Arrhythmic Mitral Valve Prolapse: A Comprehensive Review
Abstract
1. Introduction
1.1. Historical Background
1.2. Epidemiology
1.3. Pathology
1.4. Clinical Manifestations
1.5. Electrocardiography
1.6. Mechanism of VAs Occurrence
2. Multimodality Imaging of MVP
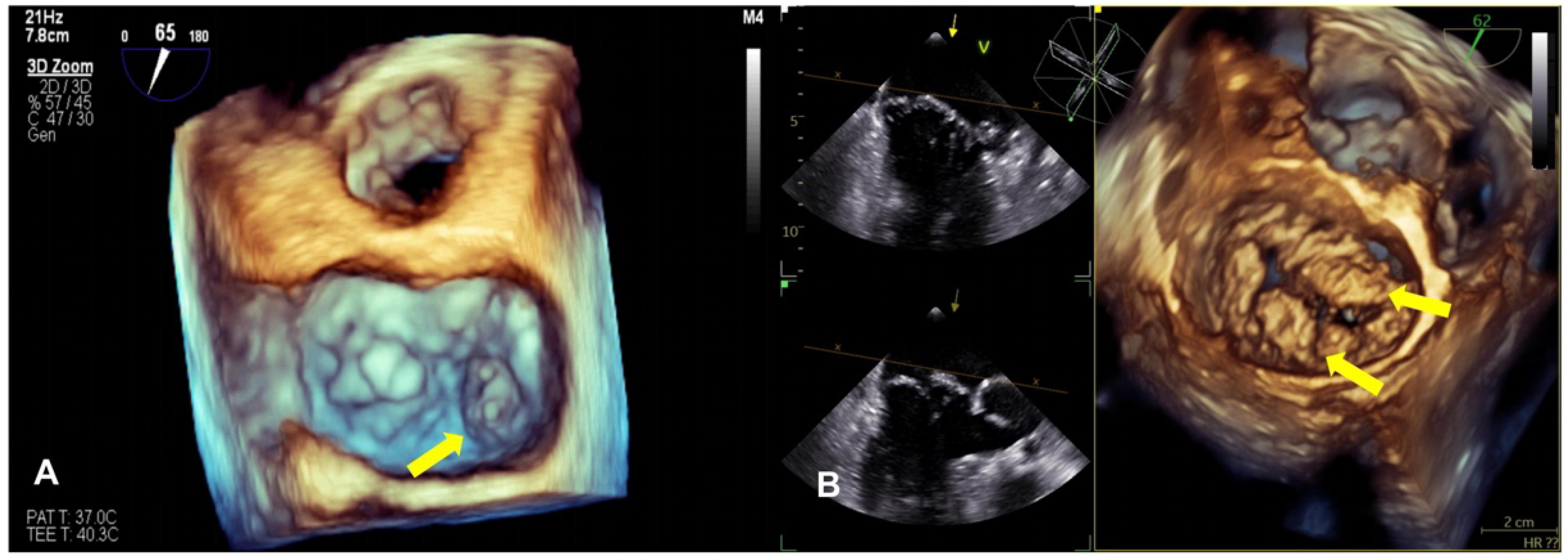
2.1. Echocardiography
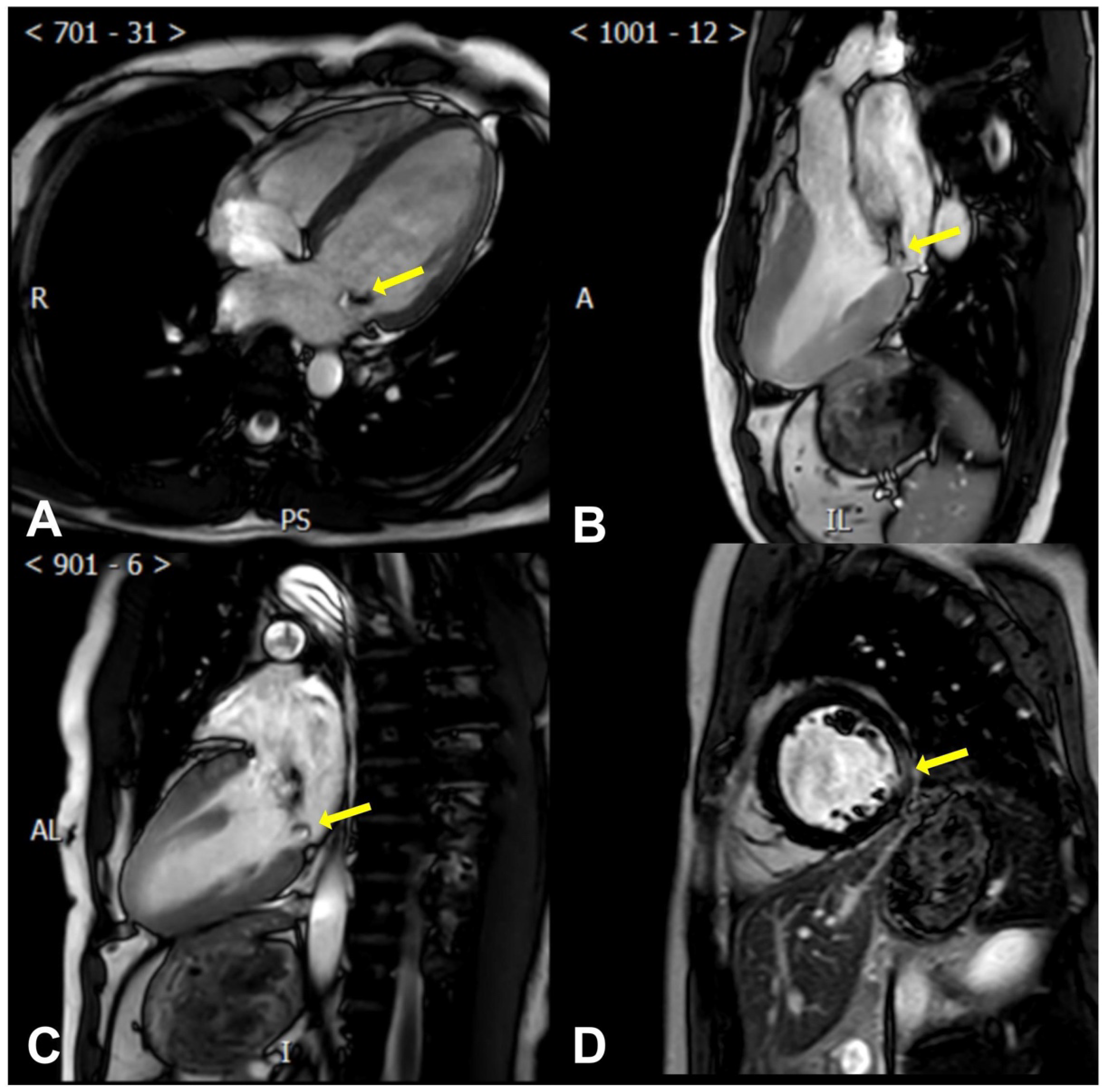
2.2. Cardiac Magnetic Resonance
2.3. Computed Tomography
2.4. Mitral Annular Disjunction
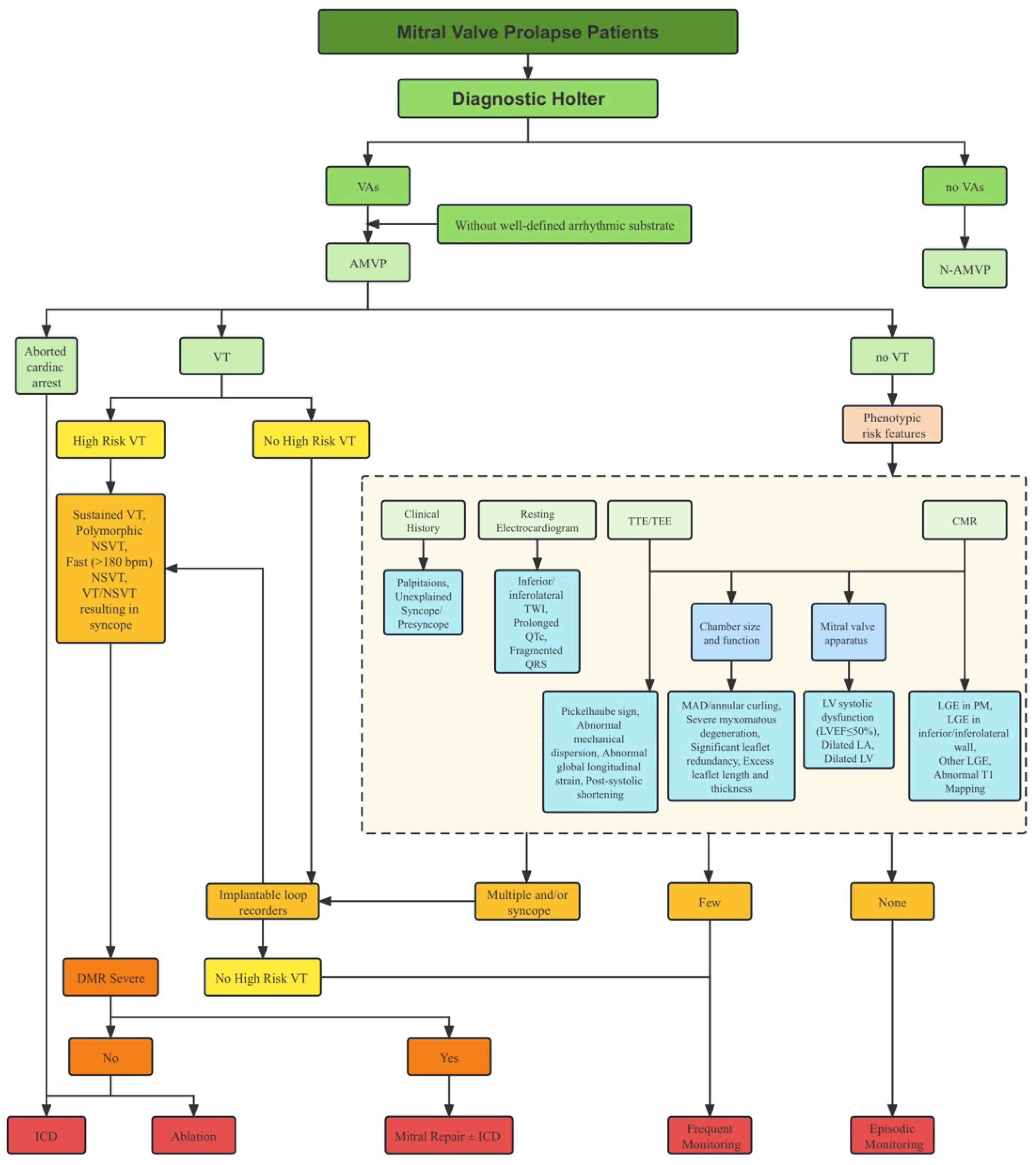
2.5. Risk Stratification
2.6. Management
3. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AMVP | arrhythmic mitral valve prolapse |
| BiMVP | bileaflet mitral valve prolapse |
| CA | cardiac arrest |
| CMR | cardiac magnetic resonance |
| CT | computed tomography |
| ECG | electrocardiogram |
| EPS | electrophysiologic studies |
| ICD | implantable cardioverter defibrillator |
| LA | left atrium |
| LGE | late gadolinium enhancement |
| LV | left ventricle |
| MAD | mitral annular disjunction |
| MR | mitral regurgitation |
| MVP | mitral valve prolapse |
| NSVT | non-sustained ventricular tachycardia |
| PM | papillary muscle |
| PVC | premature ventricular contraction |
| SCD | sudden cardiac death |
| SiMVP | single-leaflet mitral valve prolapse |
| TEE | transesophageal echocardiography |
| TTE | transthoracic echocardiography |
| TWI | T wave inversion |
| VA | ventricular arrhythmia |
| VF | ventricular fibrillation |
| VT | ventricular tachycardia |
References
- Freed, L.A.; Levy, D.; Levine, R.A.; Larson, M.G.; Evans, J.C.; Fuller, D.L.; Lehman, B.; Benjamin, E.J. Prevalence and clinical outcome of mitral-valve prolapse. N. Engl. J. Med. 1999, 341, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Devereux, R.B.; Jones, E.C.; Roman, M.J.; Howard, B.V.; Fabsitz, R.R.; Liu, J.E.; Palmieri, V.; Welty, T.K.; Lee, E.T. Prevalence and correlates of mitral valve prolapse in a population-based sample of American Indians: The Strong Heart Study. Am. J. Med. 2001, 111, 679–685. [Google Scholar] [CrossRef] [PubMed]
- Delling, F.N.; Vasan, R.S. Epidemiology and pathophysiology of mitral valve prolapse: New insights into disease progression, genetics, and molecular basis. Circulation 2014, 129, 2158–2170. [Google Scholar] [CrossRef] [PubMed]
- Rabkin, E.; Aikawa, M.; Stone, J.R.; Fukumoto, Y.; Libby, P.; Schoen, F.J. Activated interstitial myofibroblasts express catabolic enzymes and mediate matrix remodeling in myxomatous heart valves. Circulation 2001, 104, 2525–2532. [Google Scholar] [CrossRef]
- Tamura, K.; Fukuda, Y.; Ishizaki, M.; Masuda, Y.; Yamanaka, N.; Ferrans, V.J. Abnormalities in elastic fibers and other connective-tissue components of floppy mitral valve. Am. Heart J. 1995, 129, 1149–1158. [Google Scholar] [CrossRef] [PubMed]
- Delling, F.N.; Rong, J.; Larson, M.G.; Lehman, B.; Fuller, D.; Osypiuk, E.; Stantchev, P.; Hackman, B.; Manning, W.J.; Benjamin, E.J.; et al. Evolution of Mitral Valve Prolapse: Insights From the Framingham Heart Study. Circulation 2016, 133, 1688–1695. [Google Scholar] [CrossRef]
- Avierinos, J.F.; Gersh, B.J.; Melton, L.J., 3rd; Bailey, K.R.; Shub, C.; Nishimura, R.A.; Tajik, A.J.; Enriquez-Sarano, M. Natural history of asymptomatic mitral valve prolapse in the community. Circulation 2002, 106, 1355–1361. [Google Scholar] [CrossRef]
- Freed, L.A.; Benjamin, E.J.; Levy, D.; Larson, M.G.; Evans, J.C.; Fuller, D.L.; Lehman, B.; Levine, R.A. Mitral valve prolapse in the general population: The benign nature of echocardiographic features in the Framingham Heart Study. J. Am. Coll. Cardiol. 2002, 40, 1298–1304. [Google Scholar] [CrossRef]
- Coutsoumbas, G.V.; Di Pasquale, G. Mitral valve prolapse with ventricular arrhythmias: Does it carries a worse prognosis? Eur. Heart J. Suppl. 2021, 23 (Suppl. E), E77–E82. [Google Scholar] [CrossRef]
- Basso, C.; Iliceto, S.; Thiene, G.; Perazzolo Marra, M. Mitral Valve Prolapse, Ventricular Arrhythmias, and Sudden Death. Circulation 2019, 140, 952–964. [Google Scholar] [CrossRef]
- Hayek, E.; Gring, C.N.; Griffin, B.P. Mitral valve prolapse. Lancet 2005, 365, 507–518. [Google Scholar] [CrossRef]
- Miller, M.A.; Dukkipati, S.R.; Turagam, M.; Liao, S.L.; Adams, D.H.; Reddy, V.Y. Arrhythmic Mitral Valve Prolapse: JACC Review Topic of the Week. J. Am. Coll. Cardiol. 2018, 72, 2904–2914. [Google Scholar] [CrossRef]
- Delling, F.N.; Aung, S.; Vittinghoff, E.; Dave, S.; Lim, L.J.; Olgin, J.E.; Connolly, A.; Moffatt, E.; Tseng, Z.H. Antemortem and Post-Mortem Characteristics of Lethal Mitral Valve Prolapse Among All Countywide Sudden Deaths. JACC Clin. Electrophysiol. 2021, 7, 1025–1034. [Google Scholar] [CrossRef] [PubMed]
- Sriram, C.S.; Syed, F.F.; Ferguson, M.E.; Johnson, J.N.; Enriquez-Sarano, M.; Cetta, F.; Cannon, B.C.; Asirvatham, S.J.; Ackerman, M.J. Malignant bileaflet mitral valve prolapse syndrome in patients with otherwise idiopathic out-of-hospital cardiac arrest. J. Am. Coll. Cardiol. 2013, 62, 222–230. [Google Scholar] [CrossRef] [PubMed]
- Basso, C.; Perazzolo Marra, M.; Rizzo, S.; De Lazzari, M.; Giorgi, B.; Cipriani, A.; Frigo, A.C.; Rigato, I.; Migliore, F.; Pilichou, K.; et al. Arrhythmic Mitral Valve Prolapse and Sudden Cardiac Death. Circulation 2015, 132, 556–566. [Google Scholar] [CrossRef]
- Narayanan, K.; Uy-Evanado, A.; Teodorescu, C.; Reinier, K.; Nichols, G.A.; Gunson, K.; Jui, J.; Chugh, S.S. Mitral valve prolapse and sudden cardiac arrest in the community. Heart Rhythm. 2016, 13, 498–503. [Google Scholar] [CrossRef]
- Han, H.C.; Ha, F.J.; Teh, A.W.; Calafiore, P.; Jones, E.F.; Johns, J.; Koshy, A.N.; O’Donnell, D.; Hare, D.L.; Farouque, O.; et al. Mitral Valve Prolapse and Sudden Cardiac Death: A Systematic Review. J. Am. Heart Assoc. 2018, 7, e010584. [Google Scholar] [CrossRef] [PubMed]
- Nalliah, C.J.; Mahajan, R.; Elliott, A.D.; Haqqani, H.; Lau, D.H.; Vohra, J.K.; Morton, J.B.; Semsarian, C.; Marwick, T.; Kalman, J.M.; et al. Mitral valve prolapse and sudden cardiac death: A systematic review and meta-analysis. Heart 2019, 105, 144–151. [Google Scholar] [CrossRef] [PubMed]
- Barlow, J.B.; Bosman, C.K. Aneurysmal protrusion of the posterior leaflet of the mitral valve. An auscultatory-electrocardiographic syndrome. Am. Heart J. 1966, 71, 166–178. [Google Scholar] [CrossRef]
- Criley, J.M.; Lewis, K.B.; Humphries, J.O.; Ross, R.S. Prolapse of the mitral valve: Clinical and cine-angiocardiographic findings. Br. Heart J. 1966, 28, 488–496. [Google Scholar] [CrossRef] [PubMed]
- Winkle, R.A.; Lopes, M.G.; Fitzgerald, J.W.; Goodman, D.J.; Schroeder, J.S.; Harrison, D.C. Arrhythmias in patients with mitral valve prolapse. Circulation 1975, 52, 73–81. [Google Scholar] [CrossRef] [PubMed]
- DeMaria, A.N.; Amsterdam, E.A.; Vismara, L.A.; Neumann, A.; Mason, D.T. Arrhythmias in the mitral valve prolapse syndrome. Prevalence, nature, and frequency. Ann. Intern. Med. 1976, 84, 656–660. [Google Scholar] [CrossRef] [PubMed]
- Sabbag, A.; Essayagh, B.; Barrera, J.D.R.; Basso, C.; Berni, A.; Cosyns, B.; Deharo, J.C.; Deneke, T.; Di Biase, L.; Enriquez-Sarano, M.; et al. EHRA expert consensus statement on arrhythmic mitral valve prolapse and mitral annular disjunction complex in collaboration with the ESC Council on valvular heart disease and the European Association of Cardiovascular Imaging endorsed cby the Heart Rhythm Society, by the Asia Pacific Heart Rhythm Society, and by the Latin American Heart Rhythm Society. Europace 2022, 24, 1981–2003. [Google Scholar] [CrossRef] [PubMed]
- Hourdain, J.; Clavel, M.A.; Deharo, J.C.; Asirvatham, S.; Avierinos, J.F.; Habib, G.; Franceschi, F.; Probst, V.; Sadoul, N.; Martins, R.; et al. Common Phenotype in Patients With Mitral Valve Prolapse Who Experienced Sudden Cardiac Death. Circulation 2018, 138, 1067–1069. [Google Scholar] [CrossRef]
- Han, H.C.; Parsons, S.A.; Teh, A.W.; Sanders, P.; Neil, C.; Leong, T.; Koshy, A.N.; Vohra, J.K.; Kalman, J.M.; Smith, K.; et al. Characteristic Histopathological Findings and Cardiac Arrest Rhythm in Isolated Mitral Valve Prolapse and Sudden Cardiac Death. J. Am. Heart Assoc. 2020, 9, e015587. [Google Scholar] [CrossRef]
- Essayagh, B.; Sabbag, A.; Antoine, C.; Benfari, G.; Yang, L.T.; Maalouf, J.; Asirvatham, S.; Michelena, H.; Enriquez-Sarano, M. Presentation and Outcome of Arrhythmic Mitral Valve Prolapse. J. Am. Coll. Cardiol. 2020, 76, 637–649. [Google Scholar] [CrossRef]
- Kyndt, F.; Gueffet, J.P.; Probst, V.; Jaafar, P.; Legendre, A.; Le Bouffant, F.; Toquet, C.; Roy, E.; McGregor, L.; Lynch, S.A.; et al. Mutations in the gene encoding filamin A as a cause for familial cardiac valvular dystrophy. Circulation 2007, 115, 40–49. [Google Scholar] [CrossRef]
- Boudoulas, K.D.; Pitsis, A.A.; Mazzaferri, E.L.; Gumina, R.J.; Triposkiadis, F.; Boudoulas, H. Floppy mitral valve/mitral valve prolapse: A complex entity with multiple genotypes and phenotypes. Prog. Cardiovasc. Dis. 2020, 63, 308–326. [Google Scholar] [CrossRef]
- Adams, D.H.; Rosenhek, R.; Falk, V. Degenerative mitral valve regurgitation: Best practice revolution. Eur. Heart J. 2010, 31, 1958–1966. [Google Scholar] [CrossRef]
- Nordhues, B.D.; Siontis, K.C.; Scott, C.G.; Nkomo, V.T.; Ackerman, M.J.; Asirvatham, S.J.; Noseworthy, P.A. Bileaflet Mitral Valve Prolapse and Risk of Ventricular Dysrhythmias and Death. J. Cardiovasc. Electrophysiol. 2016, 27, 463–468. [Google Scholar] [CrossRef]
- Faletra, F.F.; Leo, L.A.; Paiocchi, V.L.; Caretta, A.; Viani, G.M.; Schlossbauer, S.A.; Demertzis, S.; Ho, S.Y. Anatomy of mitral annulus insights from non-invasive imaging techniques. Eur. Heart J. Cardiovasc. Imaging 2019, 20, 843–857. [Google Scholar] [CrossRef] [PubMed]
- Bharati, S.; Granston, A.S.; Liebson, P.R.; Loeb, H.S.; Rosen, K.M.; Lev, M. The conduction system in mitral valve prolapse syndrome with sudden death. Am. Heart J. 1981, 101, 667–670. [Google Scholar] [CrossRef] [PubMed]
- Hutchins, G.M.; Moore, G.W.; Skoog, D.K. The association of floppy mitral valve with disjunction of the mitral annulus fibrosus. N. Engl. J. Med. 1986, 314, 535–540. [Google Scholar] [CrossRef]
- Carmo, P.; Andrade, M.J.; Aguiar, C.; Rodrigues, R.; Gouveia, R.; Silva, J.A. Mitral annular disjunction in myxomatous mitral valve disease: A relevant abnormality recognizable by transthoracic echocardiography. Cardiovasc. Ultrasound 2010, 8, 53. [Google Scholar] [CrossRef] [PubMed]
- Dejgaard, L.A.; Skjølsvik, E.T.; Lie, Ø.H.; Ribe, M.; Stokke, M.K.; Hegbom, F.; Scheirlynck, E.S.; Gjertsen, E.; Andresen, K.; Helle-Valle, T.M.; et al. The Mitral Annulus Disjunction Arrhythmic Syndrome. J. Am. Coll. Cardiol. 2018, 72, 1600–1609. [Google Scholar] [CrossRef] [PubMed]
- Perazzolo Marra, M.; Basso, C.; De Lazzari, M.; Rizzo, S.; Cipriani, A.; Giorgi, B.; Lacognata, C.; Rigato, I.; Migliore, F.; Pilichou, K.; et al. Morphofunctional Abnormalities of Mitral Annulus and Arrhythmic Mitral Valve Prolapse. Circ. Cardiovasc. Imaging 2016, 9, e005030. [Google Scholar] [CrossRef] [PubMed]
- Faletra, F.F.; Leo, L.A.; Paiocchi, V.L.; Schlossbauer, S.A.; Pavon, A.G.; Ho, S.Y.; Maisano, F. Morphology of Mitral Annular Disjunction in Mitral Valve Prolapse. J. Am. Soc. Echocardiogr. 2022, 35, 176–186. [Google Scholar] [CrossRef]
- Lee, A.P.; Jin, C.N.; Fan, Y.; Wong, R.H.L.; Underwood, M.J.; Wan, S. Functional Implication of Mitral Annular Disjunction in Mitral Valve Prolapse: A Quantitative Dynamic 3D Echocardiographic Study. JACC Cardiovasc. Imaging 2017, 10, 1424–1433. [Google Scholar] [CrossRef]
- Essayagh, B.; Sabbag, A.; Antoine, C.; Benfari, G.; Batista, R.; Yang, L.T.; Maalouf, J.; Thapa, P.; Asirvatham, S.; Michelena, H.I.; et al. The Mitral Annular Disjunction of Mitral Valve Prolapse: Presentation and Outcome. JACC Cardiovasc. Imaging 2021, 14, 2073–2087. [Google Scholar] [CrossRef]
- Chakrabarti, A.K.; Deshmukh, A.; Liang, J.J.; Madamanchi, C.; Ghannam, M.; Morady, F.; Bogun, F. Mitral Annular Substrate and Ventricular Arrhythmias in Arrhythmogenic Mitral Valve Prolapse With Mitral Annular Disjunction. JACC Clin. Electrophysiol. 2023, 9, 1265–1275. [Google Scholar] [CrossRef]
- Konda, T.; Tani, T.; Suganuma, N.; Nakamura, H.; Sumida, T.; Fujii, Y.; Kawai, J.; Kitai, T.; Kim, K.; Kaji, S.; et al. The analysis of mitral annular disjunction detected by echocardiography and comparison with previously reported pathological data. J. Echocardiogr. 2017, 15, 176–185. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, B.W.; Schatz, R.A.; VonRamm, O.T.; Behar, V.S.; Kisslo, J.A. Mitral valve prolapse. Two-dimensional echocardiographic and angiographic correlation. Circulation 1976, 54, 716–723. [Google Scholar] [CrossRef] [PubMed]
- Savage, D.D.; Devereux, R.B.; Garrison, R.J.; Castelli, W.P.; Anderson, S.J.; Levy, D.; Thomas, H.E.; Kannel, W.B.; Feinleib, M. Mitral valve prolapse in the general population. 2. Clinical features: The Framingham Study. Am. Heart J. 1983, 106, 577–581. [Google Scholar] [CrossRef] [PubMed]
- Devereux, R.B.; Kramer-Fox, R.; Kligfield, P. Mitral valve prolapse: Causes, clinical manifestations, and management. Ann. Intern. Med. 1989, 111, 305–317. [Google Scholar] [CrossRef]
- Zouridakis, E.G.; Parthenakis, F.I.; Kochiadakis, G.E.; Kanoupakis, E.M.; Vardas, P.E. QT dispersion in patients with mitral valve prolapse is related to the echocardiographic degree of the prolapse and mitral leaflet thickness. Europace 2001, 3, 292–298. [Google Scholar] [CrossRef]
- O’Neal, W.T.; Singleton, M.J.; Roberts, J.D.; Tereshchenko, L.G.; Sotoodehnia, N.; Chen, L.Y.; Marcus, G.M.; Soliman, E.Z. Association Between QT-Interval Components and Sudden Cardiac Death: The ARIC Study (Atherosclerosis Risk in Communities). Circ. Arrhythm Electrophysiol. 2017, 10, e005485. [Google Scholar] [CrossRef]
- Giudicessi, J.R.; Rohatgi, R.K.; Bos, J.M.; Ackerman, M.J. Prevalence and clinical phenotype of concomitant long QT syndrome and arrhythmogenic bileaflet mitral valve prolapse. Int. J. Cardiol. 2019, 274, 175–178. [Google Scholar] [CrossRef]
- Kaya, Ü.; Eren, H. Fragmented QRS may be associated with complex ventricular arrhythmias in mitral valve prolapse. Minerva Cardioangiol. 2020, 68, 577–585. [Google Scholar] [CrossRef]
- Kligfield, P.; Hochreiter, C.; Kramer, H.; Devereux, R.B.; Niles, N.; Kramer-Fox, R.; Borer, J.S. Complex arrhythmias in mitral regurgitation with and without mitral valve prolapse: Contrast to arrhythmias in mitral valve prolapse without mitral regurgitation. Am. J. Cardiol. 1985, 55 Pt 1, 1545–1549. [Google Scholar] [CrossRef]
- Miller, M.A.; Devesa, A.; Robson, P.M.; Liao, S.L.; Pyzik, R.; El-Eshmawi, A.; Boateng, P.; Pandis, D.; Dukkipati, S.R.; Reddy, V.Y.; et al. Arrhythmic Mitral Valve Prolapse With Only Mild or Moderate Mitral Regurgitation: Characterization of Myocardial Substrate. JACC Clin. Electrophysiol. 2023, 9, 1709–1716. [Google Scholar] [CrossRef]
- Korovesis, T.G.; Koutrolou-Sotiropoulou, P.; Katritsis, D.G. Arrhythmogenic Mitral Valve Prolapse. Arrhythm Electrophysiol. Rev. 2022, 11, e16. [Google Scholar] [CrossRef] [PubMed]
- Lichstein, E. Site of origin of ventricular premature beats in patients with mitral valve prolapse. Am. Heart J. 1980, 100, 450–457. [Google Scholar] [CrossRef] [PubMed]
- Boudoulas, H.; Schaal, S.F.; Stang, J.M.; Fontana, M.E.; Kolibash, A.J.; Wooley, C.F. Mitral valve prolapse: Cardiac arrest with long-term survival. Int. J. Cardiol. 1990, 26, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Hohnloser, S.; Weiss, M.; Zeiher, A.; Wollschläger, H.; Hust, M.H.; Just, H. Sudden cardiac death recorded during ambulatory electrocardiographic monitoring. Clin. Cardiol. 1984, 7, 517–523. [Google Scholar] [CrossRef] [PubMed]
- Pradella, S.; Grazzini, G.; Brandani, M.; Calistri, L.; Nardi, C.; Mori, F.; Miele, V.; Colagrande, S. Cardiac magnetic resonance in patients with mitral valve prolapse: Focus on late gadolinium enhancement and T1 mapping. Eur. Radiol. 2019, 29, 1546–1554. [Google Scholar] [CrossRef]
- Basso, C.; Calabrese, F.; Corrado, D.; Thiene, G. Postmortem diagnosis in sudden cardiac death victims: Macroscopic, microscopic and molecular findings. Cardiovasc. Res. 2001, 50, 290–300. [Google Scholar] [CrossRef]
- Cobbs, B.W., Jr.; King, S.B., 3rd. Ventricular buckling: A factor in the abnormal ventriculogram and peculiar hemodynamics associated with mitral valve prolapse. Am. Heart J. 1977, 93, 741–758. [Google Scholar] [CrossRef]
- Huttin, O.; Pierre, S.; Venner, C.; Voilliot, D.; Sellal, J.M.; Aliot, E.; Sadoul, N.; Juillière, Y.; Selton-Suty, C. Interactions between mitral valve and left ventricle analysed by 2D speckle tracking in patients with mitral valve prolapse: One more piece to the puzzle. Eur. Heart J. Cardiovasc. Imaging 2017, 18, 323–331. [Google Scholar] [CrossRef]
- Chesler, E.; King, R.A.; Edwards, J.E. The myxomatous mitral valve and sudden death. Circulation 1983, 67, 632–639. [Google Scholar] [CrossRef]
- Bello, D.; Fieno, D.S.; Kim, R.J.; Pereles, F.S.; Passman, R.; Song, G.; Kadish, A.H.; Goldberger, J.J. Infarct morphology identifies patients with substrate for sustained ventricular tachycardia. J. Am. Coll. Cardiol. 2005, 45, 1104–1108. [Google Scholar] [CrossRef]
- Bogun, F.M.; Desjardins, B.; Good, E.; Gupta, S.; Crawford, T.; Oral, H.; Ebinger, M.; Pelosi, F.; Chugh, A.; Jongnarangsin, K.; et al. Delayed-enhanced magnetic resonance imaging in nonischemic cardiomyopathy: Utility for identifying the ventricular arrhythmia substrate. J. Am. Coll. Cardiol. 2009, 53, 1138–1145. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, A.; Azevedo, C.F.; Cheng, A.; Gupta, S.N.; Bluemke, D.A.; Foo, T.K.; Gerstenblith, G.; Weiss, R.G.; Marbán, E.; Tomaselli, G.F.; et al. Infarct tissue heterogeneity by magnetic resonance imaging identifies enhanced cardiac arrhythmia susceptibility in patients with left ventricular dysfunction. Circulation 2007, 115, 2006–2014. [Google Scholar] [CrossRef] [PubMed]
- Maruyama, T.; Fukata, M. Increased coupling interval variability-mechanistic, diagnostic and prognostic implication of premature ventricular contractions and underlying heart diseases. Circ. J. 2015, 79, 2317–2319. [Google Scholar] [CrossRef] [PubMed]
- Franz, M.R. Mechano-electrical feedback. Cardiovasc. Res. 2000, 45, 263–266. [Google Scholar] [CrossRef] [PubMed]
- Syed, F.F.; Ackerman, M.J.; McLeod, C.J.; Kapa, S.; Mulpuru, S.K.; Sriram, C.S.; Cannon, B.C.; Asirvatham, S.J.; Noseworthy, P.A. Sites of Successful Ventricular Fibrillation Ablation in Bileaflet Mitral Valve Prolapse Syndrome. Circ. Arrhythm Electrophysiol. 2016, 9, e004005. [Google Scholar] [CrossRef]
- Marano, P.J.; Lim, L.J.; Sanchez, J.M.; Alvi, R.; Nah, G.; Badhwar, N.; Gerstenfeld, E.P.; Tseng, Z.H.; Marcus, G.M.; Delling, F.N. Long-term outcomes of ablation for ventricular arrhythmias in mitral valve prolapse. J. Interv. Card. Electrophysiol. 2021, 61, 145–154. [Google Scholar] [CrossRef]
- Levine, R.A.; Stathogiannis, E.; Newell, J.B.; Harrigan, P.; Weyman, A.E. Reconsideration of echocardiographic standards for mitral valve prolapse: Lack of association between leaflet displacement isolated to the apical four chamber view and independent echocardiographic evidence of abnormality. J. Am. Coll. Cardiol. 1988, 11, 1010–1019. [Google Scholar] [CrossRef]
- Fulton, B.L.; Liang, J.J.; Enriquez, A.; Garcia, F.C.; Supple, G.E.; Riley, M.P.; Schaller, R.D.; Dixit, S.; Callans, D.J.; Marchlinski, F.E.; et al. Imaging characteristics of papillary muscle site of origin of ventricular arrhythmias in patients with mitral valve prolapse. J. Cardiovasc. Electrophysiol. 2018, 29, 146–153. [Google Scholar] [CrossRef]
- Lancellotti, P.; Tribouilloy, C.; Hagendorff, A.; Popescu, B.A.; Edvardsen, T.; Pierard, L.A.; Badano, L.; Zamorano, J.L. Recommendations for the echocardiographic assessment of native valvular regurgitation: An executive summary from the European Association of Cardiovascular Imaging. Eur. Heart J. Cardiovasc. Imaging 2013, 14, 611–644. [Google Scholar] [CrossRef]
- Levine, R.A.; Handschumacher, M.D.; Sanfilippo, A.J.; Hagege, A.A.; Harrigan, P.; Marshall, J.E.; Weyman, A.E. Three-dimensional echocardiographic reconstruction of the mitral valve, with implications for the diagnosis of mitral valve prolapse. Circulation 1989, 80, 589–598. [Google Scholar] [CrossRef]
- Vahanian, A.; Beyersdorf, F.; Praz, F.; Milojevic, M.; Baldus, S.; Bauersachs, J.; Capodanno, D.; Conradi, L.; De Bonis, M.; De Paulis, R.; et al. 2021 ESC/EACTS Guidelines for the management of valvular heart disease. Eur. Heart J. 2022, 43, 561–632. [Google Scholar] [CrossRef] [PubMed]
- Otto, C.M.; Nishimura, R.A.; Bonow, R.O.; Carabello, B.A.; Erwin, J.P., 3rd; Gentile, F.; Jneid, H.; Krieger, E.V.; Mack, M.; McLeod, C.; et al. 2020 ACC/AHA Guideline for the Management of Patients With Valvular Heart Disease: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 2021, 143, e72–e227. [Google Scholar] [CrossRef] [PubMed]
- Topilsky, Y.; Michelena, H.; Bichara, V.; Maalouf, J.; Mahoney, D.W.; Enriquez-Sarano, M. Mitral valve prolapse with mid-late systolic mitral regurgitation: Pitfalls of evaluation and clinical outcome compared with holosystolic regurgitation. Circulation 2012, 125, 1643–1651. [Google Scholar] [CrossRef]
- Boudoulas, K.D.; Boudoulas, H. Floppy mitral valve (FMV)/mitral valve prolapse (MVP) and the FMV/MVP syndrome: Pathophysiologic mechanisms and pathogenesis of symptoms. Cardiology 2013, 126, 69–80. [Google Scholar] [CrossRef]
- Lang, R.M.; Tsang, W.; Weinert, L.; Mor-Avi, V.; Chandra, S. Valvular heart disease. The value of 3-dimensional echocardiography. J. Am. Coll. Cardiol. 2011, 58, 1933–1944. [Google Scholar] [CrossRef]
- Mantegazza, V.; Gripari, P.; Tamborini, G.; Muratori, M.; Fusini, L.; Ghulam Ali, S.; Garlaschè, A.; Pepi, M. 3D echocardiography in mitral valve prolapse. Front. Cardiovasc. Med. 2022, 9, 1050476. [Google Scholar] [CrossRef]
- Muthukumar, L.; Rahman, F.; Jan, M.F.; Shaikh, A.; Kalvin, L.; Dhala, A.; Jahangir, A.; Tajik, A.J. The Pickelhaube Sign: Novel Echocardiographic Risk Marker for Malignant Mitral Valve Prolapse Syndrome. JACC Cardiovasc. Imaging 2017, 10, 1078–1080. [Google Scholar] [CrossRef] [PubMed]
- Ignatowski, D.; Schweitzer, M.; Pesek, K.; Jain, R.; Muthukumar, L.; Khandheria, B.K.; Tajik, A.J. Pickelhaube Spike, a High-Risk Marker for Bileaflet Myxomatous Mitral Valve Prolapse: Sonographer’s Quest for the Highest Spike. J. Am. Soc. Echocardiogr. 2020, 33, 639–640. [Google Scholar] [CrossRef]
- Lauretta, L.; Casalino, G.; Amzulescu, M.; David-Cojocariu, A.; Unger, P. How to improve tissue Doppler imaging sensitivity to detect the Pickelhaube sign. Eur. Heart J. Cardiovasc. Imaging 2020, 21, 746. [Google Scholar] [CrossRef]
- Pace, N.; Sellal, J.M.; Venner, C.; Mandry, D.; Marie, P.Y.; Filippetti, L.; Echivard, M.; Fraix, A.; Girerd, N.; Lamiral, Z.; et al. Myocardial deformation in malignant mitral valve prolapse: A shifting paradigm to dynamic mitral valve-ventricular interactions. Front. Cardiovasc. Med. 2023, 10, 1140216. [Google Scholar] [CrossRef]
- Florescu, M.; Benea, D.C.; Rimbas, R.C.; Cerin, G.; Diena, M.; Lanzzillo, G.; Enescu, O.A.; Cinteza, M.; Vinereanu, D. Myocardial systolic velocities and deformation assessed by speckle tracking for early detection of left ventricular dysfunction in asymptomatic patients with severe primary mitral regurgitation. Echocardiography 2012, 29, 326–333. [Google Scholar] [CrossRef]
- Ermakov, S.; Gulhar, R.; Lim, L.; Bibby, D.; Fang, Q.; Nah, G.; Abraham, T.P.; Schiller, N.B.; Delling, F.N. Left ventricular mechanical dispersion predicts arrhythmic risk in mitral valve prolapse. Heart 2019, 105, 1063–1069. [Google Scholar] [CrossRef] [PubMed]
- van Wijngaarden, A.L.; de Riva, M.; Hiemstra, Y.L.; van der Bijl, P.; Fortuni, F.; Bax, J.J.; Delgado, V.; Ajmone Marsan, N. Parameters associated with ventricular arrhythmias in mitral valve prolapse with significant regurgitation. Heart 2021, 107, 411–418. [Google Scholar] [CrossRef] [PubMed]
- Brainin, P.; Biering-Sørensen, S.R.; Møgelvang, R.; Søgaard, P.; Jensen, J.S.; Biering-Sørensen, T. Postsystolic Shortening by Speckle Tracking Echocardiography Is an Independent Predictor of Cardiovascular Events and Mortality in the General Population. J. Am. Heart Assoc. 2018, 7, e008367. [Google Scholar] [CrossRef] [PubMed]
- Muthukumar, L.; Jahangir, A.; Jan, M.F.; Galazka, P.; Umland, M.; Schweitzer, M.R.; Perez Moreno, A.C.; Singh, M.; Khandheria, B.K.; Tajik, A.J. Left Ventricular Global and Regional Deformation in Arrhythmic Myxomatous Bileaflet Mitral Valve Prolapse Syndrome. JACC Cardiovasc. Imaging 2020, 13, 1842–1844. [Google Scholar] [CrossRef]
- Feuchtner, G.M.; Alkadhi, H.; Karlo, C.; Sarwar, A.; Meier, A.; Dichtl, W.; Leschka, S.; Blankstein, R.; Gruenenfelder, J.; Stolzmann, P.; et al. Cardiac CT angiography for the diagnosis of mitral valve prolapse: Comparison with echocardiography1. Radiology 2010, 254, 374–383. [Google Scholar] [CrossRef]
- Chuang, M.L.; Hibberd, M.G.; Salton, C.J.; Beaudin, R.A.; Riley, M.F.; Parker, R.A.; Douglas, P.S.; Manning, W.J. Importance of imaging method over imaging modality in noninvasive determination of left ventricular volumes and ejection fraction: Assessment by two- and three-dimensional echocardiography and magnetic resonance imaging. J. Am. Coll. Cardiol. 2000, 35, 477–484. [Google Scholar] [CrossRef]
- Salton, C.J.; Chuang, M.L.; O’Donnell, C.J.; Kupka, M.J.; Larson, M.G.; Kissinger, K.V.; Edelman, R.R.; Levy, D.; Manning, W.J. Gender differences and normal left ventricular anatomy in an adult population free of hypertension. A cardiovascular magnetic resonance study of the Framingham Heart Study Offspring cohort. J. Am. Coll. Cardiol. 2002, 39, 1055–1060. [Google Scholar] [CrossRef]
- Han, Y.; Peters, D.C.; Salton, C.J.; Bzymek, D.; Nezafat, R.; Goddu, B.; Kissinger, K.V.; Zimetbaum, P.J.; Manning, W.J.; Yeon, S.B. Cardiovascular magnetic resonance characterization of mitral valve prolapse. JACC Cardiovasc. Imaging 2008, 1, 294–303. [Google Scholar] [CrossRef]
- Garg, P.; Swift, A.J.; Zhong, L.; Carlhäll, C.J.; Ebbers, T.; Westenberg, J.; Hope, M.D.; Bucciarelli-Ducci, C.; Bax, J.J.; Myerson, S.G. Assessment of mitral valve regurgitation by cardiovascular magnetic resonance imaging. Nat. Rev. Cardiol. 2020, 17, 298–312. [Google Scholar] [CrossRef]
- Lee, J.H.; Uhm, J.S.; Suh, Y.J.; Kim, M.; Kim, I.S.; Jin, M.N.; Cho, M.S.; Yu, H.T.; Kim, T.H.; Hong, Y.J.; et al. Usefulness of cardiac magnetic resonance images for prediction of sudden cardiac arrest in patients with mitral valve prolapse: A multicenter retrospective cohort study. BMC Cardiovasc. Disord. 2021, 21, 546. [Google Scholar] [CrossRef] [PubMed]
- Garbi, M.; Lancellotti, P.; Sheppard, M.N. Mitral valve and left ventricular features in malignant mitral valve prolapse. Open Heart 2018, 5, e000925. [Google Scholar] [CrossRef] [PubMed]
- Mewton, N.; Liu, C.Y.; Croisille, P.; Bluemke, D.; Lima, J.A. Assessment of myocardial fibrosis with cardiovascular magnetic resonance. J. Am. Coll. Cardiol. 2011, 57, 891–903. [Google Scholar] [CrossRef]
- Sheppard, M.N.; Steriotis, A.K.; Sharma, S. Letter by Sheppard et al. Regarding Article, “Arrhythmic Mitral Valve Prolapse and Sudden Cardiac Death”. Circulation 2016, 133, e458. [Google Scholar] [CrossRef]
- Kitkungvan, D.; Nabi, F.; Kim, R.J.; Bonow, R.O.; Khan, M.A.; Xu, J.; Little, S.H.; Quinones, M.A.; Lawrie, G.M.; Zoghbi, W.A.; et al. Myocardial Fibrosis in Patients With Primary Mitral Regurgitation With and Without Prolapse. J. Am. Coll. Cardiol. 2018, 72, 823–834. [Google Scholar] [CrossRef] [PubMed]
- White, J.A.; Fine, N.M.; Gula, L.; Yee, R.; Skanes, A.; Klein, G.; Leong-Sit, P.; Warren, H.; Thompson, T.; Drangova, M.; et al. Utility of Cardiovascular Magnetic Resonance in Identifying Substrate for Malignant Ventricular Arrhythmias. Circ. Cardiovasc. Imaging 2012, 5, 12–20. [Google Scholar] [CrossRef] [PubMed]
- Koo, H.J.; Kang, J.W.; Oh, S.Y.; Kim, D.H.; Song, J.M.; Kang, D.H.; Song, J.K.; Kim, J.B.; Jung, S.H.; Choo, S.J.; et al. Cardiac computed tomography for the localization of mitral valve prolapse: Scallop-by-scallop comparisons with echocardiography and intraoperative findings. Eur. Heart J. Cardiovasc. Imaging 2019, 20, 550–557. [Google Scholar] [CrossRef]
- Serruys, P.W.; Hara, H.; Garg, S.; Kawashima, H.; Nørgaard, B.L.; Dweck, M.R.; Bax, J.J.; Knuuti, J.; Nieman, K.; Leipsic, J.A.; et al. Coronary Computed Tomographic Angiography for Complete Assessment of Coronary Artery Disease: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2021, 78, 713–736. [Google Scholar] [CrossRef]
- Comparative effectiveness of initial computed tomography and invasive coronary angiography in women and men with stable chest pain and suspected coronary artery disease: Multicentre randomised trial. BMJ 2022, 379, e071133. [CrossRef]
- Alkadhi, H.; Wildermuth, S.; Bettex, D.A.; Plass, A.; Baumert, B.; Leschka, S.; Desbiolles, L.M.; Marincek, B.; Boehm, T. Mitral regurgitation: Quantification with 16-detector row CT-initial experience. Radiology 2006, 238, 454–463. [Google Scholar] [CrossRef]
- Delgado, V.; Tops, L.F.; Schuijf, J.D.; de Roos, A.; Brugada, J.; Schalij, M.J.; Thomas, J.D.; Bax, J.J. Assessment of mitral valve anatomy and geometry with multislice computed tomography. JACC Cardiovasc. Imaging 2009, 2, 556–565. [Google Scholar] [CrossRef] [PubMed]
- Morris, M.F.; Maleszewski, J.J.; Suri, R.M.; Burkhart, H.M.; Foley, T.A.; Bonnichsen, C.R.; Anavekar, N.S.; Young, P.M.; Williamson, E.E.; Glockner, J.F.; et al. CT and MR imaging of the mitral valve: Radiologic-pathologic correlation. Radiographics 2010, 30, 1603–1620. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, N.; Al-Shehri, H.; Chan, K.; Mesana, T.; Chan, V.; Chen, L.; Yam, Y.; Chow, B.J. Characterization of mitral valve prolapse with cardiac computed tomography: Comparison to echocardiographic and intraoperative findings. Int. J. Cardiovasc. Imaging 2012, 28, 855–863. [Google Scholar] [CrossRef]
- Parwani, P.; Avierinos, J.F.; Levine, R.A.; Delling, F.N. Mitral Valve Prolapse: Multimodality Imaging and Genetic Insights. Prog. Cardiovasc. Dis. 2017, 60, 361–369. [Google Scholar] [CrossRef] [PubMed]
- Tribouilloy, C.; Grigioni, F.; Avierinos, J.F.; Barbieri, A.; Rusinaru, D.; Szymanski, C.; Ferlito, M.; Tafanelli, L.; Bursi, F.; Trojette, F.; et al. Survival implication of left ventricular end-systolic diameter in mitral regurgitation due to flail leaflets a long-term follow-up multicenter study. J. Am. Coll. Cardiol. 2009, 54, 1961–1968. [Google Scholar] [CrossRef]
- Mantegazza, V.; Volpato, V.; Gripari, P.; Ghulam Ali, S.; Fusini, L.; Italiano, G.; Muratori, M.; Pontone, G.; Tamborini, G.; Pepi, M. Multimodality imaging assessment of mitral annular disjunction in mitral valve prolapse. Heart 2021, 107, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Toh, H.; Mori, S.; Izawa, Y.; Fujita, H.; Miwa, K.; Suzuki, M.; Takahashi, Y.; Toba, T.; Watanabe, Y.; Kono, A.K.; et al. Prevalence and extent of mitral annular disjunction in structurally normal hearts: Comprehensive 3D analysis using cardiac computed tomography. Eur. Heart J. Cardiovasc. Imaging 2021, 22, 614–622. [Google Scholar] [CrossRef] [PubMed]
- Enriquez-Sarano, M. Mitral Annular Disjunction: The Forgotten Component of Myxomatous Mitral Valve Disease. JACC Cardiovasc. Imaging 2017, 10, 1434–1436. [Google Scholar] [CrossRef]
- Zeppenfeld, K.; Tfelt-Hansen, J.; de Riva, M.; Winkel, B.G.; Behr, E.R.; Blom, N.A.; Charron, P.; Corrado, D.; Dagres, N.; de Chillou, C.; et al. 2022 ESC Guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death. Eur. Heart J. 2022, 43, 3997–4126. [Google Scholar] [CrossRef]
- Cantillon, D.J. Evaluation and management of premature ventricular complexes. Cleve. Clin. J. Med. 2013, 80, 377–387. [Google Scholar] [CrossRef]
- Hong, T.; Yang, M.; Zhong, L.; Lee, Y.H.; Vaidya, V.R.; Asirvatham, S.J.; Ackerman, M.J.; Pislaru, S.V.; Suri, R.M.; Slusser, J.P.; et al. Ventricular premature contraction associated with mitral valve prolapse. Int. J. Cardiol. 2016, 221, 1144–1149. [Google Scholar] [CrossRef] [PubMed]
- Abbadi, D.R.; Purbey, R.; Poornima, I.G. Mitral valve repair is an effective treatment for ventricular arrhythmias in mitral valve prolapse syndrome. Int. J. Cardiol. 2014, 177, e16–e18. [Google Scholar] [CrossRef] [PubMed]
- Alqarawi, W.; Birnie, D.H.; Burwash, I.G. Mitral valve repair results in suppression of ventricular arrhythmias and normalization of repolarization abnormalities in mitral valve prolapse. Heart Rhythm Case Rep. 2018, 4, 191–194. [Google Scholar] [CrossRef] [PubMed]

| Classification of MVP-Related VAs | |
|---|---|
| High risk | Sustained VT not originating from the right or left ventricular outflow tract |
| Spontaneous polymorphic NSVT | |
| Rapid NSVT monomorphic (>180 bpm) has been associated with subsequent excess-mortality | |
| Intermediate risk | Polymorphic PVCs |
| NSVT monomorphic, of lower rate (<180 bpm) | |
| Highly frequent or complex PVCs (bigamy and couplets) | |
| Low risk | Patients with frequent PVCs but not complex VAs (and no morphological higher risk features) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Deng, Y.; Liu, J.; Wu, S.; Li, X.; Yu, H.; Tang, L.; Xie, M.; Zhang, C. Arrhythmic Mitral Valve Prolapse: A Comprehensive Review. Diagnostics 2023, 13, 2868. https://doi.org/10.3390/diagnostics13182868
Deng Y, Liu J, Wu S, Li X, Yu H, Tang L, Xie M, Zhang C. Arrhythmic Mitral Valve Prolapse: A Comprehensive Review. Diagnostics. 2023; 13(18):2868. https://doi.org/10.3390/diagnostics13182868
Chicago/Turabian StyleDeng, Yuyan, Jinfeng Liu, Shan Wu, Xiaoming Li, Huimei Yu, Lili Tang, Meng Xie, and Chun Zhang. 2023. "Arrhythmic Mitral Valve Prolapse: A Comprehensive Review" Diagnostics 13, no. 18: 2868. https://doi.org/10.3390/diagnostics13182868
APA StyleDeng, Y., Liu, J., Wu, S., Li, X., Yu, H., Tang, L., Xie, M., & Zhang, C. (2023). Arrhythmic Mitral Valve Prolapse: A Comprehensive Review. Diagnostics, 13(18), 2868. https://doi.org/10.3390/diagnostics13182868






