostics. BioMed. Res. Int. 2015, 2015, 490531. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Silsirivanit, A. Glycosylation markers in cancer. Adv. Clin. Chem. 2019, 89, 189–213. [Google Scholar]
- Bottoni, P.; Scatena, R. The Role of CA 125 as Tumor Marker: Biochemical and Clinical Aspects. Adv. Cancer Biomark. 2015, 867, 229–244. [Google Scholar]
- Giamougiannis, P.; Martin-Hirsch, P.L.; Martin, F.L. The evolving role of MUC16 (CA125) in the transformation of ovarian cells and the progression of neoplasia. Carcinogenesis 2021, 42, 327–343. [Google Scholar] [CrossRef] [PubMed]
- Bergan, L.; Gross, J.A.; Nevin, B.; Urban, N.; Scholler, N. Development and in vitro validation of anti-mesothelin biobodies that prevent CA125/Mesothelin-dependent cell attachment. Cancer Lett. 2007, 255, 263–274. [Google Scholar] [CrossRef] [PubMed]
- Seelenmeyer, C.; Wegehingel, S.; Lechner, J.; Nickel, W. The cancer antigen CA125 represents a novel counter receptor for galectin-1. J. Cell Sci. 2003, 116, 1305–1318. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bast, R.C., Jr.; Klug, T.L.; St. John, E.; Jenison, E.; Niloff, J.M.; Lazarus, H.; Berkowitz, R.S.; Leavitt, T.; Griffiths, C.T.; Parker, L.; et al. A Radioimmunoassay Using a Monoclonal Antibody to Monitor the Course of Epithelial Ovarian Cancer. N. Engl. J. Med. 1983, 309, 883–887. [Google Scholar] [CrossRef]
- Wanyama, F.; Blanchard, V. Glycomic-Based Biomarkers for Ovarian Cancer: Advances and Challenges. Diagnostics 2021, 11, 643. [Google Scholar] [CrossRef]
- Ferraro, S.; Braga, F.; Lanzoni, M.; Boracchi, P.; Biganzoli, E.; Panteghini, M. Serum human epididymis protein 4 vs carbohydrate antigen 125 for ovarian cancer diagnosis: A systematic review. J. Clin. Pathol. 2013, 66, 273–281. [Google Scholar] [CrossRef]
- Ledermann, J.A.; Raja, F.A.; Fotopoulou, C.; Gonzalez-Martin, A.; Colombo, N.; Sessa, C. Newly diagnosed and relapsed epithelial ovarian carcinoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2013, 24 (Suppl. S6), vi24–vi32. [Google Scholar] [CrossRef] [PubMed]
- Grisham, R.N.; Berek, J.; Pfisterer, J.; Sabbatini, P. Abagovomab: An anti-idiotypic CA-125 targeted immunotherapeutic agent for ovarian cancer. Immunotherapy 2011, 3, 153–162. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sabbatini, P.; Harter, P.; Scambia, G.; Sehouli, J.; Meier, W.; Wimberger, P.; Baumann, K.H.; Kurzeder, C.; Schmalfeldt, B.; Cibula, D.; et al. Abagovomab As Maintenance Therapy in Patients with Epithelial Ovarian Cancer: A Phase III Trial of the AGO OVAR, COGI, GINECO, and GEICO—The MIMOSA Study. J. Clin. Oncol. 2013, 31, 1554–1561. [Google Scholar] [CrossRef] [PubMed]
- Coll-de la Rubia, E.; Martinez-Garcia, E.; Dittmar, G.; Gil-Moreno, A.; Cabrera, S.; Colas, E. Prognostic Biomarkers in Endometrial Cancer: A Systematic Review and Meta-Analysis. J. Clin. Med. 2020, 9, 1900. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, P.; Kehoe, S. Serum tumour markers in gynaecological cancers. Maturitas 2010, 67, 46–53. [Google Scholar] [CrossRef]
- Kakimoto, S.; Miyamoto, M.; Einama, T.; Takihata, Y.; Matsuura, H.; Iwahashi, H.; Ishibashi, H.; Sakamoto, T.; Hada, T.; Suminokura, J.; et al. Significance of mesothelin and CA125 expression in endometrial carcinoma: A retrospective analysis. Diagn. Pathol. 2021, 16, 28. [Google Scholar] [CrossRef]
- Haridas, D.; Ponnusamy, M.P.; Chugh, S.; Lakshmanan, I.; Seshacharyulu, P.; Batra, S.K. MUC16: Molecular analysis and its functional implications in benign and malignant conditions. FASEB J. 2014, 28, 4183–4199. [Google Scholar] [CrossRef]
- Togami, S.; Nomoto, M.; Higashi, M.; Goto, M.; Yonezawa, S.; Tsuji, T.; Batra, S.K.; Douchi, T. Expression of mucin antigens (MUC1 and MUC16) as a prognostic factor for mucinous adenocarcinoma of the uterine cervix. J. Obstet. Gynaecol. Res. 2010, 36, 588–597. [Google Scholar] [CrossRef]
- Li, X.; Xu, Y.; Zhang, L. Serum CA153 as biomarker for cancer and noncancer diseases. Prog. Mol. Biol. Transl. Sci. 2019, 162, 265–276. [Google Scholar]
- Von Mensdorff-Pouilly, S.; Snijdewint, F.G.; Verstraeten, A.A.; Verheijen, R.H.; Kenemans, P. Human MUC1 mucin: A multifaceted glycoprotein. Int. J. Biol. Markers 2000, 15, 343–356. [Google Scholar] [CrossRef]
- Deng, J.; Wang, L.; Chen, H.; Li, L.; Ma, Y.; Ni, J.; Li, Y. The role of tumour-associated MUC1 in epithelial ovarian cancer metastasis and progression. Cancer Metastasis Rev. 2013, 32, 535–551. [Google Scholar] [CrossRef] [PubMed]
- Feng, H.; Ghazizadeh, M.; Konishi, H.; Araki, T. Expression of MUC1 and MUC2 Mucin Gene Products in Human Ovarian Carcinomas. Jpn. J. Clin. Oncol. 2002, 32, 525–529. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wesseling, J.; van der Valk, S.W.; Vos, H.L.; Sonnenberg, A.; Hilkens, J. Episialin (MUC1) overexpression inhibits integrin-mediated cell adhesion to extracellular matrix components. J. Cell Biol. 1995, 129, 255–265. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ren, J.; Agata, N.; Chen, D.; Li, Y.; Yu, W.-H.; Huang, L.; Raina, D.; Chen, W.; Kharbanda, S.; Kufe, D. Human MUC1 carcinoma-associated protein confers resistance to genotoxic anticancer agents. Cancer Cell 2004, 5, 163–175. [Google Scholar] [CrossRef]
- Engel, B.J.; Bowser, J.L.; Broaddus, R.R.; Carson, D.D. MUC1 stimulates EGFR expression and function in endometrial cancer. Oncotarget 2016, 7, 32796–32809. [Google Scholar] [CrossRef]
- Skates, S.J.; Horick, N.; Yu, Y.; Xu, F.-J.; Berchuck, A.; Havrilesky, L.J.; de Bruijn, H.W.; van der Zee, A.G.; Woolas, R.P.; Jacobs, I.J.; et al. Preoperative Sensitivity and Specificity for Early-Stage Ovarian Cancer When Combining Cancer Antigen CA-125II, CA 15–3, CA 72–4, and Macrophage Colony-Stimulating Factor Using Mixtures of Multivariate Normal Distributions. J. Clin. Oncol. 2004, 22, 4059–4066. [Google Scholar] [CrossRef]
- Loveland, B.E.; Zhao, A.; White, S.; Gan, H.; Hamilton, K.; Xing, P.-X.; Pietersz, G.A.; Apostolopoulos, V.; Vaughan, H.; Karanikas, V.; et al. Mannan-MUC1–Pulsed Dendritic Cell Immunotherapy: A Phase I Trial in Patients with Adenocarcinoma. Clin. Cancer Res. 2006, 12, 869–877. [Google Scholar] [CrossRef] [Green Version]
- Chen, C.-H.; Wang, S.-W.; Huang, M.-R.; Hung, J.-S.; Huang, H.-C.; Lin, H.-H.; Chen, R.-J.; Shyu, M.-K.; Huang, M.-C. MUC20 overexpression predicts poor prognosis and enhances EGF-induced malignant phenotypes via activation of the EGFR–STAT3 pathway in endometrial cancer. Gynecol. Oncol. 2013, 128, 560–567. [Google Scholar] [CrossRef]
- Chen, S.-T.; Kuo, T.-C.; Liao, Y.-Y.; Lin, M.-C.; Tien, Y.-W.; Huang, M.-C. Silencing of MUC20 suppresses the malignant character of pancreatic ductal adenocarcinoma cells through inhibition of the HGF/MET pathway. Oncogene 2018, 37, 6041–6053. [Google Scholar] [CrossRef] [Green Version]
- Zheng, F.; Yu, H.; Lu, J. High expression of MUC20 drives tumorigenesis and predicts poor survival in endometrial cancer. J. Cell Biochem. 2019, 120, 11859–11866. [Google Scholar] [CrossRef]
- Bialecki, E.S.; di Bisceglie, A.M. Diagnosis of hepatocellular carcinoma. HPB 2005, 7, 26–34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, F.; Zhang, Z.-L. The Diagnostic Value of Transvaginal Sonograph (TVS), Color Doppler, and Serum Tumor Marker CA125, CEA, and AFP in Ovarian Cancer. Cell Biophys. 2015, 72, 353–357. [Google Scholar] [CrossRef]
- Rabban, J.T.; Zaloudek, C.J. A practical approach to immunohistochemical diagnosis of ovarian germ cell tumours and sex cord-stromal tumours. Histopathology 2012, 62, 71–88. [Google Scholar] [CrossRef]
- Nicolini, A.; Ferrari, P.; Rossi, G. Mucins and Cytokeratins as Serum Tumor Markers in Breast Cancer. Adv. Cancers Biomark. 2015, 867, 197–225. [Google Scholar]
- Dasari, S.; Wudayagiri, R.; Valluru, L. Cervical cancer: Biomarkers for diagnosis and treatment. Clin. Chim. Acta 2015, 445, 7–11. [Google Scholar] [CrossRef] [PubMed]
- Wan, Q.; Liu, Y.; Lv, B.; Chen, X. Correlation of Molecular Tumor Markers CA125, HE4, and CEA with the Development and Progression of Epithelial Ovarian Cancer. Iran. J. Public Health 2021, 50, 1197–1205. [Google Scholar] [CrossRef]
- Sagi-Dain, L.; Lavie, O.; Auslander, R.; Sagi, S. CEA in Evaluation of Adnexal Mass: Retrospective Cohort Analysis and Review of the Literature. Int. J. Biol. Markers 2015, 30, 394–400. [Google Scholar] [CrossRef]
- Lertkhachonsuk, A.A.; Buranawongtrakoon, S.; Lekskul, N.; Rermluk, N.; Wee-Stekly, W.W.; Charakorn, C. Serum CA19–9, CA-125 and CEA as tumor markers for mucinous ovarian tumors. J. Obstet. Gynaecol. Res. 2020, 46, 2287–2291. [Google Scholar] [CrossRef] [PubMed]
- Barr, C.E.; Funston, G.; Mounce, L.T.A.; Pemberton, P.W.; Howe, J.D.; Crosbie, E.J. Comparison of two immunoassays for the measurement of serum HE4 for ovarian cancer. Pract. Lab. Med. 2021, 26, e00235. [Google Scholar] [CrossRef]
- Piovano, E.; Attamante, L.; Macchi, C.; Cavallero, C.; Romagnolo, C.; Maggino, T.; Landoni, F.; Gadducci, A.; Sartori, E.; Gion, M.; et al. The Role of HE4 in Ovarian Cancer Follow-up: A Review. Int. J. Gynecol. Cancer 2014, 24, 1359–1365. [Google Scholar] [CrossRef] [Green Version]
- Ferraro, S.; Panteghini, M. Is serum human epididymis protein 4 ready for prime time? Ann. Clin. Biochem. 2014, 51 Pt 2, 128–136. [Google Scholar] [CrossRef] [Green Version]
- Dochez, V.; Caillon, H.; Vaucel, E.; Dimet, J.; Winer, N.; Ducarme, G. Biomarkers and algorithms for diagnosis of ovarian cancer: CA125, HE4, RMI and ROMA, a review. J. Ovarian Res. 2019, 12, 28. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moore, R.G.; McMeekin, D.S.; Brown, A.K.; DiSilvestro, P.; Miller, M.C.; Allard, W.J.; Gajewski, W.; Kurman, R.; Bast, R.C., Jr.; Skates, S.J. A novel multiple marker bioassay utilizing HE4 and CA125 for the prediction of ovarian cancer in patients with a pelvic mass. Gynecol. Oncol. 2009, 112, 40–46. [Google Scholar] [CrossRef] [Green Version]
- Nolen, B.; Velikokhatnaya, L.; Marrangoni, A.; de Geest, K.; Lomakin, A.; Bast, R.C.; Lokshin, A. Serum biomarker panels for the discrimination of benign from malignant cases in patients with an adnexal mass. Gynecol. Oncol. 2010, 117, 440–445. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moore, R.G.; Miller, M.C.; DiSilvestro, P.; Landrum, L.M.; Gajewski, W.; Ball, J.J.; Skates, S.J. Evaluation of the Diagnostic Accuracy of the Risk of Ovarian Malignancy Algorithm in Women With a Pelvic Mass. Obstet. Gynecol. 2011, 118, 280–288. [Google Scholar] [CrossRef] [PubMed]
- Shen, H.; Hsu, H.-C.; Tai, Y.-J.; Kuo, K.-T.; Wu, C.-Y.; Lai, Y.-L.; Chiang, Y.-C.; Chen, Y.-L.; Cheng, W.-F. Factors Influencing the Discordancy Between Intraoperative Frozen Sections and Final Paraffin Pathologies in Ovarian Tumors. Front. Oncol. 2021, 11, 694441. [Google Scholar] [CrossRef]
- Behrouzi, R.; Barr, C.E.; Crosbie, E.J. HE4 as a Biomarker for Endometrial Cancer. Cancers 2021, 13, 4764. [Google Scholar] [CrossRef] [PubMed]
- Dai, C.; Zheng, Y.; Li, Y.; Tian, T.; Wang, M.; Xu, P.; Deng, Y.; Hao, Q.; Wu, Y.; Zhai, Z.; et al. Prognostic values of HE4 expression in patients with cancer: A meta-analysis. Cancer Manag. Res. 2018, 10, 4491–4500. [Google Scholar] [CrossRef] [Green Version]
- Kato, H.; Torigoe, T. Radioimmunoassay for tumor antigen of human cervical squamous cell carcinoma. Cancer 1977, 40, 1621–1628. [Google Scholar] [CrossRef]
- Izuhara, K.; Yamaguchi, Y.; Ohta, S.; Nunomura, S.; Nanri, Y.; Azuma, Y.; Nomura, N.; Noguchi, Y.; Aihara, M. Squamous Cell Carcinoma Antigen 2 (SCCA2, SERPINB4): An Emerging Biomarker for Skin Inflammatory Diseases. Int. J. Mol. Sci. 2018, 19, 1102. [Google Scholar] [CrossRef] [Green Version]
- Liu, Z.; Shi, H. Prognostic Role of Squamous Cell Carcinoma Antigen in Cervical Cancer: A Meta-analysis. Dis. Markers 2019, 2019, 6710352. [Google Scholar] [CrossRef] [Green Version]
- Li, N.; Spetz, M.R.; Ho, M. The Role of Glypicans in Cancer Progression and Therapy. J. Histochem. Cytochem. 2020, 68, 841–862. [Google Scholar] [CrossRef] [PubMed]
- Matsuzaki, S.; Serada, S.; Hiramatsu, K.; Nojima, S.; Matsuzaki, S.; Ueda, Y.; Ohkawara, T.; Mabuchi, S.; Fujimoto, M.; Morii, E.; et al. Anti-glypican-1 antibody-drug conjugate exhibits potent preclinical antitumor activity against glypican-1 positive uterine cervical cancer. Int. J. Cancer 2018, 142, 1056–1066. [Google Scholar] [CrossRef]
- Capurro, M.; Wanless, I.R.; Sherman, M.; Deboer, G.; Shi, W.; Miyoshi, E.; Filmus, J. Glypican-3: A novel serum and histochemical marker for hepatocellular carcinoma. Gastroenterology 2003, 125, 89–97. [Google Scholar] [CrossRef]
- Zynger, D.L.; Everton, M.J.; Dimov, N.D.; Chou, P.M.; Yang, X.J. Expression of Glypican 3 in Ovarian and Extragonadal Germ Cell Tumors. Am. J. Clin. Pathol. 2008, 130, 224–230. [Google Scholar] [CrossRef] [PubMed]
- Stadlmann, S.; Gueth, U.; Baumhoer, D.; Moch, H.; Terracciano, L.; Singer, G. Glypican-3 expression in primary and recurrent ovarian carcinomas. Int. J. Gynecol. Pathol. 2007, 26, 341–344. [Google Scholar] [CrossRef]
- Wiedemeyer, K.; Köbel, M.; Koelkebeck, H.; Xiao, Z.; Vashisht, K. High glypican-3 expression characterizes a distinct subset of ovarian clear cell carcinomas in Canadian patients: An opportunity for targeted therapy. Hum. Pathol. 2020, 98, 56–63. [Google Scholar] [CrossRef] [PubMed]
- Breij, E.C.; de Goeij, B.E.; Verploegen, S.; Schuurhuis, D.H.; Amirkhosravi, A.; Francis, J.; Miller, V.B.; Houtkamp, M.; Bleeker, W.K.; Satijn, D.; et al. An Antibody–Drug Conjugate That Targets Tissue Factor Exhibits Potent Therapeutic Activity against a Broad Range of Solid Tumors. Cancer Res 2014, 74, 1214–1226. [Google Scholar] [CrossRef] [Green Version]
- Cocco, E.; Varughese, J.; Buza, N.; Bellone, S.; Glasgow, M.; Bellone, M.; Todeschini, P.; Carrara, L.; Silasi, D.A.; Azodi, M.; et al. Expression of tissue factor in adenocarcinoma and squamous cell carcinoma of the uterine cervix: Implications for immunotherapy with hI-con1, a factor VII-IgGFc chimeric protein targeting tissue factor. BMC Cancer 2011, 11, 263. [Google Scholar] [CrossRef] [Green Version]
- Zhao, X.; Cheng, C.; Gou, J.; Yi, T.; Qian, Y.; Du, X.; Zhao, X. Expression of tissue factor in human cervical carcinoma tissue. Exp. Ther. Med. 2018, 16, 4075–4081. [Google Scholar] [CrossRef] [Green Version]
- Ju, T.; Wang, Y.; Aryal, R.P.; Lehoux, S.D.; Ding, X.; Kudelka, M.R.; Cutler, C.; Zeng, J.; Wang, J.; Sun, X.; et al. Tn and sialyl-Tn antigens, aberrant O-glycomics as human disease markers. Proteomics Clin. Appl. 2013, 7, 618–631. [Google Scholar] [CrossRef] [Green Version]
- Munkley, J. The glycosylation landscape of pancreatic cancer (Review). Oncol. Lett. 2019, 17, 2569–2575. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scara’, S.; Bottoni, P.; Scatena, R. CA 19–9: Biochemical and Clinical Aspects. Adv. Exp. Med. Biol. 2015, 867, 247–260. [Google Scholar] [PubMed]
- Haglund, C.; Roberts, P.J.; Kuusela, P.; Scheinin, T.M.; Mäkelä, O.; Jalanko, H. Evaluation of CA 19–9 as a serum tumour marker in pancreatic cancer. Br. J. Cancer 1986, 53, 197–202. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kelly, P.J.; Archbold, P.; Price, J.H.; Cardwell, C.; McCluggage, W.G. Serum CA19.9 levels are commonly elevated in primary ovarian mucinous tumours but cannot be used to predict the histological subtype. J. Clin. Pathol. 2010, 63, 169–173. [Google Scholar] [CrossRef]
- Bian, J.; Sun, X.; Li, B.; Ming, L. Clinical Significance of Serum HE4, CA125, CA724, and CA19-9 in Patients With Endometrial Cancer. Technol. Cancer Res. Treat. 2016, 16, 435–439. [Google Scholar] [CrossRef]
- Niimi, K.; Yamamoto, E.; Fujiwara, S.; Shinjo, K.; Kotani, T.; Umezu, T.; Kajiyama, H.; Shibata, K.; Ino, K.; Kikkawa, F. High expression of N-acetylglucosaminyltransferase IVa promotes invasion of choriocarcinoma. Br. J. Cancer 2012, 107, 1969–1977. [Google Scholar] [CrossRef] [Green Version]
- Nishino, K.; Yamamoto, E.; Niimi, K.; Sekiya, Y.; Yamashita, Y.; Kikkawa, F. N-acetylglucosaminyltransferase IVa promotes invasion of choriocarcinoma. Oncol. Rep. 2017, 38, 440–448. [Google Scholar] [CrossRef] [Green Version]
- Bennett, E.P.; Mandel, U.; Clausen, H.; Gerken, T.A.; Fritz, T.A.; Tabak, L.A. Control of mucin-type O-glycosylation: A classification of the polypeptide GalNAc-transferase gene family. Glycobiology 2012, 22, 736–756. [Google Scholar] [CrossRef] [Green Version]
- Wu, Y.-M.; Liu, C.-H.; Hu, R.-H.; Huang, M.-J.; Lee, J.-J.; Chen, C.-H.; Huang, J.; Lai, H.-S.; Lee, P.-H.; Hsu, W.-M.; et al. Mucin Glycosylating Enzyme GALNT2 Regulates the Malignant Character of Hepatocellular Carcinoma by Modifying the EGF Receptor. Cancer Res. 2011, 71, 7270–7279. [Google Scholar] [CrossRef] [Green Version]
- Hussain, M.R.M.; Hoessli, D.C.; Fang, M. N-acetylgalactosaminyltransferases in cancer. Oncotarget 2016, 7, 54067–54081. [Google Scholar] [CrossRef] [Green Version]
- Hu, W.-T.; Yeh, C.-C.; Liu, S.-Y.; Huang, M.-C.; Lai, I.-R. The O-glycosylating enzyme GALNT2 suppresses the malignancy of gastric adenocarcinoma by reducing EGFR activities. Am. J. Cancer Res. 2018, 8, 1739–1751. [Google Scholar] [PubMed]
- Liao, W.-C.; Chen, C.-H.; Liu, C.-H.; Huang, M.-J.; Hung, J.-S.; Chou, C.-H.; Che, M.-I.; Chang, H.-M.; Lan, C.-T.; Huang, H.-C.; et al. Expression of GALNT2 in human extravillous trophoblasts and its suppressive role in trophoblast invasion. Placenta 2012, 33, 1005–1011. [Google Scholar] [CrossRef] [PubMed]
- Park, J.-H.; Nishidate, T.; Kijima, K.; Ohashi, T.; Takegawa, K.; Fujikane, T.; Hirata, K.; Nakamura, Y.; Katagiri, T. Critical Roles of Mucin 1 Glycosylation by Transactivated Polypeptide N-Acetylgalactosaminyltransferase 6 in Mammary Carcinogenesis. Cancer Res 2010, 70, 2759–2769. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wagner, K.W.; Punnoose, E.; Januario, T.; Lawrence, D.; Pitti, R.M.; Lancaster, K.; Lee, D.; von Goetz, M.; Yee, S.F.; Totpal, K.; et al. Death-receptor O-glycosylation controls tumor-cell sensitivity to the proapoptotic ligand Apo2L/TRAIL. Nat. Med. 2007, 13, 1070–1077. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Yu, C.; Zhao, D.; Wu, M.; Yang, Z. The mucin-type glycosylating enzyme polypeptide N-acetylgalactosaminyltransferase 14 promotes the migration of ovarian cancer by modifying mucin 13. Oncol. Rep. 2013, 30, 667–676. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.-Q.; Bachvarova, M.; Morin, C.; Plante, M.; Gregoire, J.; Renaud, M.-C.; Sebastianelli, A.; Bachvarov, D. Role of the polypeptide N-acetylgalactosaminyltransferase 3 in ovarian cancer progression: Possible implications in abnormal mucin O-glycosylation. Oncotarget 2014, 5, 544–560. [Google Scholar] [CrossRef] [Green Version]
- Lin, T.-C.; Chen, S.-T.; Huang, M.-C.; Huang, J.; Hsu, C.-L.; Juan, H.-F.; Lin, H.-H.; Chen, C.-H. GALNT6 expression enhances aggressive phenotypes of ovarian cancer cells by regulating EGFR activity. Oncotarget 2017, 8, 42588–42601. [Google Scholar] [CrossRef]
- Sheta, R.; Bachvarova, M.; Plante, M.; Gregoire, J.; Renaud, M.-C.; Sebastianelli, A.; Popa, I.; Bachvarov, D. Altered expression of different GalNAc-transferases is associated with disease progression and poor prognosis in women with high-grade serous ovarian cancer. Int. J. Oncol. 2017, 51, 1887–1897. [Google Scholar] [CrossRef] [Green Version]
- Li, Z.; Yamada, S.; Wu, Y.; Wang, K.-Y.; Liu, Y.-P.; Uramoto, H.; Kohno, K.; Sasaguri, Y. Polypeptide N-acetylgalactosaminyltransferase-6 expression independently predicts poor overall survival in patients with lung adenocarcinoma after curative resection. Oncotarget 2016, 7, 54463–54473. [Google Scholar] [CrossRef] [Green Version]
- Liu, C.; Li, Z.; Xu, L.; Shi, Y.; Zhang, X.; Shi, S.; Hou, K.; Fan, Y.; Li, C.; Wang, X.; et al. GALNT6 promotes breast cancer metastasis by increasing mucin-type O-glycosylation of α2M. Aging 2020, 12, 11794–11811. [Google Scholar] [CrossRef]
- Nguyen, T.T.; Kurita, T.; Koi, C.; Murakami, M.; Kagami, S.; Hachisuga, T.; Hou, K.; Fan, Y.; Li, C.; Wang, X.; et al. GalNAc-T6 in the relationship with invasion ability of endometrial carcinomas and prognostic significance. Am. J. Cancer Res. 2017, 7, 1188–1197. [Google Scholar] [PubMed]
- Hung, J.-S.; Huang, J.; Lin, Y.-C.; Huang, M.-J.; Lee, P.-H.; Lai, H.-S.; Liang, J.-T.; Huang, M.-C. C1GALT1 overexpression promotes the invasive behavior of colon cancer cells through modifying O-glycosylation of FGFR2. Oncotarget 2014, 5, 2096–2106. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chou, C.-H.; Huang, M.-J.; Chen, C.-H.; Shyu, M.-K.; Huang, J.; Hung, J.-S.; Huang, C.-S.; Huang, M.-C. Up-regulation of C1GALT1 promotes breast cancer cell growth through MUC1-C signaling pathway. Oncotarget 2015, 6, 6123–6135. [Google Scholar] [CrossRef] [Green Version]
- Lee, P.C.; Chen, S.-T.; Kuo, T.-C.; Lin, T.-C.; Lin, M.-C.; Huang, J.; Hung, J.-S.; Hsu, C.-L.; Juan, H.-F.; Huang, M.-C. C1GALT1 is associated with poor survival and promotes soluble Ephrin A1-mediated cell migration through activation of EPHA2 in gastric cancer. Oncogene 2020, 39, 2724–2740. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shen, H.; Lee, C.-Y.; Chen, C.-H. Protein Glycosylation as Biomarkers in Gynecologic Cancers. Diagnostics 2022, 12, 3177. https://doi.org/10.3390/diagnostics12123177
Shen H, Lee C-Y, Chen C-H. Protein Glycosylation as Biomarkers in Gynecologic Cancers. Diagnostics. 2022; 12(12):3177. https://doi.org/10.3390/diagnostics12123177
Chicago/Turabian StyleShen, Hung, Chia-Yi Lee, and Chi-Hau Chen. 2022. "Protein Glycosylation as Biomarkers in Gynecologic Cancers" Diagnostics 12, no. 12: 3177. https://doi.org/10.3390/diagnostics12123177
APA StyleShen, H., Lee, C.-Y., & Chen, C.-H. (2022). Protein Glycosylation as Biomarkers in Gynecologic Cancers. Diagnostics, 12(12), 3177. https://doi.org/10.3390/diagnostics12123177





