Abstract
(1) Background: Although transcatheter aortic valve replacement (TAVR) significantly improves long-term outcomes of symptomatic severe aortic stenosis (AS) patients, long-term mortality rates are still high. The aim of our study was to identify potential inflammatory biomarkers with predictive capacity for post-TAVR adverse events from a wide panel of routine biomarkers by employing ML techniques. (2) Methods: All patients diagnosed with symptomatic severe AS and treated by TAVR since January 2016 in a tertiary center were included in the present study. Three separate analyses were performed: (a) using only inflammatory biomarkers, (b) using inflammatory biomarkers, age, creatinine, and left ventricular ejection fraction (LVEF), and (c) using all collected parameters. (3) Results: A total of 338 patients were included in the study, of which 56 (16.5%) patients died during follow-up. Inflammatory biomarkers assessed using ML techniques have predictive value for adverse events post-TAVR with an AUC-ROC of 0.743 and an AUC-PR of 0.329; most important variables were CRP, WBC count and Neu/Lym ratio. When adding age, creatinine and LVEF to inflammatory panel, the ML performance increased to an AUC-ROC of 0.860 and an AUC-PR of 0.574; even though LVEF was the most important predictor, inflammatory parameters retained their value. When using the entire dataset (inflammatory parameters and complete patient characteristics), the ML performance was the highest with an AUC-ROC of 0.916 and an AUC-PR of 0.676; in this setting, the CRP and Neu/Lym ratio were also among the most important predictors of events. (4) Conclusions: ML models identified the CRP, Neu/Lym ratio, WBC count and fibrinogen as important variables for adverse events post-TAVR.
1. Introduction
Degenerative aortic valve stenosis (AS) is the most commonly acquired valvular heart disease and its prevalence increases with an ageing population [1]. Once AS becomes symptomatic, a poor prognosis is observed, with a survival rate of 30% at 5 years [2]. The only treatment option for decades was surgical aortic valve replacement (SAVR) with good long-term prognosis in ideal candidates. However, the operative risk is heterogenous, significantly increasing with old age and association of cardiac or non-cardiac comorbidities [3], leading to a deferral from SAVR in a third of the patients with symptomatic AS [4]. Transcatheter aortic valve replacement (TAVR) procedure, which was only relatively recently introduced in clinical practice, is nowadays generally accepted as the new standard of care for patients with symptomatic severe AS who are not candidates for open surgery [5]. Although TAVR significantly improves the long-term outcomes of symptomatic severe AS patients, reported 3-years mortality rates are roughly 40% [6,7]. Thus, identifying predictors of adverse events post-TAVR, especially modifiable parameters, is a major clinical desiderate.
Severe AS diagnosis is performed using transthoracic echocardiographic evaluation of the mean aortic transvalvular gradient, peak aortic transvalvular velocity, and aortic valve area [8]. In certain clinical conditions, echocardiography is not enough, and cardiac computed tomography contributes to the final diagnosis. Severe AS is diagnosed in the following three clinical presentations [8]: (1) High-gradient AS—mean aortic transvalvular gradient above 40 mmHg, peak aortic transvalvular velocity above 4.0 m/s, and aortic valve area less than 1 cm2. All high-gradient AS cases are considered severe AS, irrespective of left ventricular ejection fraction (LVEF) or LV flow conditions. (2) Low-flow, low-gradient AS, reduced LVEF—mean aortic transvalvular gradient below 40 mmHg, aortic valve area less than 1 cm2, a LVEF below 50% and an indexed stroke volume less than 35 mL/m2. This clinical instance requires further investigation to determine whether the low aortic valve area is due to low-flow conditions and a dobutamine test should be performed. If under dobutamine, the aortic valve area remains under 1 cm2 with a minimum of 20% increase in stroke volume, severe AS can be considered. (3) Low-flow, low-gradient AS, preserved LVEF—mean aortic transvalvular gradient below 40 mmHg, aortic valve area less than 1 cm2, a LVEF above 50% and an indexed stroke volume less than 35 mL/m2. The definite diagnosis of severe AS is relatively more difficult and prognosis of this clinical form of AS is similar to high-gradient AS [9], although this clinical instance is less frequent. High degrees of aortic valve calcifications at cardiac computed tomography provide important further diagnostic elements [8].
Machine learning (ML) techniques were described decades ago [10], but only recently gained exponential attention because of the increase in computational power and the availability of big data [11]. Machine learning techniques include, but are not limited to, algorithms such as random forest, gradient boosting machines or support vector machines [12]. Machine learning models differ from classical statistical methods such as logistical regression by their capacity to make predictions on unseen data [12]. Machine learning models can be used to perform either classification (binary or multiclass predictions) or regression (predicting a value). The use of ML techniques is appealing because it can effectively handle non-linearity and find complex interaction patterns among numerous variables, thus offering the potential to improve prediction accuracy [12,13]. However, due to its underlying mathematical complexity, ML models are difficult to interpret, being considered a black box [14]. In cardiovascular medicine, ML models can identify complex interactions among clinical variables and make an accurate event prediction [15]. The aim of our study was to identify potential inflammatory biomarkers with predictive capacity for post-TAVR event prediction from a wide panel of routine biomarkers by employing ML techniques.
2. Materials and Methods
All patients diagnosed with symptomatic severe AS and treated by TAVR since January 2016 at the Emergency Institute for Cardiovascular Diseases and Transplantation of Târgu Mureş were included in the present study. Patient data was retrospectively collected and included baseline demographic characteristics, cardiovascular risk factors, comorbidities, laboratory parameters on admission, echocardiographic parameters, coronary anatomy parameters, TAVR-related parameters, and clinical post-procedural evolution. A total of 93 clinical parameters were included in the ML analysis. Patients were not eligible for TAVR procedure if certain criteria were present, such as active infection, severe comorbidities, a high grade of frailty, severely reduced cognitive function, or limited life expectancy, consistent with our institutional TAVR protocol. The Romanian National Health Insurance System database supplied mortality rates for all the patients. For patients who had died during follow-up, the Regional Statistics Office of the Romanian National Institute of Statistics supplied the exact date and cause of death according to the tenth revision of the International Classification of Diseases (ICD-10). All included patients completed informed consent forms. The study was approved by the ethical committee of our institution. The protocol was carried out in accordance with the ethical principles for medical research involving human subjects established by the Declaration of Helsinki, protecting the confidentiality of personal information of the patients.
2.1. Machine Learning
A gradient boosting algorithm (XGBoost) was used to train (1) a model as a binary classifier for predicting 3-year all-cause cause mortality and (2) an accelerated failure time (AFT) model to predict survival. Open-source XGBoost native package was implemented in Python version 3.9. The model was trained using a 5-fold cross-validation technique. Predictions from the testing dataset for all 5 folds were pooled when performance was assessed. Hyperparameter optimization was obtained using grid search technique. No conversion of any data to a specific format was performed and one-hot encoding was used when dealing with categorical variables. Prediction interpretation and visualization was performed using open-source Shapley additive explanations (SHAP) framework that was also implemented in Python version 3.9. Three separate analyses were performed: (1) an analysis using only inflammatory biomarkers, (2) an analysis using inflammatory biomarkers, age, creatinine, and left ventricular ejection fraction (LVEF), and (3) an analysis using all collected parameters.
2.2. Statistical Analysis
A significance level α of 0.05 and a 95% confidence interval (CI) were considered. Continuous variables were evaluated for normal distribution using the Shapiro-Wilk test. Continuous variables with parametric distributions were reported as mean ± standard deviation and compared using a non-paired or paired Student t-test, while continuous variables with non-parametric distributions and discrete variables were reported as the median (interquartile range) and compared using a Mann–Whitney or Wilcoxon test. Categorical variables were reported as absolute and relative frequencies and compared using Fisher exact test for variables with frequencies of less than 5, and a Chi2 test otherwise. The prediction performance of ML models were evaluated using multiple performance metrics: area under the receiver–operator characteristic (AUC-ROC), area under the precision–recall curve (AUC-PR). Statistical analysis was performed using R version 4.1.1 and R Studio version 1.4.17.
3. Results
A total number of 338 patients were included, of which 204 (60.3%) were males, with a median age of 76 (72–80) years and median body mass index of 29.01 ± 4.48 kg/m2. The baseline characteristics of the studied population are reported in Table 1 and the survival curve is illustrated in Figure 1.

Table 1.
Baseline characteristics of the studied population.
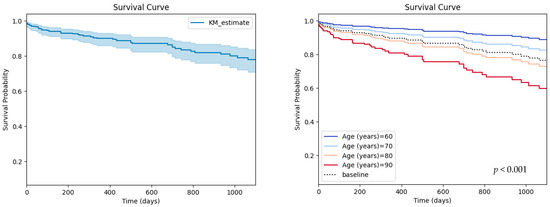
Figure 1.
Survival curve for the studied population (left) and partial effects of age on survival (right).
During follow-up, a total of 56 (16.5%) patients died, of which 3 (0.8%) patients suffered in-hospital death during their initial hospitalization for the TAVR procedure. There was no patient–prosthesis mismatch in the studied population.
Echocardiographic parameters are reported in Table 2. Among significant echocardiographic parameters besides LVEF, left ventricular end-diastolic diameter (LVEDD) was also higher among patients who died during follow-up, while baseline aortic gradients were not predictive of death.

Table 2.
Comparison between pre-procedural echocardiographic parameters among studied groups.
Cardiac computed tomography parameters relevant for the TAVR population are reported in Table 3. Interestingly, none of the baseline LVOT or aortic root parameters were predictive of adverse events, while a higher calcium score of the left main coronary artery was predictive of impaired survival.

Table 3.
Comparison between cardiac computed tomography parameters among studied groups.
A wide range of routinely performed laboratory parameters were determined. Of those, inflammatory related parameters (Table 4) had a predictive value for clinical evolution after TAVR.

Table 4.
Comparison between inflammatory markers among studied groups.
Biochemical parameters with potentially important survival effects are reported in Table 5. Serum creatinine and serum albumin were significantly higher and lower, respectively, in patients who suffered all-cause death during follow-up.

Table 5.
Comparison between biochemical parameters among studied groups.
Machine Learning Assessment
Inflammatory biomarkers assessed using ML techniques had a predictive value for adverse events post-TAVR, with an AUC-ROC of 0.743 and an AUC-PR of 0.329 (Figure 2). When adding age, creatinine and LVEF to the inflammatory panel, the ML performance increased to an AUC-ROC of 0.860 and an AUC-PR of 0.574 (Figure 2). If using the entire dataset (inflammatory parameters and complete patient characteristics), the ML performance was the highest with an AUC-ROC of 0.916 and an AUC-PR of 0.676 (Figure 2).
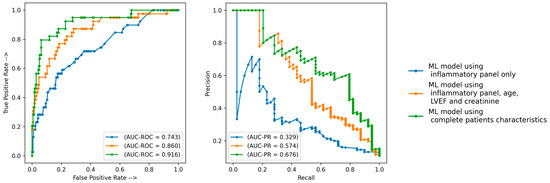
Figure 2.
Prediction performance of the ML models. AUC-PR—area under precision recall curve; AUC-ROC—area under receiver operator curve; ML—machine learning.
Of note, the tuned hyperparameters of the final models included (1) a total of 300 decision trees aggregated, (2) a tree depth of four levels and (3) a learning rate of 0.01. The ML decision process can be understood using Shapley values [16]. Initially, the ML model ranks the most important variables for the prediction of mortality (Figure 3). On the dataset with only inflammatory markers, C-reactive protein (CRP), white blood cells (WBC) count and Neu/Lym ratio were the three most important features (Figure 3A). On the dataset with inflammatory markers, age, creatinine and left ventricular ejection fraction (LVEF), LVEF, CRP and WBC were the three most important features (Figure 3B). On the dataset with complete patient characteristics, left ventricular end-diastolic diameter (LVEDD), LVEF, CRP and Neu/Lym ratio were the most important features (Figure 3C). Afterwards, each variable was assigned a SHAP value for a particular variable value. A lower SHAP value is protective, while a higher score reflects impaired prognosis. In Figure 3, the x-axis reflects SHAP values, while each parameter has a blue and red side reflecting lower and higher parameter values, respectively. For instance, the blue side of the LVEDD parameter reflects lower LVEDD values and is on negative side of SHAP values; thus, there is a better prognosis when the LVEDD is lower. In contrast, the blue side of the LVEF parameter reflects higher LVEF values and is on the positive side of SHAP values; thus, there is a worse prognosis when the LVEF is lower. The dependence plots between predictor value and SHAP value for the most important variables are illustrated in Figure 4.
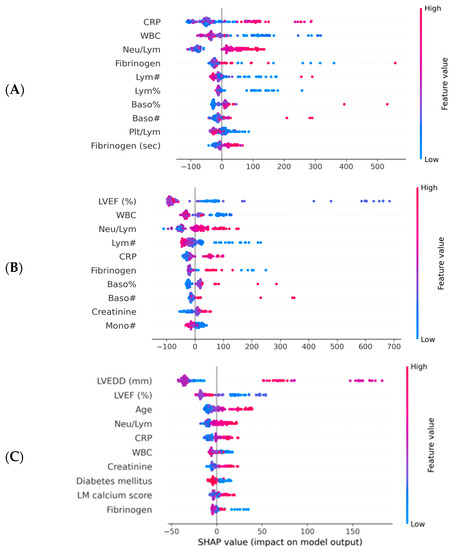
Figure 3.
Importance plots for the ML models. CRP—C reactive protein; LM—left main artery; LVEF—left ventricular ejection fraction; LVEDD—left ventricular end diastolic diameter; ML—machine learning; WBC—white blood cells. (A) Importance plot for ML model applied to dataset with inflammatory markers. (B) Importance plot for ML model applied to dataset with inflammatory markers, LFEV, age and creatinine. (C) Importance plot for ML model applied to entire dataset.
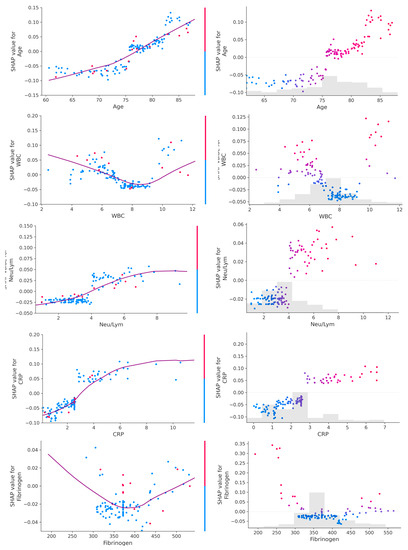
Figure 4.
Dependance plot (left) and scatter plot (right) for age and inflammatory parameters. A linear relationship can be observed between values of age, CRP and Neu/Lym ratio and their SHAP values, while a bimodal relationship can be observed between values of WBC and fibrinogen and their SHAP values.
4. Discussion
The main findings of our study can be summarized as follows: (1) inflammatory biomarkers assessed using ML techniques have predictive value for adverse events post-TAVR, with an AUC-ROC of 0.743 and an AUC-PR of 0.329; the most important variables were CRP, WBC count and the Neu/Lym ratio. (2) When adding age, creatinine and LVEF to an inflammatory panel, the ML performance increased to an AUC-ROC of 0.860 and an AUC-PR of 0.574; even though the LVEF was the most important predictor, inflammatory parameters retained their value. (3) When using the entire dataset (inflammatory parameters and complete patient characteristics), the ML performance was the highest, with an AUC-ROC of 0.916 and an AUC-PR of 0.676; in this setting, the CRP and Neu/Lym ratio were also among the most important predictors of events. (4) When using SHAP values to explain the outcomes, with the increase in age, CRP and Neu/Lym ratio, an increase in event risk was also observed, while for WBC count and fibrinogen levels, a bimodal relationship was observed—the event risk was higher for both low and high levels of both WBC and fibrinogen. Even though numerous echocardiographic and cardiac computed tomography parameters were also included in the final analysis, only the LVEF and LVEDD were among the most important predictors. Besides inflammatory markers, it is not surprising that age, echocardiographic parameters, and creatinine are other important clinical parameters, as it is commonly known that they are the main survival determinants of heart disease populations. Our study supports the concept of precision phenotyping—AI and ML techniques can find complex patterns and interactions among clinical parameters that are invisible or unimportant to the clinician. The performance analysis of the ML models showed that an accurate prognosis estimate was given from routine biomarkers. The performance in survival prediction was reflected not only by the AUC-ROC, but also by the AUC-PR, a better metric for imbalanced datasets (e.g., deceased patients were fewer than alive patients) [17,18,19].
Undisputedly, TAVR offers both short-term and long-term advantages over SAVR, especially in high-risk patients, but post-TAVR evolution does not lack adverse events. Growing evidence suggests that inflammation status both before and after TAVR is an important predictor of adverse outcomes. High levels of biomarkers such as CRP, GDF-15 or IL-8 were associated with a 1-year mortality after TAVR [20]. Similarly, impaired platelet activity after TAVR was also a predictor of mortality [21]. In our study, the CRP and Neu/Lym ratio were higher in patients who died during follow-up. Moreover, by employing ML models, a bimodal relationship was observed for WBC count—lower and higher values were associated with impaired survival (Table 3). This relationship was not observed when alive versus deceased patients were compared (Table 2). All included patients in the present study underwent transfemoral TAVR approach. A recent study reported that the inflammatory response was significantly lower in transfemoral TAVR compared to both SAVR and apical TAVR [22]. This reduced response in the context of transfemoral TAVR may also be responsible for the improved evolution of patients treated with this strategy. Noteworthy, patients were not subjected to TAVR procedure if there was an active infection or inflammation as per our institutional protocol and clinical guidelines. Our findings suggest that even subclinical inflammation assessed by routine biomarkers has important prognostic value.
Inflammation is an important element in atherosclerotic disease that leads to aortic valve degeneration, thus being a potential and attractive pharmacologic target. In current clinical practice, pharmacological treatment in the context of symptomatic AS is directed to treating comorbidities since no pharmacological agent improves the clinical course of AS per se. The same principle is applied post-TAVR, with the exception of empirical double antiplatelet therapy for 3–6 months followed by indefinite single platelet inhibitor treatment [23]. Our observations, along with other evidence from literature, could sustain the hypothesis of a beneficial effect exerted by anti-inflammatory medication. Indeed, certain anti-inflammatory agents reduced the risk of cardiovascular events, such as colchicine, in the context of coronary artery disease [24].
The 3-year all-cause mortality or stroke rate was roughly 9% lower in TAVR versus SAVR high-risk patients [25]. However, TAVR mortality during follow-up was still high, at 32.9% in the same study [25]. In our study, mortality during follow-up was lower; however, the included population was also smaller. Nevertheless, the ML model, using all the clinical characteristics, predicted mortality with an AUC-ROC of 0.916. Low LVEF and high LVEDD were the two most important predictors, followed by a CRP and Neu/Lym ratio. A large meta-analysis also showed the impaired prognosis associated with low LVEF [26]. This is not surprising, since LVEF is the most important parameter of cardiac systolic function.
Our study is limited by the relatively small size of the study population, using all-cause instead of cardiovascular-cause mortality, and a lack of frailty scores. Including more patients would increase the statistical power of the study. However, some clear trends were observed for the studied parameters (Table 3).
5. Conclusions
Identifying predictors, especially modifiable ones, for impaired survival after TAVR for symptomatic severe AS is an important objective in contemporary cardiovascular medicine, since post-TAVR mortality is still relatively high. Inflammatory status assessment could provide such predictors. In our study, the ML models identified the CRP, Neu/Lym ratio, WBC count and fibrinogen as important variables for adverse events.
Author Contributions
Conceptualization, M.M.; methodology, P.-A.C.; software, P.-A.C.; formal analysis, P.-A.C., M.M. and A.S.; investigation, A.S. and H.S.; resources, A.S. and H.S.; data curation, R.-K.D.; writing—original draft preparation, P.-A.C. and A.S.; writing—review and editing, all authors; visualization, P.-A.C.; supervision, M.M. and H.S.; project administration, H.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
This study was conducted in accordance with the Declaration of Helsinki and approved by the Institutional Ethics Committee of the Emergency Institute for Cardiovascular Diseases and Transplantation Târgu Mureș, Târgu Mureș, Romania (1397/20 February 2023).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Data available on request. The data and scripts underlying this article will be shared upon reasonable request by the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Nkomo, V.T.; Gardin, J.M.; Skelton, T.N.; Gottdiener, J.S.; Scott, C.G.; Enriquez-Sarano, M. Burden of valvular heart diseases: A population-based study. Lancet Lond Engl. 2006, 368, 1005–1011. [Google Scholar] [CrossRef]
- Ross, J.; Braunwald, E. Aortic stenosis. Circulation 1968, 38, 61–67. [Google Scholar] [CrossRef] [PubMed]
- Otto, C.M. Valvular aortic stenosis: Disease severity and timing of intervention. J. Am. Coll. Cardiol. 2006, 47, 2141–2151. [Google Scholar] [CrossRef] [PubMed]
- Iung, B.; Baron, G.; Butchart, E.G.; Delahaye, F.; Gohlke-Bärwolf, C.; Levang, O.W.; Tornos, P.; Vanoverschelde, J.-L.; Vermeer, F.; Boersma, E.; et al. A prospective survey of patients with valvular heart disease in Europe: The Euro Heart Survey on Valvular Heart Disease. Eur. Heart J. 2003, 24, 1231–1243. [Google Scholar] [CrossRef]
- Webb, J.G.; Wood, D.A. Current Status of Transcatheter Aortic Valve Replacement. J. Am. Coll. Cardiol. 2012, 60, 483–492. [Google Scholar] [CrossRef] [PubMed]
- Gurvitch, R.; Wood, D.A.; Tay, E.L.; Leipsic, J.; Ye, J.; Lichtenstein, S.V.; Thompson, C.R.; Carere, R.G.; Wijesinghe, N.; Nietlispach, F.; et al. Transcatheter Aortic Valve Implantation. Circulation 2010, 122, 1319–1327. [Google Scholar] [CrossRef] [PubMed]
- Ussia, G.P.; Barbanti, M.; Petronio, A.S.; Tarantini, G.; Ettori, F.; Colombo, A.; Violini, R.; Ramondo, A.; Santoro, G.; Klugmann, S.; et al. Transcatheter aortic valve implantation: 3-year outcomes of self-expanding CoreValve prosthesis. Eur. Heart J. 2012, 33, 969–976. [Google Scholar] [CrossRef]
- Vahanian, A.; Beyersdorf, F.; Praz, F.; Milojevic, M.; Baldus, S.; Bauersachs, J.; Capodanno, D.; Conradi, L.; De Bonis, M.; De Paulis, R.; et al. 2021 ESC/EACTS Guidelines for the management of valvular heart disease: Developed by the Task Force for the management of valvular heart disease of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS). Eur. Heart J. 2022, 43, 561–632. [Google Scholar] [CrossRef]
- Tribouilloy, C.; Rusinaru, D.; Maréchaux, S.; Castel, A.-L.; Debry, N.; Maizel, J.; Mentaverri, R.; Kamel, S.; Slama, M.; Lévy, F. Low-Gradient, Low-Flow Severe Aortic Stenosis With Preserved Left Ventricular Ejection Fraction. J. Am. Coll. Cardiol. 2015, 65, 55–66. [Google Scholar] [CrossRef]
- Breiman, L. Classification and Regression Trees; Routledge: New York, NY, USA, 1984; ISBN 978-1-315-13947-0. [Google Scholar] [CrossRef]
- Hussain, I.; Park, S.J. Big-ECG: Cardiographic Predictive Cyber-Physical System for Stroke Management. IEEE Access 2021, 9, 123146–123164. [Google Scholar] [CrossRef]
- Jordan, M.I.; Mitchell, T.M. Machine learning: Trends, perspectives, and prospects. Science 2015, 349, 255–260. [Google Scholar] [CrossRef] [PubMed]
- Ghahramani, Z. Probabilistic machine learning and artificial intelligence. Nature 2015, 521, 452–459. [Google Scholar] [CrossRef] [PubMed]
- Savage, N. Breaking into the black box of artificial intelligence. Nature 2022. [Google Scholar] [CrossRef] [PubMed]
- Călburean, P.-A.; Grebenișan, P.; Nistor, I.-A.; Pal, K.; Vacariu, V.; Drincal, R.-K.; Țepes, O.; Bârlea, I.; Șuș, I.; Somkereki, C.; et al. Prediction of 3-year all-cause and cardiovascular cause mortality in a prospective percutaneous coronary intervention registry: Machine learning model outperforms conventional clinical risk scores. Atherosclerosis 2022, 350, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Lundberg, S.; Lee, S.-I. A Unified Approach to Interpreting Model Predictions. arXiv 2017, arXiv:1705.07874. [Google Scholar]
- Saito, T.; Rehmsmeier, M. The Precision-Recall Plot Is More Informative than the ROC Plot When Evaluating Binary Classifiers on Imbalanced Datasets. PLoS ONE 2015, 10, e0118432. [Google Scholar] [CrossRef]
- Călburean, P.-A.; Osorio, T.G.; Sorgente, A.; Almorad, A.; Pannone, L.; Monaco, C.; Miraglia, V.; Al Housari, M.; Mojica, J.; Bala, G. High vagal tone predicts pulmonary vein reconnection after cryoballoon ablation for paroxysmal atrial fibrillation. Pacing Clin. Electrophysiol. 2021, 44, 2075–2083. [Google Scholar] [CrossRef]
- Călburean, P.-A.; Pannone, L.; Sorgente, A.; Gauthey, A.; Monaco, C.; Strazdas, A.; Almorad, A.; Bisignani, A.; Bala, G.; Ramak, R.; et al. Heart rate variability and microvolt T wave alternans changes during ajmaline test may predict prognosis in Brugada syndrome. Clin. Auton. Res. 2023, 33, 51–62. [Google Scholar] [CrossRef]
- Sinning, J.-M.; Wollert, K.C.; Sedaghat, A.; Widera, C.; Radermacher, M.-C.; Descoups, C.; Hammerstingl, C.; Weber, M.; Stundl, A.; Ghanem, A.; et al. Risk scores and biomarkers for the prediction of 1-year outcome after transcatheter aortic valve replacement. Am. Heart J. 2015, 170, 821–829. [Google Scholar] [CrossRef]
- Sexton, T.R.; Wallace, E.L.; Chen, A.; Charnigo, R.J.; Reda, H.K.; Ziada, K.M.; Gurley, J.C.; Smyth, S.S. Thromboinflammatory response and predictors of outcomes in patients undergoing transcatheter aortic valve replacement. J. Thromb. Thrombolysis 2016, 41, 384–393. [Google Scholar] [CrossRef]
- Uhle, F.; Castrup, C.; Necaev, A.-M.; Grieshaber, P.; Lichtenstern, C.; Weigand, M.A.; Böning, A. Inflammation and Its Consequences After Surgical Versus Transcatheter Aortic Valve Replacement. Artif. Organs 2018, 42, E1–E12. [Google Scholar] [CrossRef] [PubMed]
- Guedeney, P.; Mehran, R.; Collet, J.-P.; Claessen, B.E.; ten Berg, J.; Dangas, G.D. Antithrombotic Therapy After Transcatheter Aortic Valve Replacement. Circ. Cardiovasc. Interv. 2019, 12, e007411. [Google Scholar] [CrossRef] [PubMed]
- Nidorf, S.M.; Fiolet, A.T.L.; Mosterd, A.; Eikelboom, J.W.; Schut, A.; Opstal, T.S.J.; The, S.H.K.; Xu, X.-F.; Ireland, M.A.; Lenderink, T.; et al. Colchicine in Patients with Chronic Coronary Disease. N. Engl. J. Med. 2020, 383, 1838–1847. [Google Scholar] [CrossRef] [PubMed]
- Deeb, G.M.; Reardon, M.J.; Chetcuti, S.; Patel, H.J.; Grossman, P.M.; Yakubov, S.J.; Kleiman, N.S.; Coselli, J.S.; Gleason, T.G.; Lee, J.S.; et al. 3-Year Outcomes in High-Risk Patients Who Underwent Surgical or Transcatheter Aortic Valve Replacement. J. Am. Coll. Cardiol. 2016, 67, 2565–2574. [Google Scholar] [CrossRef]
- Sannino, A.; Gargiulo, G.; Schiattarella, G.G.; Brevetti, L.; Perrino, C.; Stabile, E.; Losi, M.A.; Toscano, E.; Giugliano, G.; Scudiero, F.; et al. Increased mortality after transcatheter aortic valve implantation (TAVI) in patients with severe aortic stenosis and low ejection fraction: A meta-analysis of 6898 patients. Int. J. Cardiol. 2014, 176, 32–39. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).