IVDR: Analysis of the Social, Economic, and Practical Consequences of the Application of an Ordinance of the In Vitro Diagnostic Ordinance in Switzerland
Abstract
:1. Introduction
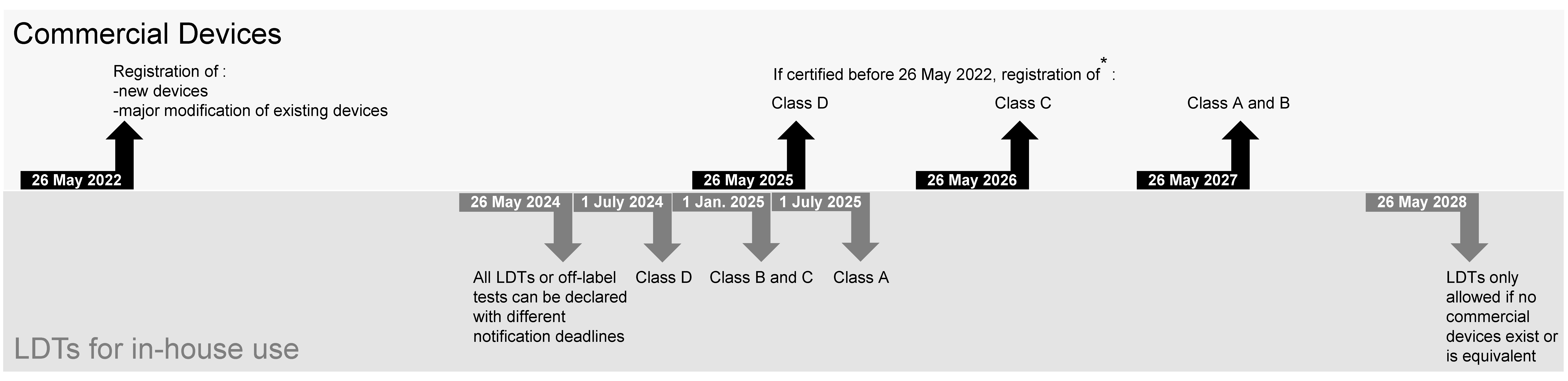
2. What IVDR and IvDO Imply
3. Difficulties Encountered in Clinical Microbiology Laboratories
4. Foreseen Impact of the Law
5. How to Face the Problems Raised by the Regulation
- ISO-15189-accredited laboratories may replace all LDT notifications with submission of their accreditation certificate.
- The IvDO 2028 milestone, which requires the superiority of an LDT against any CE-IVD test to be proven, should not be implemented. Mitigation measures could, for instance, include scientific surveillance and comparison with commercial tests, up to the level of equivalence (but not superiority).
6. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- European Union. In vitro Diagnostic regulation. 2017. Available online: https://eur-lex.europa.eu/eli/reg/2017/746/oj (accessed on 1 July 2023).
- The Swiss Federal Council. Ordinance on In Vitro Diagnostic Medical Devices (IvDO). 2022. Available online: https://www.fedlex.admin.ch/filestore/fedlex.data.admin.ch/eli/cc/2022/291/20220526/en/doc/fedlex-data-admin-ch-eli-cc-2022-291-20220526-en-doc.doc (accessed on 1 July 2023).
- Kahles, A.; Goldschmid, H.; Volckmar, A.L.; Ploeger, C.; Kazdal, D.; Penzel, R.; Budczies, J.; Kempny, G.; Kazmierczak, M. Structure and content of the EU-IVDR: Current status and implications for pathology. Pathologie 2023, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Bank, P.C.D.; Jacobs, L.H.J.; van den Berg, S.A.A.; van Deutekom, H.W.M.; Hamann, D.; Molenkamp, R.; Ruivenkamp, C.A.L.; Swen, J.J.; Tops, B.B.J.; Wamelink, M.M.C.; et al. The end of the laboratory developed test as we know it? Recommendations from a national multidisciplinary taskforce of laboratory specialists on the interpretation of the IVDR and its complications. Clin. Chem. Lab. Med. 2020, 59, 491–497. [Google Scholar] [CrossRef] [PubMed]
- van Deutekom, H.W.M.; Haitjema, S. Recommendations for IVDR compliant in-house software development in clinical practice: A how-to paper with three use cases. Clin. Chem. Lab. Med. 2022, 60, 982–988. [Google Scholar] [CrossRef] [PubMed]
- European Union. Guidance on Classification Rules for In Vitro Diagnostic Medical Devices under Regulation (EU) 2017/746. 2023. Available online: https://health.ec.europa.eu/latest-updates/update-mdcg-2020-16-rev2-guidance-classification-rules-vitro-diagnostic-medical-devices-under-2023-02-10_en (accessed on 1 July 2023).
- Pandey, S.; Yadav, B.; Pandey, A.; Tripathi, T.; Khawary, M.; Kant, S.; Tripathi, D. Lessons from SARS-CoV-2 Pandemic: Evolution, Disease Dynamics and Future. Biology 2020, 9, 141. [Google Scholar] [CrossRef] [PubMed]
- Desoubeaux, G.; Coste, A.T.; Imbert, C.; Hennequin, C. Overview about Candida auris: What’s up 12 years after its first description? J. Mycol. Med. 2022, 32, 101248. [Google Scholar] [CrossRef] [PubMed]
- Marions, L.; Rotzen-Ostlund, M.; Grillner, L.; Edgardh, K.; Tiveljung-Lindell, A.; Wikstrom, A.; Lidbrink, P. High occurrence of a new variant of Chlamydia trachomatis escaping diagnostic tests among STI clinic patients in Stockholm, Sweden. Sex. Transm. Dis. 2008, 35, 61–64. [Google Scholar] [CrossRef] [PubMed]
- Diene, S.M.; Bertelli, C.; Pillonel, T.; Jacquier, N.; Croxatto, A.; Jaton, K.; Greub, G. Comparative genomics of Neisseria meningitidis strains: New targets for molecular diagnostics. Clin. Microbiol. Infect. 2016, 22, 568-e1. [Google Scholar] [CrossRef] [PubMed]
- Hraib, M.; Jouni, S.; Albitar, M.M.; Alaidi, S.; Alshehabi, Z. The outbreak of monkeypox 2022: An overview. Ann. Med. Surg. 2022, 79, 104069. [Google Scholar] [CrossRef] [PubMed]
- Kumar, N.; Acharya, A.; Gendelman, H.E.; Byrareddy, S.N. The 2022 outbreak and the pathobiology of the monkeypox virus. J. Autoimmun. 2022, 131, 102855. [Google Scholar] [CrossRef] [PubMed]
- Petersen, E.; Kantele, A.; Koopmans, M.; Asogun, D.; Yinka-Ogunleye, A.; Ihekweazu, C.; Zumla, A. Human Monkeypox: Epidemiologic and Clinical Characteristics, Diagnosis, and Prevention. Infect. Dis. Clin. N. Am. 2019, 33, 1027–1043. [Google Scholar] [CrossRef] [PubMed]
- Stegmuller, S.; Fraefel, C.; Kubacki, J. Genome Sequence of Alongshan Virus from Ixodes ricinus Ticks Collected in Switzerland. Microbiol. Resour. Announc. 2023, 12, e0128722. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.D.; Wang, W.; Wang, N.N.; Qiu, K.; Zhang, X.; Tana, G.; Liu, Q.; Zhu, X.Q. Prevalence of the emerging novel Alongshan virus infection in sheep and cattle in Inner Mongolia, northeastern China. Parasit. Vectors 2019, 12, 450. [Google Scholar] [CrossRef] [PubMed]
- Bertelli, C.; Greub, G. Rapid bacterial genome sequencing: Methods and applications in clinical microbiology. Clin. Microbiol. Infect. 2013, 19, 803–813. [Google Scholar] [CrossRef] [PubMed]
- de Vries, H.J.C.; Pannekoek, Y.; Dean, D.; Bavoil, P.M.; Borel, N.; Greub, G.; Morre, S.A.; on behalf ofthe ICSP Subcommittee on the Taxonomy of Chlamydiae. Call for consensus in Chlamydia trachomatis nomenclature: Moving from biovars, serovars, and serotypes to genovariants and genotypes. Clin. Microbiol. Infect. 2022, 28, 761–763. [Google Scholar] [CrossRef] [PubMed]
- Neves, A.; Walther, D.; Martin-Campos, T.; Barbie, V.; Bertelli, C.; Blanc, D.; Bouchet, G.; Erard, F.; Greub, G.; Hirsch, H.H.; et al. The Swiss Pathogen Surveillance Platform—Towards a nation-wide One Health data exchange platform for bacterial, viral and fungal genomics and associated metadata. Microb. Genom. 2023, 9, mgen001001. [Google Scholar] [CrossRef] [PubMed]
- Pillonel, T.; Bertelli, C.; Salamin, N.; Greub, G. Taxogenomics of the order Chlamydiales. Int. J. Syst. Evol. Microbiol. 2015, 65, 1381–1393. [Google Scholar] [CrossRef] [PubMed]
- Tagini, F.; Greub, G. Bacterial genome sequencing in clinical microbiology: A pathogen-oriented review. Eur. J. Clin. Microbiol. Infect. Dis. 2017, 36, 2007–2020. [Google Scholar] [CrossRef] [PubMed]
- Vanstapel, F.; Orth, M.; Streichert, T.; Capoluongo, E.D.; Oosterhuis, W.P.; Cubukcu, H.C.; Bernabeu-Andreu, F.A.; Thelen, M.; Jacobs, L.H.J.; Linko, S.; et al. ISO 15189 is a sufficient instrument to guarantee high-quality manufacture of laboratory developed tests for in-house-use conform requirements of the European In-Vitro-Diagnostics Regulation. Clin. Chem. Lab. Med. 2023, 61, 608–626. [Google Scholar] [CrossRef] [PubMed]
- Dombrink, I.; Lubbers, B.R.; Simulescu, L.; Doeswijk, R.; Tkachenko, O.; Dequeker, E.; Fraser, A.G.; van Dongen, J.J.M.; Cobbaert, C.; Bruggemann, M.; et al. Critical Implications of IVDR for Innovation in Diagnostics: Input from the BioMed Alliance Diagnostics Task Force. Hemasphere 2022, 6, e724. [Google Scholar] [CrossRef] [PubMed]
| Definition | Designation in EU | Designation in CH |
|---|---|---|
| New regulation text and denomination for in vitro diagnostic medical devices | IVDR | IvDO * |
| Definition of in-house or laboratory-developed tests (LDTs) and obligation to notify | Article 5.5 | Articles 9 (definition) and 10 (notification) |
| Authority to which the notification must be sent | Notified Bodies | Swissmedic |
| In vitro devices | IVDs | IVDs ** |
| Former regulation | IVDD | MedDO |
| IVD classes | IVDR Chapter V, Section 1, Article 47 | IvDO Section 2, art. 14 |
| Type of Devices | Targeted | Unaffected |
|---|---|---|
| Microscope | Gram examination | Confocal microscopy for research purposes |
| Thermocyler | In-house diagnostic PCR | Amplification of a gene for cloning purposes (research use only) |
| Excel file or R pipeline | Calculation of a concentration or parasitemia, comparison of values for test validation | Statistics of ticks Statistical evaluation of results on individual pathogens |
| DNA extraction kit | DNA extracted from a patient sample for diagnostic purposes | DNA extracted from soil to detect microorganisms |
| CE-marked devices |
| Commercial IVD devices used following manufacturer recommendations, including intended use and kit protocol |
| Class | Definition | Examples | |
|---|---|---|---|
| D |
|
| |
|
| ||
|
| ||
| C |
|
|
|
|
| ||
|
| ||
|
|
| |
|
| ||
| B | Devices not covered by the other-mentioned classification rules are classified as class B |
| |
| A |
|
| |
| Instruments intended by the manufacturer specifically to be used for in vitro diagnostic procedures | Enzyme immunoassay analyzer, PCR thermocycler, sequencer for NGS applications, clinical chemistry analyzer.
| ||
| Risk | Reasons Foreseen |
|---|---|
| Costs | Increased workload; additional reagents and control costs |
| Reduction of quality | Less test diversity, fewer laboratories, less innovation, reduced capacity to rapidly react to emerging microbes, decreased skills of co-workers |
| Lack of tests | Decreased diversity of commercial tests and strong pressure against LDTs will increase the demand for the remaining commercially available tests |
| Loss of specialists | The regulatory burden and reduced scope for innovation may make the profession seem unattractive. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Coste, A.T.; Egli, A.; Schrenzel, J.; Nickel, B.; Zbinden, A.; Lienhard, R.; Dumoulin, A.; Risch, M.; Greub, G., on behalf of Coordinated Clinical Commission of Microbiology (CCCM). IVDR: Analysis of the Social, Economic, and Practical Consequences of the Application of an Ordinance of the In Vitro Diagnostic Ordinance in Switzerland. Diagnostics 2023, 13, 2910. https://doi.org/10.3390/diagnostics13182910
Coste AT, Egli A, Schrenzel J, Nickel B, Zbinden A, Lienhard R, Dumoulin A, Risch M, Greub G on behalf of Coordinated Clinical Commission of Microbiology (CCCM). IVDR: Analysis of the Social, Economic, and Practical Consequences of the Application of an Ordinance of the In Vitro Diagnostic Ordinance in Switzerland. Diagnostics. 2023; 13(18):2910. https://doi.org/10.3390/diagnostics13182910
Chicago/Turabian StyleCoste, Alix T., Adrian Egli, Jacques Schrenzel, Beatrice Nickel, Andrea Zbinden, Reto Lienhard, Alexis Dumoulin, Martin Risch, and Gilbert Greub on behalf of Coordinated Clinical Commission of Microbiology (CCCM). 2023. "IVDR: Analysis of the Social, Economic, and Practical Consequences of the Application of an Ordinance of the In Vitro Diagnostic Ordinance in Switzerland" Diagnostics 13, no. 18: 2910. https://doi.org/10.3390/diagnostics13182910
APA StyleCoste, A. T., Egli, A., Schrenzel, J., Nickel, B., Zbinden, A., Lienhard, R., Dumoulin, A., Risch, M., & Greub, G., on behalf of Coordinated Clinical Commission of Microbiology (CCCM). (2023). IVDR: Analysis of the Social, Economic, and Practical Consequences of the Application of an Ordinance of the In Vitro Diagnostic Ordinance in Switzerland. Diagnostics, 13(18), 2910. https://doi.org/10.3390/diagnostics13182910






