Predicting the Probability of the Incidence of Maxillary Sinus Fungus Ball in Patients Using Nomogram Models
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Participants
2.2. Clinical Characteristics and CT Imaging Features
2.3. Statistical Analysis
3. Results
3.1. Descriptive Characteristics of the Participants
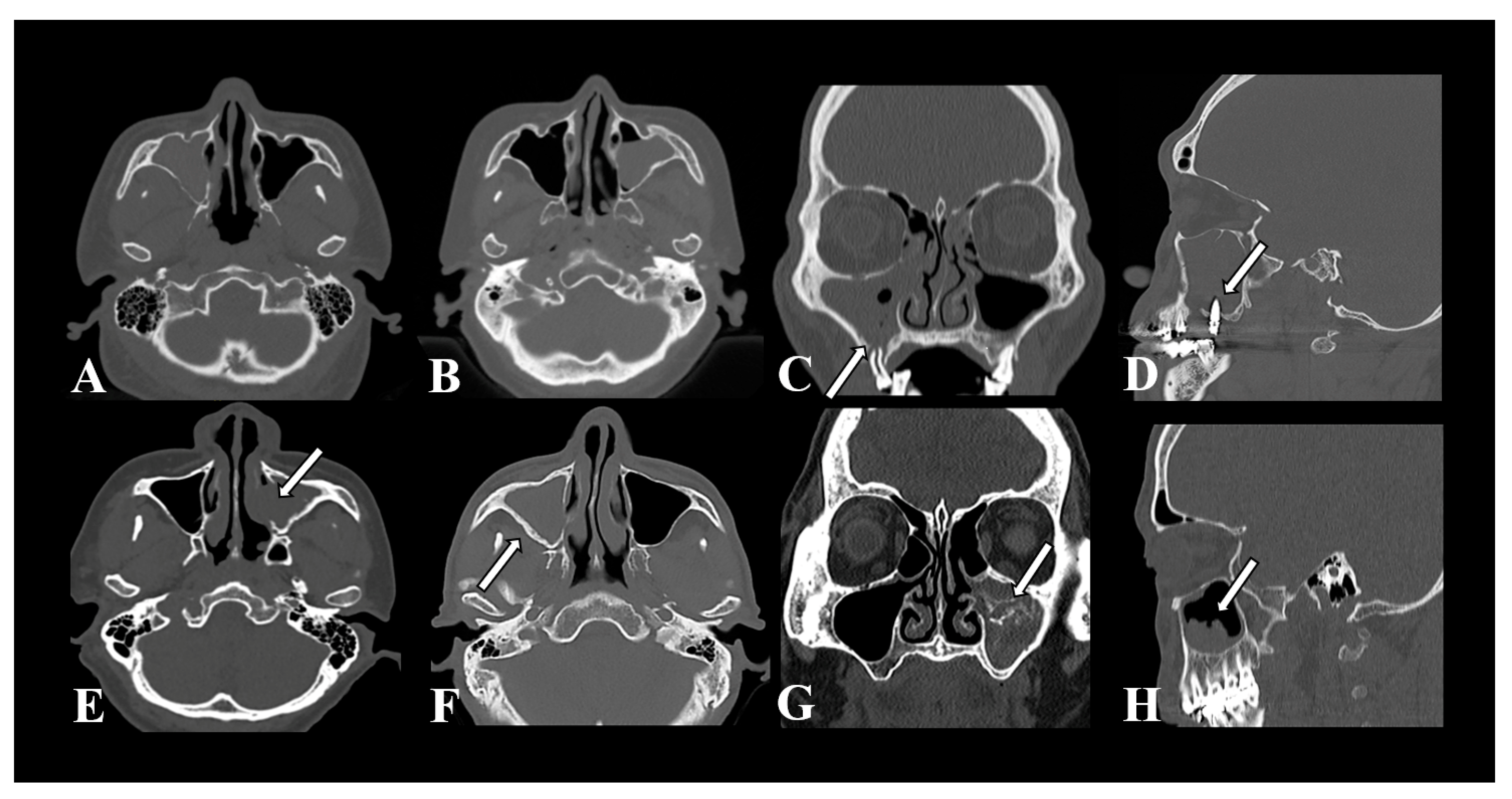
3.2. Features of CT Imaging
3.3. Logistic Regression Analysis
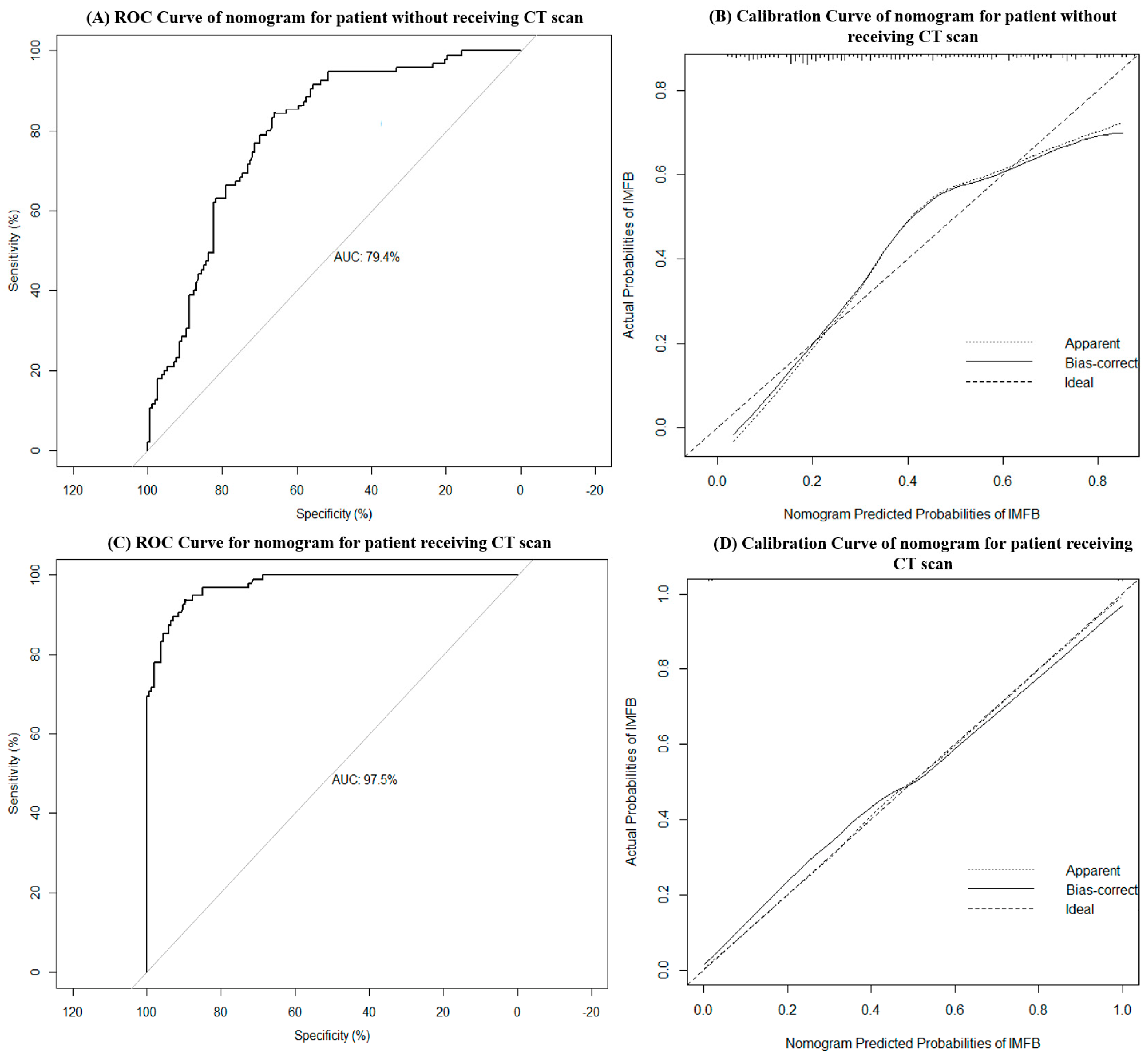
3.4. Nomograms for Predicting the Probability of the Incidence of MSFB
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chakrabarti, A.; Denning, D.W.; Ferguson, B.J.; Ponikau, J.; Buzina, W.; Kita, H.; Marple, B.; Panda, N.; Vlaminck, S.; Kauffmann-Lacroix, C.; et al. Fungal rhinosinusitis: A categorization and definitional schema addressing current controversies. Laryngoscope 2009, 119, 1809–1818. [Google Scholar] [CrossRef] [PubMed]
- deShazo, R.D.; O’Brien, M.; Chapin, K.; Soto-Aguilar, M.; Swain, R.; Lyons, M.; Bryars, W.C., Jr.; Alsip, S. Criteria for the diagnosis of sinus mycetoma. J. Allergy Clin. Immunol. 1997, 99, 475–485. [Google Scholar] [CrossRef] [PubMed]
- Wu, P.W.; Huang, Y.L.; Yang, S.W.; Lee, Y.S.; Huang, C.C.; Chang, P.H.; Lee, T.J. Acute invasive fungal rhinosinusitis in twenty-one diabetic patients. Clin. Otolaryngol. 2018, 43, 1163–1167. [Google Scholar] [CrossRef] [PubMed]
- Roland, L.T.; Humphreys, I.M.; Le, C.H.; Babik, J.M.; Bailey, C.E.; Ediriwickrema, L.S.; Fung, M.; Lieberman, J.A.; Magliocca, K.R.; Nam, H.H.; et al. Diagnosis, Prognosticators, and Management of Acute Invasive Fungal Rhinosinusitis: Multidisciplinary Consensus Statement and Evidence-Based Review with Recommendations. Int. Forum Allergy Rhinol. 2023; epub ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Liu, C.; Wei, H.; He, S.; Dong, S.; Zhou, B.; Zhang, L.; Li, Y. A retrospective analysis of 1717 paranasal sinus fungus ball cases from 2008 to 2017. Laryngoscope 2020, 130, 75–79. [Google Scholar] [CrossRef] [PubMed]
- Paknezhad, H.; Borchard, N.A.; Charville, G.W.; Ayoub, N.F.; Choby, G.W.; Thamboo, A.; Nayak, J.V. Evidence for a ‘preinvasive’ variant of fungal sinusitis: Tissue invasion without angioinvasion. World J. Otorhinolaryngol. Head Neck Surg. 2017, 3, 37–43. [Google Scholar] [CrossRef]
- Yoon, Y.H.; Xu, J.; Park, S.K.; Heo, J.H.; Kim, Y.M.; Rha, K.S. A retrospective analysis of 538 sinonasal fungus ball cases treated at a single tertiary medical center in Korea (1996–2015). Int. Forum Allergy Rhinol. 2017, 7, 1070–1075. [Google Scholar] [CrossRef]
- Wu, P.W.; Lee, T.J.; Yang, S.W.; Huang, Y.; Lee, Y.S.; Ho, C.F.; Huang, C.C. Differences in clinical and imaging presentation of maxillary sinus fungus ball with and without intralesional hyperdensity. Sci. Rep. 2021, 11, 23945. [Google Scholar] [CrossRef]
- Dhong, H.J.; Jung, J.Y.; Park, J.H. Diagnostic accuracy in sinus fungus balls: CT scan and operative findings. Am. J. Rhinol. 2000, 14, 227–231. [Google Scholar] [CrossRef]
- Doo, J.G.; Min, H.K.; Choi, G.W.; Kim, S.W.; Min, J.Y. Analysis of predisposing factors in unilateral maxillary sinus fungal ball: The predictive role of odontogenic and anatomical factors. Rhinology 2022, 60, 377–383. [Google Scholar] [CrossRef]
- Ho, C.F.; Lee, T.J.; Wu, P.W.; Huang, C.C.; Chang, P.H.; Huang, Y.L.; Lee, Y.L.; Huang, C.C. Diagnosis of a maxillary sinus fungus ball without intralesional hyperdensity on computed tomography. Laryngoscope 2019, 129, 1041–1045. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.C.; Ho, C.Y. The significance of computed tomographic findings in the diagnosis of fungus ball in the paranasal sinuses. Am. J. Rhinol. Allergy 2012, 26, 117–119. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.H.; Wu, P.W.; Huang, Y.L.; Lee, C.C.; Lee, T.J.; Huang, C.C.; Chang, P.H. Identifying a sphenoid sinus fungus ball using a nomogram model. Rhinology 2023, 61, 153–160. [Google Scholar] [CrossRef] [PubMed]
- Gumussoy, S.; Gumussoy, M.; Hortu, I.; Ergenoglu, A.M. The effect of surgical menopause after bilateral oophorectomy on hormonal changes, mucociliary clearance, and quality of life. Eur. Arch. Otorhinolaryngol. 2020, 277, 2793–2800. [Google Scholar] [CrossRef] [PubMed]
- Soylu Özler, G.; Akbay, E.; Akkoca, A.N.; Karapınar, O.S.; Şimşek, G.Ö. Does menopause effect nasal mucociliary clearance time? Eur. Arch. Otorhinolaryngol. 2015, 272, 363–366. [Google Scholar] [CrossRef] [PubMed]
- Haeggström, A.; Östberg, B.; Stjerna, P.; Graf, P.; Hallén, H. Nasal mucosal swelling and reactivity during a menstrual cycle. ORL J. Otorhinolaryngol. Relat. Spec. 2000, 62, 39–42. [Google Scholar] [CrossRef] [PubMed]
- Lao, M.; Li, C.; Li, J.; Chen, D.; Ding, M.; Gong, Y. Opportunistic invasive fungal disease in patients with type 2 diabetes mellitus from Southern China: Clinical features and associated factors. J. Diabetes Investig. 2020, 11, 731–744. [Google Scholar] [CrossRef]
- Bachert, C.; Zhang, N.; Hellings, P.W.; Bousquet, J. Endotype-driven care pathways in patients with chronic rhinosinusitis. J. Allergy Clin. Immunol. 2018, 141, 1543–1551. [Google Scholar] [CrossRef]
- Fokkens, W.J.; Lund, V.J.; Hopkins, C.; Hellings, P.W.; Kern, R.; Reitsma, S.; Toppila-Salmi, S.; Bernal-Sprekelsen, M.; Mullol, J.; Alobid, I.; et al. European Position Paper on Rhinosinusitis and Nasal Polyps 2020. Rhinology 2020, 58, 1–464. [Google Scholar] [CrossRef]
- Park, H.J.; Seoh, J.Y.; Han, K.H.; Moon, K.R.; Lee, S.S. The role of mucosal immunity in fungus ball of the paranasal sinuses. Acta Otolaryngol. 2012, 132 (Suppl. 1), S58–S62. [Google Scholar] [CrossRef]
- Speth, C.; Rambach, G.; Lass-Flörl, C. Platelet immunology in fungal infections. Thromb. Haemost. 2014, 112, 632–639. [Google Scholar] [CrossRef]
- Hamzeh-Cognasse, H.; Damien, P.; Chabert, A.; Pozzetto, B.; Cognasse, F.; Garraud, O. Platelets and infections-complex interactions with bacteria. Front. Immunol. 2015, 6, 82. [Google Scholar] [CrossRef]
- Jiang, Z.; Zhang, K.; Huang, W.; Yuan, Q. A Preliminary Study on Sinus Fungus Ball with MicroCT and X-ray Fluorescence Technique. PLoS ONE 2016, 11, e0148515. [Google Scholar] [CrossRef]
- Little, R.E.; Long, C.M.; Loehrl, T.A.; Poetker, D.M. Odontogenic sinusitis: A review of the current literature. Laryngoscope Investig. Otolaryngol. 2018, 3, 110–114. [Google Scholar] [CrossRef]
- Melén, I.; Lindahl, L.; Andréasson, L.; Rundcrantz, H. Chronic maxillary sinusitis. Definition, diagnosis and relation to dental infections and nasal polyposis. Acta Otolaryngol. 1986, 101, 320–327. [Google Scholar] [CrossRef]
- Mensi, M.; Piccioni, M.; Marsili, F.; Nicolai, P.; Sapelli, P.L.; Latronico, N. Risk of maxillary fungus ball in patients with endodontic treatment on maxillary teeth: A case-control study. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontol. 2007, 103, 433–436. [Google Scholar] [CrossRef] [PubMed]

| Variables | MSFB Group | UMRS Group | p Value |
|---|---|---|---|
| (n = 97) | (n = 158) | ||
| Age, years (mean ± SD) | 59.61 ± 13.2 | 46.31 ± 14.8 | <0.001 *** |
| Sex | |||
| Male, n (%) | 33 (34.0) | 97 (61.4) | <0.001 *** |
| Female, n (%) | 64 (66.0) | 61 (38.6) | |
| Laboratory data | |||
| WBC (×103/μL) | 6.69 ± 1.90 | 7.87 ± 2.41 | <0.001 *** |
| Neutrophils count (×103/μL) | 3.97 ± 1.56 | 4.95 ± 2.13 | <0.001 *** |
| Lymphocytes count (×103/μL) | 2.20 ± 0.722 | 2.27 ± 7.04 | 0.489 |
| Eosinophils count (×102/μL) | 1.83 ± 1.91 | 1.78 ± 1.67 | 0.796 |
| Platelet (×106/μL) | 2.50 ± 0.58 | 2.72 ± 0.63 | 0.006 ** |
| Underlying condition | |||
| Diabetes mellitus, n (%) | 19 (19.6) | 20 (12.7) | 0.136 |
| Clinical presentations | |||
| Rhinorrhea, n (%) | 60 (61.9) | 113 (71.5) | 0.109 |
| Post nasal dripping, n (%) | 47 (48.5) | 85 (53.8) | 0.407 |
| Nasal obstruction, n (%) | 43 (44.3) | 107 (67.7) | <0.001 *** |
| Headache and facial pain, n (%) | 28 (28.9) | 43 (27.2) | 0.775 |
| Hyposmia, n (%) | 25 (25.8) | 42 (26.6) | 0.887 |
| CT Imaging Features | MSFB Group | UMRS Group | p Value |
|---|---|---|---|
| (n = 97) | (n = 158) | ||
| Total opacification, n (%) | 47 (48.5) | 84 (53.2) | 0.465 |
| Partial opacification, n (%) | 50 (51.5) | 74 (46.8) | 0.465 |
| Irregular surface, n (%) | 41 (42.3) | 14 (8.9) | <0.001 *** |
| Maxillary odontogenic pathology, n (%) | 16 (16.5) | 53 (33.5) | 0.003 ** |
| Erosion of medial sinus wall, n (%) | 53 (54.6) | 12 (7.6) | <0.001 *** |
| Sclerosis of lateral sinus wall, n (%) | 88 (90.7) | 109 (69.0) | <0.001 *** |
| Intralesional hyperdensity, n (%) | 79 (81.4) | 9 (5.7) | <0.001 *** |
| Variables | Univariate Regression Analysis | |
|---|---|---|
| Odds Ratio (95% CI) | p Value | |
| Characteristics of patients | ||
| Age (years) | 1.07 (1.04−1.09) | <0.001 *** |
| Diabetes mellitus | 1.68 (0.85−3.33) | 0.138 |
| Female sex (female versus male) | 3.08 (1.82−5.23) | <0.001 ** |
| Laboratory data | ||
| WBC count (×103/μL) | 0.77 (0.68−0.88) | 0.001 ** |
| Neutrophils count (×103/μL) | 0.73 (0.62−0.87) | <0.001 *** |
| Lymphocytes count (×103/μL) | 0.88 (0.61−1.27) | 0.480 |
| Eosinophils count (×102/μL) | 1.00 (0.99−1.02) | 0.761 |
| Platelet (×106/μL) | 0.55 (0.36−0.86) | 0.008 ** |
| CT imaging features | ||
| Total opacification | 0.83 (0.50−1.37) | 0.465 |
| Partial opacification | 1.21 (0.73−2.00) | 0.465 |
| Maxillary odontogenic pathology | 0.39 (0.21−0.73) | 0.003 ** |
| Irregular surface | 7.53 (3.81−14.88) | <0.001 *** |
| Erosion of medial sinus wall | 14.66 (7.19−29.85) | <0.001 *** |
| Sclerosis of lateral sinus wall | 4.40 (2.05−9.44) | <0.001 *** |
| Intralesional hyperdensity | 72.66 (31.20−169.21) | <0.001 *** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fan, Y.-H.; Shih, K.-Y.; Wu, P.-W.; Huang, Y.-L.; Lee, T.-J.; Huang, C.-C.; Chang, P.-H.; Huang, C.-C. Predicting the Probability of the Incidence of Maxillary Sinus Fungus Ball in Patients Using Nomogram Models. Diagnostics 2023, 13, 3156. https://doi.org/10.3390/diagnostics13193156
Fan Y-H, Shih K-Y, Wu P-W, Huang Y-L, Lee T-J, Huang C-C, Chang P-H, Huang C-C. Predicting the Probability of the Incidence of Maxillary Sinus Fungus Ball in Patients Using Nomogram Models. Diagnostics. 2023; 13(19):3156. https://doi.org/10.3390/diagnostics13193156
Chicago/Turabian StyleFan, Yu-Hsi, Kai-Yi Shih, Pei-Wen Wu, Yen-Lin Huang, Ta-Jen Lee, Chi-Che Huang, Po-Hung Chang, and Chien-Chia Huang. 2023. "Predicting the Probability of the Incidence of Maxillary Sinus Fungus Ball in Patients Using Nomogram Models" Diagnostics 13, no. 19: 3156. https://doi.org/10.3390/diagnostics13193156
APA StyleFan, Y.-H., Shih, K.-Y., Wu, P.-W., Huang, Y.-L., Lee, T.-J., Huang, C.-C., Chang, P.-H., & Huang, C.-C. (2023). Predicting the Probability of the Incidence of Maxillary Sinus Fungus Ball in Patients Using Nomogram Models. Diagnostics, 13(19), 3156. https://doi.org/10.3390/diagnostics13193156









