Cardiac Magnetic Resonance Imaging with Myocardial Strain Assessment Correlates with Cardiopulmonary Exercise Testing in Patients with Pectus Excavatum
Abstract
1. Introduction
2. Materials and Methods
2.1. MRI
2.2. Cardiopulmonary Exercise Testing
2.3. Statistics
3. Results
3.1. Cardiac MRI
3.2. Cardiopulmonary Exercise Testing
3.3. Correlations Between Cardiac MRI and CPET Data
4. Discussion
Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cartoski, M.J.; Nuss, D.; Goretsky, M.J.; Proud, V.K.; Croitoru, D.P.; Gustin, T.; Mitchell, K.; Vasser, E.; Kelly, R.E., Jr. Classification of the dysmorphology of pectus excavatum. J. Pediatr. Surg. 2006, 41, 1573–1581. [Google Scholar] [CrossRef] [PubMed]
- Lollert, A.; Funk, J.; Tietze, N.; Turial, S.; Laudemann, K.; Düber, C.; Staatz, G. Morphologic assessment of thoracic deformities for the preoperative evaluation of pectus excavatum by magnetic resonance imaging. Eur. Radiol. 2015, 25, 785–791. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Granillo, G.A.; Raggio, I.M.; Deviggiano, A.; Bellia-Munzon, G.; Capunay, C.; Nazar, M.; Martinez, J.L.; Carrascosa, P.; Martinez-Ferro, M. Impact of pectus excavatum on cardiac morphology and function according to the site of maximum compression: Effect of physical exertion and respiratory cycle. Eur. Heart J. Cardiovasc. Imaging 2020, 21, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Abu-Tair, T.; Turial, S.; Willershausen, I.; Alkassar, M.; Staatz, G.; Kampmann, C. Evaluating Cardiac Lateralization by MRI to Simplify Estimation of Cardiopulmonary Impairment in Pectus Excavatum. Diagnostics 2023, 13, 844. [Google Scholar] [CrossRef]
- Dore, M.; Triana Junco, P.; Bret, M.; Gomez Cervantes, M.; Muñoz Romo, M.; Jimenez Gomez, J.; Perez Vigara, A.; Parron Pajares, M.; Luis Encinas, J.; Hernandez, F.; et al. Advantages of Cardiac Magnetic Resonance Imaging for Severe Pectus Excavatum Assessment in Children. Eur. J. Pediatr. Surg. 2018, 28, 34–38. [Google Scholar] [CrossRef]
- Monti, L.; Montini, O.; Voulaz, E.; Maagaard, M.; Morenghi, E.; Pilegaard, H.K.; Infante, M. Cardiovascular magnetic resonance assessment of biventricular changes during vacuum bell correction of pectus excavatum. J. Thorac. Dis. 2019, 11, 5398–5406. [Google Scholar] [CrossRef]
- Saleh, R.S.; Finn, J.P.; Fenchel, M.; Moghadam, A.N.; Krishnam, M.; Abrazado, M.; Ton, A.; Habibi, R.; Fonkalsrud, E.W.; Cooper, C.B. Cardiovascular magnetic resonance in patients with pectus excavatum compared with normal controls. J. Cardiovasc. Magn. Reson. 2010, 12, 73. [Google Scholar] [CrossRef]
- Hor, K.N.; Baumann, R.; Pedrizzetti, G.; Tonti, G.; Gottliebson, W.M.; Taylor, M.; Benson, D.W.; Mazur, W. Magnetic resonance derived myocardial strain assessment using feature tracking. J. Vis. Exp. JoVE 2011, 48, 2356. [Google Scholar] [CrossRef]
- Scatteia, A.; Baritussio, A.; Bucciarelli-Ducci, C. Strain imaging using cardiac magnetic resonance. Heart Fail. Rev. 2017, 22, 465–476. [Google Scholar] [CrossRef]
- Lollert, A.; Emrich, T.; Eichstädt, J.; Kampmann, C.; Abu-Tair, T.; Turial, S.; Düber, C.; Kreitner, K.F.; Staatz, G. Differences in myocardial strain between pectus excavatum patients and healthy subjects assessed by cardiac MRI: A pilot study. Eur. Radiol. 2018, 28, 1276–1284. [Google Scholar] [CrossRef]
- Truong, V.T.; Li, C.Y.; Brown, R.L.; Moore, R.A.; Garcia, V.F.; Crotty, E.J.; Taylor, M.D.; Ngo, T.M.N.; Mazur, W. Occult RV systolic dysfunction detected by CMR derived RV circumferential strain in patients with pectus excavatum. PLoS ONE 2017, 12, e0189128. [Google Scholar] [CrossRef] [PubMed]
- Sonaglioni, A.; Nicolosi, G.L.; Granato, A.; Lombardo, M.; Anzà, C.; Ambrosio, G. Reduced Myocardial Strain Parameters in Subjects With Pectus Excavatum: Impaired Myocardial Function or Methodological Limitations Due to Chest Deformity? Semin. Thorac. Cardiovasc. Surg. 2021, 33, 251–262. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Granillo, G.A.; Toselli, L.; Farina, J.; Raggio, I.; Diluca, P.; Fontana, L.; Valle-Anziani, M.; Bordoli, I.; Bellia-Munzon, G.; Martinez-Ferro, M. Usefulness of strain cardiac magnetic resonance for the exposure of mild left ventricular systolic abnormalities in pectus excavatum. J. Pediatr. Surg. 2022, 57, 319–324. [Google Scholar] [CrossRef] [PubMed]
- Abu-Tair, T.; Turial, S.; Hess, M.; Wiethoff, C.M.; Staatz, G.; Lollert, A.; Kampmann, C. Impact of Pectus Excavatum on Cardiopulmonary Function. Ann Thorac Surg 2018, 105, 455–460. [Google Scholar] [CrossRef]
- Maagaard, M.; Heiberg, J. Improved cardiac function and exercise capacity following correction of pectus excavatum: A review of current literature. Ann. Cardiothorac. Surg. 2016, 5, 485–492. [Google Scholar] [CrossRef]
- Kelly, R.E., Jr.; Obermeyer, R.J.; Nuss, D. Diminished pulmonary function in pectus excavatum: From denying the problem to finding the mechanism. Ann. Cardiothorac. Surg. 2016, 5, 466–475. [Google Scholar] [CrossRef]
- Janssen, N.; Daemen, J.H.T.; van Polen, E.J.; Coorens, N.A.; Jansen, Y.J.L.; Franssen, A.J.P.M.; Hulsewé, K.W.E.; Vissers, Y.L.J.; Haecker, F.; Milanez de Campos, J.R.; et al. Pectus Excavatum: Consensus and Controversies in Clinical Practice. Ann. Thorac. Surg. 2023, 116, 191–199. [Google Scholar] [CrossRef]
- Dahle, G.O.; Stangeland, L.; Moen, C.A.; Salminen, P.R.; Haaverstad, R.; Matre, K.; Grong, K. The influence of acute unloading on left ventricular strain and strain rate by speckle tracking echocardiography in a porcine model. Am. J. Physiol. Heart Circ. Physiol. 2016, 310, H1330–H1339. [Google Scholar] [CrossRef]
- Dubowy, K.O.; Baden, W.; Bernitzki, S.; Peters, B. A practical and transferable new protocol for treadmill testing of children and adults. Cardiol. Young 2008, 18, 615–623. [Google Scholar] [CrossRef]
- Deviggiano, A.; Carrascosa, P.; Vallejos, J.; Bellia-Munzon, G.; Vina, N.; Rodríguez-Granillo, G.A.; Martinez-Ferro, M. Relationship between cardiac MR compression classification and CT chest wall indexes in patients with pectus excavatum. J. Pediatr. Surg. 2018, 53, 2294–2298. [Google Scholar] [CrossRef]
- Levine, B.D. VO2max: What do we know, and what do we still need to know? J. Physiol. 2008, 586, 25–34. [Google Scholar] [CrossRef] [PubMed]
- Zens, T.J.; Casar Berazaluce, A.M.; Jenkins, T.M.; Hardie, W.; Alsaied, T.; Tretter, J.T.; Moore, R.; Foster, K.; Fleck, R.J.; Hanke, R.E.; et al. The Severity of Pectus Excavatum Defect Is Associated With Impaired Cardiopulmonary Function. Ann. Thorac. Surg. 2022, 114, 1015–1021. [Google Scholar] [CrossRef]
- Wald, R.M.; Haber, I.; Wald, R.; Valente, A.M.; Powell, A.J.; Geva, T. Effects of regional dysfunction and late gadolinium enhancement on global right ventricular function and exercise capacity in patients with repaired tetralogy of Fallot. Circulation 2009, 119, 1370–1377. [Google Scholar] [CrossRef]
- Albouaini, K.; Egred, M.; Alahmar, A.; Wright, D.J. Cardiopulmonary exercise testing and its application. Heart Br. Card. Soc. 2007, 93, 1285–1292. [Google Scholar] [CrossRef] [PubMed]
- Jaroszewski, D.E.; Velazco, C.S.; Pulivarthi, V.; Arsanjani, R.; Obermeyer, R.J. Cardiopulmonary Function in Thoracic Wall Deformities: What Do We Really Know? Eur. J. Pediatr. Surg. 2018, 28, 327–346. [Google Scholar] [CrossRef] [PubMed]
- Koumbourlis, A.C. Pectus excavatum: Pathophysiology and clinical characteristics. Paediatr. Respir. Rev. 2009, 10, 3–6. [Google Scholar] [CrossRef]
- Casar Berazaluce, A.M.; Jenkins, T.M.; Garrison, A.P.; Hardie, W.D.; Foster, K.E.; Alsaied, T.; Tretter, J.; Moore, R.A.; Fleck, R.J.; Garcia, V.F.; et al. The chest wall gender divide: Females have better cardiopulmonary function and exercise tolerance despite worse deformity in pectus excavatum. Pediatr. Surg. Int. 2020, 36, 1281–1286. [Google Scholar] [CrossRef]
- Raman, S.V.; Dickerson, J.A.; Mazur, W.; Wong, T.C.; Schelbert, E.B.; Min, J.K.; Scandling, D.; Bartone, C.; Craft, J.T.; Thavendiranathan, P.; et al. Diagnostic Performance of Treadmill Exercise Cardiac Magnetic Resonance: The Prospective, Multicenter Exercise CMR’s Accuracy for Cardiovascular Stress Testing (EXACT) Trial. J. Am. Heart Assoc. 2016, 5, 8. [Google Scholar] [CrossRef]
- Jaroszewski, D.E.; Farina, J.M.; Gotway, M.B.; Stearns, J.D.; Peterson, M.A.; Pulivarthi, V.; Bostoros, P.; Abdelrazek, A.S.; Gotimukul, A.; Majdalany, D.S.; et al. Cardiopulmonary Outcomes After the Nuss Procedure in Pectus Excavatum. J. Am. Heart Assoc. 2022, 11, e022149. [Google Scholar] [CrossRef]
- Das, B.B.; Recto, M.R.; Yeh, T. Improvement of cardiopulmonary function after minimally invasive surgical repair of pectus excavatum (Nuss procedure) in children. Ann. Pediatr. Cardiol. 2019, 12, 77–82. [Google Scholar] [CrossRef]
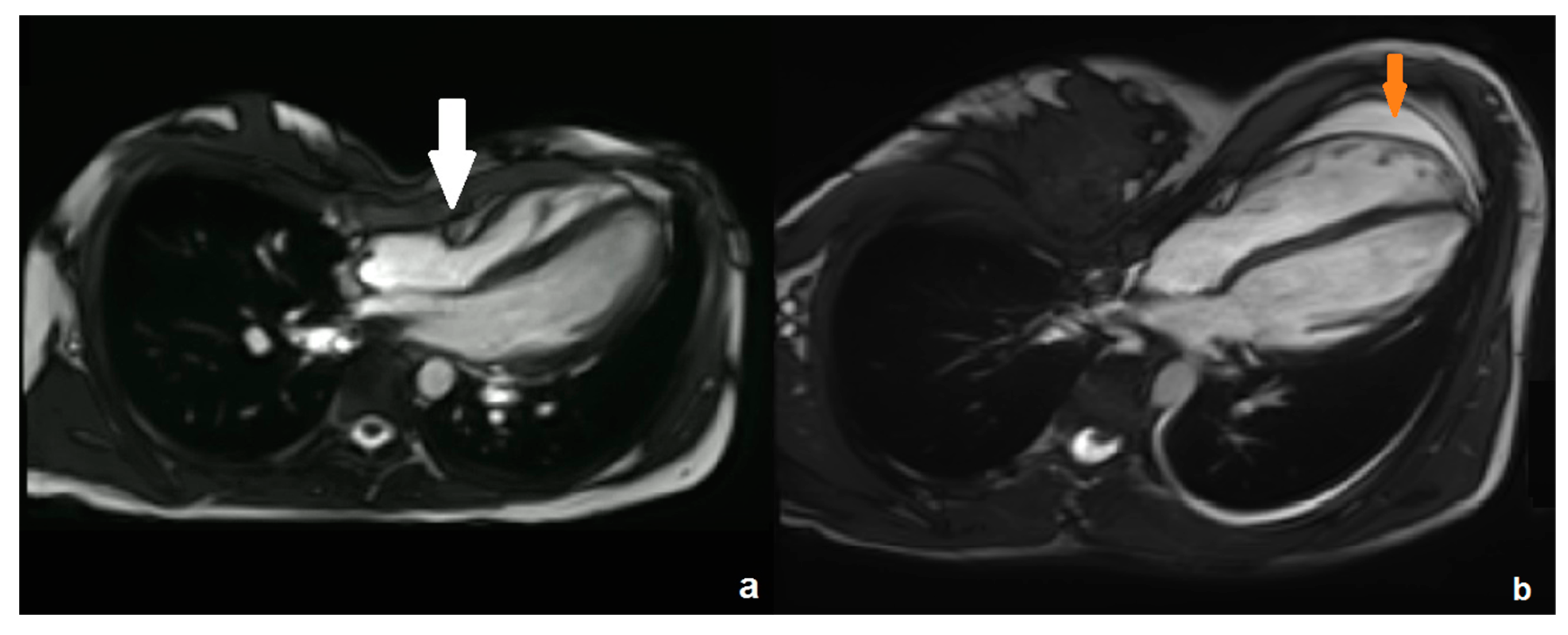
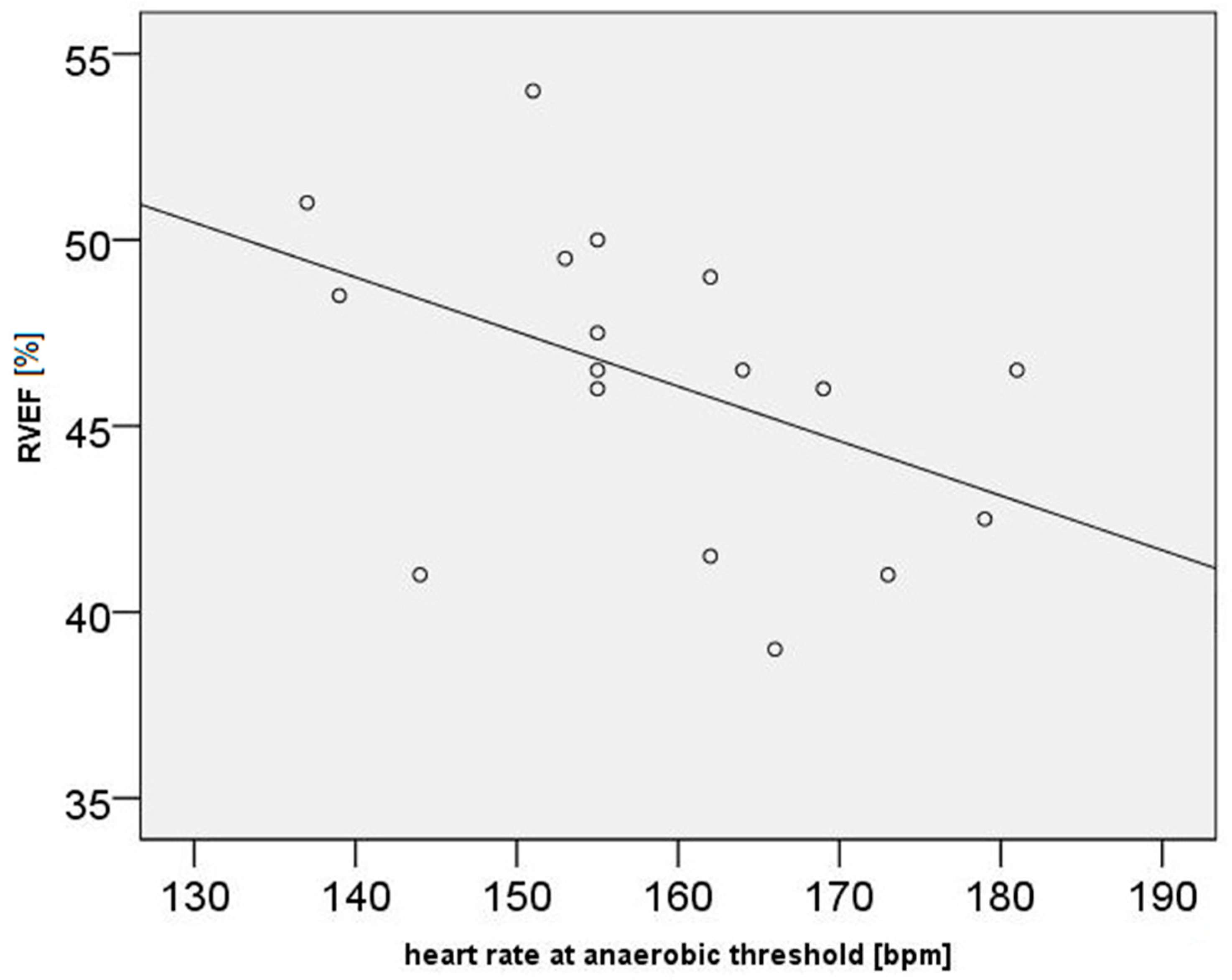
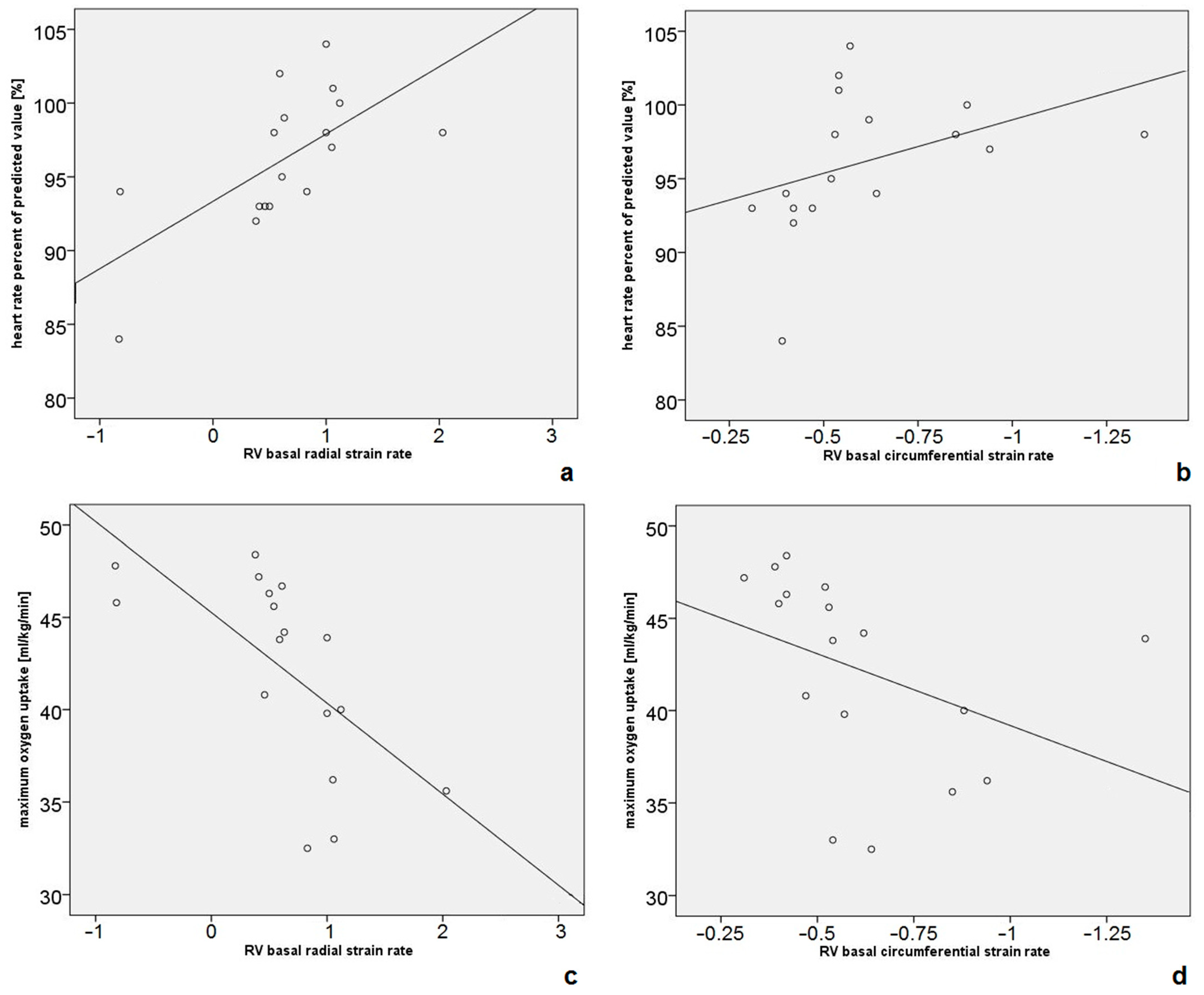
| Parameter | Mean | Standard Deviation | Minimum | Maximum |
|---|---|---|---|---|
| Heart rate [beats per minute] | 74 | 10 | 61 | 95 |
| Left ventricle | ||||
| - normalized end-diastolic volume [mL/m2] | 78.5 | 9.8 | 62 | 92 |
| - normalized end-systolic volume [mL/m2] | 28.2 | 5 | 20 | 36 |
| - normalized stroke volume [mL/m2] | 50.2 | 6 | 42 | 61 |
| - cardiac index [L/min/m2] | 3.83 | 0.63 | 3.1 | 5.6 |
| - ejection fraction [%] | 64 | 3.4 | 58 | 70 |
| Right ventricle | ||||
| - normalized end-diastolic volume [mL/m2] | 94.6 | 11.6 | 72 | 115 |
| - normalized end-systolic volume [mL/m2] | 50.8 | 8.1 | 36 | 67 |
| - normalized stroke volume [mL/m2] | 42.8 | 5.7 | 34 | 54 |
| - cardiac index [L/min/m2] | 3.26 | 0.37 | 2.8 | 4.5 |
| - ejection fraction [%] | 46 | 4.1 | 39 | 54 |
| Parameter | Mean | Standard Deviation | Minimum | Maximum |
|---|---|---|---|---|
| Left ventricle | ||||
| Circumferential strain rate [s−1] | ||||
| - global | −1.43 | 0.2 | −1.83 | −1.09 |
| - basal | −1.23 | 0.27 | −1.76 | −0.74 |
| - mid-ventricular | −1.33 | 0.26 | −2.02 | −0.95 |
| - apical | −2.08 | 0.47 | −3.06 | −1.02 |
| Radial strain rate [s−1] | ||||
| - global | 2.68 | 0.49 | 2.05 | 3.61 |
| - basal | 2.3 | 0.8 | 1.39 | 3.99 |
| - mid-ventricular | 2.67 | 0.56 | 1.82 | 3.8 |
| - apical | 3.63 | 0.78 | 2.36 | 5.68 |
| Global longitudinal strain rate [s−1] | −1.09 | 0.33 | −1.88 | −0.57 |
| Right ventricle | ||||
| Circumferential strain rate [s−1] | ||||
| - global | −0.72 | 0.2 | −1.07 | −0.39 |
| - basal | −0.61 | 0.26 | −1.35 | −0.31 |
| - mid-ventricular | −0.71 | 0.21 | −1.21 | −0.29 |
| - apical | −1.03 | 0.24 | −1.56 | −0.68 |
| Radial strain rate [s−1] | ||||
| - global | 1.06 | 0.32 | 0.54 | 1.47 |
| - basal | 0.62 | 0.67 | 0.83 | 2.03 |
| - mid-ventricular | 0.95 | 0.32 | 0.32 | 1.7 |
| - apical | 1.55 | 0.4 | 0.92 | 2.28 |
| Global longitudinal strain rate [s−1] | −1.15 | 0.24 | −1.51 | −0.82 |
| Parameter | Mean | Standard Deviation | Minimum | Maximum |
|---|---|---|---|---|
| Maximum oxygen uptake (VO2max, mL/kg × min) | 42.2 | 5.2 | 32.5 | 48.4 |
| Percentage of predicted oxygen uptake (VO2max%) | 98.3 | 16.2 | 69.6 | 127.7 |
| Oxygen uptake at anaerobic threshold (VO2AT, mL/kg × min) | 26.3 | 4.4 | 17.7 | 34.7 |
| Maximum oxygen pulse (O2-pulsemax, mL/beat) | 11.1 | 2.6 | 7.2 | 15.9 |
| Percentage of predicted oxygen pulse (O2-pulsemax%) | 87.7 | 15.3 | 53.6 | 110.3 |
| Oxygen pulse at anaerobic threshold (O2-pulseAT, mL/beat) | 8.5 | 1.9 | 5.3 | 12 |
| Maximum physical work capacity (Wattmax, Watt/kg) | 4.6 | 0.56 | 3.5 | 5.5 |
| Maximum heart rate (HRmax, beats/min) | 197 | 8 | 173 | 206 |
| Percentage of predicted heart rate (HRmax%) | 96.2 | 4.7 | 84 | 104 |
| Heart rate at anaerobic threshold (HRAT, beats/min) | 159 | 12.6 | 137 | 181 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lollert, A.; Abu-Tair, T.; Emrich, T.; Kreitner, K.-F.; Sterlin, A.; Kampmann, C.; Staatz, G. Cardiac Magnetic Resonance Imaging with Myocardial Strain Assessment Correlates with Cardiopulmonary Exercise Testing in Patients with Pectus Excavatum. Diagnostics 2024, 14, 2758. https://doi.org/10.3390/diagnostics14232758
Lollert A, Abu-Tair T, Emrich T, Kreitner K-F, Sterlin A, Kampmann C, Staatz G. Cardiac Magnetic Resonance Imaging with Myocardial Strain Assessment Correlates with Cardiopulmonary Exercise Testing in Patients with Pectus Excavatum. Diagnostics. 2024; 14(23):2758. https://doi.org/10.3390/diagnostics14232758
Chicago/Turabian StyleLollert, André, Tariq Abu-Tair, Tilman Emrich, Karl-Friedrich Kreitner, Alexander Sterlin, Christoph Kampmann, and Gundula Staatz. 2024. "Cardiac Magnetic Resonance Imaging with Myocardial Strain Assessment Correlates with Cardiopulmonary Exercise Testing in Patients with Pectus Excavatum" Diagnostics 14, no. 23: 2758. https://doi.org/10.3390/diagnostics14232758
APA StyleLollert, A., Abu-Tair, T., Emrich, T., Kreitner, K.-F., Sterlin, A., Kampmann, C., & Staatz, G. (2024). Cardiac Magnetic Resonance Imaging with Myocardial Strain Assessment Correlates with Cardiopulmonary Exercise Testing in Patients with Pectus Excavatum. Diagnostics, 14(23), 2758. https://doi.org/10.3390/diagnostics14232758






